ABSTRACT
Capsicums (bell peppers) are an important international crop that has specific postharvest handling requirements due to a high susceptibility to quality deterioration from water loss. When harvested green, capsicums are susceptible to chilling injury if stored below ∼7°C for long periods, although red fruit can withstand lower temperatures. Chilling injury usually manifests as spots of surface pitting that can develop into large regions of pitted areas. Extending storage life to enable sea freight from countries distant from their final market (such as New Zealand) means that fruit must be exceptionally well cleaned to allow high humidity to be maintained during storage so that rots do not develop. A range of postharvest treatments that might reduce chilling injury is discussed, although few if any of these have entered commercial practice. This review also covers biology and consumer attributes including colour, taste and flavour, and texture.
Introduction
Sweet capsicums (Capsicum annuum L.), also known as bell or sweet peppers, are a member of the Solanaceae family originating in South America and now an important food crop internationally. Domestication of capsicum occurred in two areas: in the Andean region and in Central America (Bosland and Votava Citation2012). In the Capsicum genus, about 30 species have been described, 5 of which were independently domesticated and cultivated for use as spice and vegetables (C. annuum, C. pubescens, C. chinense, C. frutescens and C. baccatum) (Paran and van der Knaap Citation2007). Capsicums were introduced into Europe by the Spaniards following the expedition of Columbus in the fifteenth century and became widely cultivated (Paran and van der Knaap Citation2007; Monforte et al. Citation2014). In the eighteenth and nineteenth centuries, the sweet types (relatively large, non-pungent with fleshy fruit walls) became dominant in Western Europe and North America (Paran and van der Knaap Citation2007).
In New Zealand, capsicum is grown under covered conditions primarily for supply to the domestic market but with increasing trade to overseas markets. This export trade provides a challenge for the postharvest supply chain. Air freight is often used because of the short transit time and the high frequency of flights, but disadvantages include volume constraints and cost. Sea freight provides an alternative, but longer transit times and lower frequency require application of the best postharvest knowledge and technologies.
The postharvest requirements of capsicums were most recently reviewed in 2012 (Gil and Tudela Citation2012) within a publication covering taxonomy, genetics and growth/development of the wider Capsicum genus (Russo Citation2012). In this review, we focus on commercially grown, fleshy, non-pungent, sweet capsicums, and summarise the recent molecular, biochemical and applied postharvest research directed at understanding the constraints to their storage life and potential for extended storage.
Genetics
Like most members of the Solanaceae family, Capsicum spp. (including the largest group C. annuum) are diploid (n = 12). The genome of C. annuum cv ‘CM33’ (a hot pepper type) has recently been sequenced (Kim et al. Citation2014). It consists of ∼3.48 billion base pairs, which is four times larger than that of tomato, although both have a similar number of protein-coding genes (∼35,000).
Selecting material for postharvest longevity has not been a target for breeders, who instead have made wide crosses to select for improvement in traits including yield, fruit colour, size, shape, pungency, pericarp thickness and disease resistance (Paran and van der Knaap Citation2007). This has led to important advances in these traits.
Natural and chemically induced mutants of capsicum are known that differ in a number of traits. One of the most defining natural mutations is a large deletion in the capsaicin synthase gene, which is responsible for the loss of capsaicinoids, the alkaloids which provide the pungency of hot peppers, unique to the Capsicum genus. This mutation underpins the sweet capsicum cultivar group, although low pungency can be found in other pepper species (Stewart et al. Citation2005). The non-softening characteristic of modern capsicums results from a mutation in the gene coding polygalacturonase (PG) resulting in loss of function of this enzyme. This single base pair change in the gene prevents the full protein sequence from being made (Kim et al. Citation2014).
There is potential for using chemical mutagenesis to generate variability leading to superior fruit quality, as reported for crop production traits (Ibiza et al. Citation2010; Hwang et al. Citation2014) but we have found no reports of this. The application of biotechnological methods for improvement of capsicum species is limited by the recalcitrant nature of the genus to transformation and regeneration, although some transgenic lines with resistance to virus or insects have been generated (Brummell and Pathirana Citation2007). However, the genetic variability inherent in the capsicum cultivars makes it possible to select for fruit with superior postharvest performance. For example, Parsons et al. (Citation2013) found unexpectedly large variation in the cuticle lipids of 50 diverse capsicum genotypes from a world collection, and these accessions differed considerably in the amounts of water loss. Maalekuu et al. (Citation2006) found that the wide variation in water loss during storage of 10 capsicum genotypes correlated with differences in amounts of total lipids, total phospholipids and phospholipid classes. These findings indicate that there is potential to breed much better water retention features into current capsicum material.
Anatomy and ripening
The capsicum fruit is classified as a berry (from a single flower, containing one ovary with numerous seeds; Zhigila et al. Citation2014). The flesh of the fruit itself is the ovary wall, termed pericarp, which can vary in thickness. The interior of the fruit may be partially divided into three to four carpels, with the dividing tissue being fragile. So-called giant cells can be present close to the interior of the pericarp, giving rise to the roughness of the internal surface (Bosland and Votava Citation2012). Numerous seeds are attached to the placenta.
On the epidermis the cuticle coverage is abundant, while stomata are rare. The epidermal cells themselves are very thick (Weryszko-Chmielewska and Michalojc Citation2011). These features are typical plant mechanisms to prevent excessive water loss. However, the epidermis of the green tissue of the peduncle has numerous stomata, and there are large intercellular spaces within this tissue.
Fruit shape trends from blocky (square/rectangular, with a thick pericarp wall) to elongated (extended and tapering, usually with a thinner wall). The blocky types dominate commercial production in New Zealand. Consistent fruit size and shape are important quality factors for commerce. Non-ideal temperature during flowering can have an impact on ovary size and locule number, affecting fruit size and shape (Ali and Kelly Citation1993; Cruz-Huerta et al. Citation2011).
In capsicum, ripening is initiated when fruit have stopped enlarging, which can be contrasted with fruit such as strawberry whose fruit ripen as the fruit enlarge. Fruit that are ready to ripen are termed ‘horticulturally mature’. If fruit are harvested before the breaker stage they do not ripen fully (Krajayklang et al. Citation2000; Aizat et al. Citation2013). During ripening, capsicums lose chlorophyll and synthesise carotenoids, the timing of which can be affected by ethylene. Flavour and aroma also change. Ripening-related textural changes appear to be highly cultivar-dependent, and in many cases can be related to water loss after harvest.
Colour development
The hue and intensity of capsicum colour are important factors in the visual appeal of the fruit as well as the consumer’s perception of ripeness, freshness and nutritional value. Much information about colour composition and development has been obtained from studies of a range of Capsicum spp., including C. annuum, but often there is no information given about the type of capsicum being studied. The assumption is that the chemistry and control of synthesis is similar between hot and sweet types of C. annuum, since the defining characteristic is the presence/absence of functional capsaicin synthase, rather than colour differences.
Capsicums sold as green are usually lines that tend to ripen slowly and can be sold before ripe colours develop. In coloured capsicums, the loss of green colour is ripening-related and genetically controlled. Several genes identified with the process may have commercial significance in new germplasm. The chlorophyll retainer (cl) capsicum is mutated in the STAY-GREEN gene and ripening-related chlorophyll breakdown is prevented (Borovsky and Paran Citation2008). In red fruit, this mutation results in overall brown colouration, while in yellow fruit it results in a persistent green colour. This may be the basis of the new ‘green ripe’ commercial lines alluded to by Brand et al. (Citation2014). The nature of the STAY-GREEN protein and how it affects chlorophyll breakdown is not yet known entirely, but in rice it is thought to be associated with breakdown of thylakoids, the internal structures of the chloroplasts that house the chlorophyll (Park et al. Citation2007). The transcription factor CaGLK has been shown to affect the size of chloroplasts in capsicum (Brand et al. Citation2014). Its discovery has led to speculation that breeding efforts can maximise the benefits of this gene to provide sweeter green fruit with higher amounts of other pigments (particularly in the ripe-green cultivars that carry the STAY-GREEN gene).
The main pigments in red capsicum are the carotenoids capsanthin and capsorubin and their ester derivatives (Minguez-Mosquera and Hornero-Mendez Citation1994; Giuffrida et al. Citation2011; Bonaccorsi et al. Citation2016). Capsanthin-capsorubin synthase (CCS), the enzyme that converts yellow antheraxanthins and violaxanthins to red capsanthin and capsorubin, is unique to Capsicum species (). In orange and yellow capsicums, the CCS gene tends to be absent (Lefebvre et al. Citation1998; Popovsky and Paran Citation2000) or has structural mutations that affect transcription (Ha et al. Citation2007; Rodriguez-Uribe et al. Citation2012). The yellow and orange colours therefore arise primarily from lutein and β-carotene, but also α-carotene, β-cryptoxanthin, zeaxanthin and in the case of some orange lines, very low levels of capsanthin (Ha et al. Citation2007; Guzman et al. Citation2010; Rodriguez-Uribe et al. Citation2012; Tian et al. Citation2015). The colour of brown capsicums arises through a lack of chlorophyll breakdown while red pigments are being synthesised. Purple and black colours arise through the enhanced synthesis of the anthocyanin delphinidin-3-(4-p-coumaroyl)-rutinoside-5-glucoside (Lightbourn et al. Citation2008). The regulatory control of the purple in capsicums is not well understood, but is believed to associate with a set of MYB transcription factors (Borovsky et al. Citation2004; Stommel et al. Citation2009, Citation2014). Purple colouration is continuously present, along with chlorophyll, in immature fruit. The anthocyanins degrade along with chlorophyll as fruit ripen.
Figure 1. Condensed version of the biosynthetic pathway of carotenoids in capsicums and tomatoes. Some steps (such as phytoene to lycopene) require several enzymes. Capsicum spp. possess unique reactions catalysed by capsanthin-capsorubin synthase (CCS). Simplified from schemes in Tanaka et al. (Citation2008) and Gomez-Garcia and Ochoa-Alejo (Citation2013).
The point of colour change in capsicum (often termed ‘breaker’) is usually marked by the appearance of small coloured patches or streaks (initially 5–10% of the fruit surface). Fruit is termed ‘turning’ when these patches extend to 10–30% (turning 1) and 30–70% (turning 2) of the fruit. In some red cultivars, the onset of colour change affects the entire fruit rather than patches and the fruit may briefly appear chocolate brown at the turning stage. Red colours can become more intense once all the chlorophyll is lost. Ha et al. (Citation2007) found that capsanthin content increased 4- to 5-fold as red fruit ripened, but that non-red capsicums did not increase total carotenoid content during maturation.
Carotenoid synthesis is affected by the light received during growth. It is thought that the fruit synthesise carotenoids as a defence against photo-oxidation (Frary and Frary Citation2012). Screening the plants with photo-selective nets can improve the colour development and other quality factors such as ascorbic acid and aroma volatiles in material growing in outdoor shade houses (Alkalai-Tuvia et al. Citation2014; Selahle et al. Citation2015). These effects extend to quality after harvest. Selahle et al. (Citation2015) found that netting colour had an effect on colour. Red and yellow capsicums grown under pearl-coloured netting had a better colour profile, greater firmness, reduced weight loss and greater sensory preference scores after storage at 7.5°C for 21 days and 3 days at 20°C in comparison to fruit grown under yellow or black nets.
Fruit harvested with 80% colour coverage can continue to develop full, even colour. However, growers have observed that green streaks present at harvest are not lost completely, and in the case of red fruit, this leaves darker streaks, so their preference is to harvest at full colour. Colour development is affected by light and temperature, which may explain this observation. Yoshida et al. (Citation2014) reported that green to red colour change was positively affected by light, with limited colour development in the dark. If red fruit were harvested with streaks of green, the loss of chlorophyll was inhibited by temperatures <10°C or >30°C. Ethylene had little positive effect on capsicum colour development in the dark.
For capsicums sold as green fruit, the decision when to harvest is problematic, as there are no external signs to indicate how mature the fruit are, and how close they might be to changing colour. The presence of coloured streaks after long-term storage reduces commercial value.
Taste and flavour
Sweet capsicums, which completely lack the pungent capsaicinoids of hot chillies (Gonzalez-Zamora et al. Citation2013), are popular worldwide for their flavour and taste as well as colour and texture. Although the flavour literature is abundant for fruit crops such as tomato, strawberry and kiwifruit, much less information is available for the flavour compound profile that gives capsicum its distinct sensory characteristics (Rodriguez-Burruezo et al. Citation2010; Eggink et al. Citation2012). Capsicum flavour research has mainly focused on the characterisation of volatile and non-volatile compounds, with few investigations attempting to correlate this information with sensory data. shows the structures and sensory attributes of several compounds key to capsicum flavour.
Table 1. Key compounds associated with flavour perception in capsicum.
The flavour profile of green fruit is less sweet and slightly more bitter than their yellow, orange or red counterparts. Green capsicum also has the ‘green note’ aroma, which is characteristic of some white wines such as Cabernet Franc or Sauvignon blanc (Parr et al. Citation2007). This note is due to 2-isobutyl-3-methoxypyrazine (IBMP; syn. 3-isobutyl-2-methoxypyrazine), which was first identified and characterised from green capsicum (Buttery et al. Citation1969), and which has a particularly low sensory threshold (2 ng L−1 in water). This compound has also been identified in the headspace of several other vegetables, including hot peppers, beans, lettuce, spinach and nasturtium, with relative amounts ranging from 5 ng in broad bean seeds to 5500 ng in hot peppers and 20,000 ng in green capsicums (Murray and Whitfield Citation1975). In capsicum, IBMP amount declines significantly during ripening (Luning et al. Citation1994a; Ryona et al. Citation2010), which may be due in part to demethylation of IBMP to 3-isobutyl-2-hydroxypyrazine, as happens in grapes (Ryona et al. Citation2010).
Sensory evaluation of three ripening stages of capsicum (green, turning and red) using a trained sensory panel confirmed that distinct flavour changes occur during ripening. Green capsicums scored comparatively strongly for bitter, grassy and cucumber, while red capsicums scored strongly for sweetness and sourness (Luning et al. Citation1994b), and turning fruit was intermediate between the two for all attributes measured. Fructose and glucose concentrations increased with capsicum ripening, and principal component analysis confirmed that these sugars, total sugar content and dry matter were related to sweetness in red capsicum, although sucrose was not (Luning et al. Citation1994b). Acid was also found to be an important sensory factor, with citric and ascorbic acid closely associated with perceived sourness and increasing with ripening. However, pH and malic, oxalic, fumaric and pyroglutamic acids appeared to be negatively correlated with sourness, suggesting that total organic acid content should not be used as a marker of capsicum sourness.
Volatile compound analysis of capsicum during ripening using a gas chromatograph equipped with a sniffing port identified several volatile odour compounds common to all ripening stages, and which formed the basis of capsicum flavour. These were 2,3-butanedione (responsible for caramel, sweet flavours), 1-penten-3-one (chemical/pungent, spicy), hexanal (grassy, spicy), 3-carene (rubbery, red capsicum), (Z)-β-ocimene (rancid/sweaty), octanal (fruity) and IBMP (red and green capsicum, lettuce) (Luning et al. Citation1994a). During fruit maturation, the concentration of most of the volatile compounds decreased, but (E)-2-hexen-1-ol (responsible for almond, fruity and sweet odours) and an unidentified compound with a geranium, spicy odour, increased in turning and red fruit (Luning et al. Citation1994a), though these increases were not necessarily detectable by the sniffing panel.
Eggink et al. (Citation2012) combined a metabolomic approach to measure non-volatile and volatile flavour-related compounds in 24 genotypes with taste evaluation by a trained descriptive sensory panel who used 14 taste attributes, of which texture attributes and sweet/sour were the most discriminatory. Using the combined non-volatile and volatile data, the group were able to predict perceived sweetness, sourness, aroma and fruity/apple flavour in the genotypes. Fructose and (E)-2-hexen-1-ol content in particular were highly correlated with sweetness, aroma and fruity/apple flavour.
There is little information available about flavour development/changes in fresh capsicum following postharvest treatments and long-term storage. Green capsicums held under controlled atmosphere (CA) storage conditions of 5 and 10% CO2 for 9 days at 2°C were slightly poorer in flavour compared to fruit stored in air (Cappellini et al. Citation1984). In another experiment, green fruit were stored in low-density polyethylene film bags at 10°C for up to 40 days, with no abnormal flavour development and senescence significantly delayed (Gonzalez and Tiznado Citation1993). Recently, 14 major aromatic volatiles were detected in red capsicum that had been harvested monthly for three months and stored loose or in microperforated bags at 95% relative humidity (RH) for 21 days at 1.5, 4 or 7°C (Lama et al. Citation2016). The aromatic compounds included those responsible for fruity, spicy, pungent, floral sweet, floral green and capsicum aroma notes. Generally, there was a decrease in aromatic production in fruit harvested in the later months. Furthermore, fruit stored at lower temperatures also had decreased amounts of some key compounds, such as IBMP. A reduction in the amount of this compound also appeared to be associated with the use of the packaging; in contrast, hexanal was found in greater quantities. Despite these changes, the authors comment that the overall aroma of the fruit was maintained.
Texture changes during ripening
The typical texture (mouthfeel) of sweet capsicums is usually described as crisp, and the general biochemical hallmark of crisp-textured fruit is that there is little if any pectin breakdown. By far the biggest contributor to firmness changes in harvested capsicums is water loss, and if water loss is prevented many capsicum cultivars are capable of remaining firm for long periods. Postharvest softening that is independent of water loss appears to be strongly cultivar-dependent, and when different studies are compared this can lead to difficulties in establishing cause-and-effect patterns between genetically controlled ripening-associated enzyme activity and cell wall breakdown/softening. We have observed that even in fruit that have lost enough water to be visibly shrivelled and soft, the pericarp texture can remain crisp rather than melting. Unlike wild peppers, softening in most capsicum cultivars occurs very slowly in association with ripening (Rao and Paran Citation2003), and may occur after visual ripening hallmarks (colour change) appear rather than accompanying it.
Cell wall modification in capsicum fruit is restrained, in contrast to melting-textured fruit where substantial depolymerisation of cell wall pectins occurs (Brummell Citation2006). In some capsicum cultivars, pectin depolymerisation was essentially absent during ripening (Harpster et al. Citation2002), and this would appear to be the situation in most domesticated cultivars bred for improved storage life. Jen and Robinson (Citation1984) found that PG activity was less than one-hundredth the activity in tomato. In a wild Indian accession and in two Chinese-developed capsicum cultivars, PG activity peaked at the turning stage but persisted into the red ripe stage, and showed some correlation with softening and increased pectin solubility (Priya Sethu et al. Citation1996; Cheng et al. Citation2008). Ramana Rao et al. (Citation2011) found postharvest increases in PG activity after storage for 18 days at either 25°C or 10°C. However, all these researchers used a PG assay that does not specifically measure endo-PG, the type that would contribute to rapid depolymerisation of pectin, and the majority of the activity was likely due to exo-PG which would have much less effect on wall cohesion. Ripening-related increases in endo-PG mRNA abundance have been noted in some cultivars (Ogasawara et al. Citation2007; Ahmed et al. Citation2011). However, unlike some wild accessions that show considerable softening, domesticated varieties of capsicum contain a mutation that prevents the endo-PG gene from producing a functional protein (Rao and Paran Citation2003; Kim et al., Citation2014).
Despite the lack of pectin depolymerisation in domesticated cultivars, other cell wall changes do occur. High mRNA abundance and activity of β-galactosidase has been detected in red ripe fruit (Ogasawara et al. Citation2007), and substantial amounts of cell wall galactose can be lost (Priya Sethu et al. Citation1996). Ripe fruit also showed depolymerisation of hemicelluloses, including xyloglucan, although this appeared to be unrelated to the ripening-related increase in endo-(1,4)-β-glucanase activity (Harpster et al. Citation2002). It seems likely that the limited softening occurring in capsicums is associated with reductions in hemicellulose molecular weight and loss of pectic galactan side chains rather than to any depolymerisation of pectins.
Ethylene
Sweet capsicum is generally considered to be a non-climacteric fruit because it does not produce a surge in ethylene production at the onset of ripening or the rise is small, and ethylene production is not autocatalytic (Pretel et al. Citation1995). Villaviciencio et al. (Citation1999) measured the ethylene production and respiration rates of 13 cultivars representative of sweet and hot pepper types and found that ethylene production rates during ripening were low (2.2–40.2 pmol kg−1 h−1). In comparison, ripe tomatoes, a climacteric fruit, have ethylene production 100-fold higher (Kader Citation2002). There is considerable variation between cultivars in patterns of ethylene and CO2 production, with some cultivars showing climacteric characteristics (Villavicencio et al. Citation1999; Tan et al. Citation2012).
Capsicum plants have multiple genes encoding enzymes involved in ethylene synthesis. The sweet capsicum ‘Aries’ has at least two different genes encoding 1-aminocyclopropane-1-carboxylic acid synthase (ACS) and six genes encoding 1-aminocyclopropane-1-carboxylate oxygenase (ACO) (Aizat et al. Citation2013). During ripening of ‘Aries’, there was no significant change in mRNA abundance of either gene encoding ACS during ripening, and similarly ACS activity did not significantly alter during ripening. Only one gene (CaACO4) was up-regulated at the onset of ripening (breaker stage), and this gene was negatively regulated by ethylene. Osorio et al. (Citation2012) compared the metabolome and transcriptome of the ripening programme of C. chilense ‘Habanero’ with that of tomato and found that they had similar ethylene-mediated signalling components, but whereas tomatoes showed an increase in accumulation of mRNA for ACS and ACO during ripening that drove climacteric ethylene production, capsicums did not. Nevertheless, there are numerous reports of particular genes showing altered expression in response to treatments with ethylene or the ethylene perception inhibitor 1-methylcyclopropene (1-MCP), indicating that capsicums are not completely unresponsive to ethylene (Harpster et al. Citation1997; Tian et al. Citation2004; Aizat et al. Citation2013).
Ethylene treatments
Ethylene treatment is accepted as a method for stimulating ripening of fruit, but usually for climacteric fruit. The effects of supplemental ethylene on capsicum ripening vary depending on cultivar and often only parts of the ripening programme are affected. In red ‘Aries’, no hastening of ripening was observed after treatment with 4500 pmol L−1 ethylene (Aizat et al. Citation2013), whereas in ‘Robusta’ a similar ethylene treatment accelerated the attainment of full red colour by ∼5 days (Fox et al. Citation2005). When ‘Prador R’ (yellow fruit) and ‘Rubia R’ (red fruit) were harvested with 15% of their surface coloured and exposed to 5400 pmol L−1 ethylene every 6 h, their colour development was accelerated and soluble solids increased, but there was no effect on ascorbic acid, titratable acidity and pH (Cerqueira-Pereira et al. Citation2007).
Continuously treating greenhouse-grown ‘Robusta’ that were harvested at five maturity states (10% red to full red) with 4500 pmol L−1 ethylene at 90% RH hastened ripening of fruit by 6.4 days for fruit harvested in winter and 4 days for fruit harvested in early spring (Fox et al. Citation2005). The accelerated ripening was independent of maturity stage at harvest. Neither ethylene exposure nor harvest maturity had appreciable effects on pulp soluble solids content, total titratable acidity or pH in the ripened fruit. There was also no significant difference in total carotenoids, total ascorbic acid and soluble phenolics at the various stages caused by ethylene exposure. However, there was a difference between the fruit harvested in the different seasons: spring-harvested fruit had twice the concentrations of these chemicals compared to winter-harvested fruit. The researchers concluded that supplemental ethylene permitted earlier harvesting as it was effective at degreening without altering phytochemical synthesis rates.
Because of the differences found in ethylene response in capsicums, it is not surprising that there are differing reports on the effectiveness and wisdom of using 1-MCP on capsicums. Ilic et al. (Citation2012) reported that 1-MCP treatment at the mature green stage of the red cultivar ‘Selika’ and ‘H1530’ (an ‘ever-green’ line) reduced reddening, weight loss and decay when stored at 7°C for 18 days followed by 20°C for 3 days. Aizat et al. (Citation2013) found that treatment of ‘Aries’ at the breaker stage with 1-MCP delayed ripening by about 7 days despite ethylene supplementation not accelerating ripening, indicating that ethylene perception was important for colour change. In contrast, Fernández-Trujillo et al. (Citation2009) found that 1-MCP treatment of the red cultivar ‘Setubal’ had small but detrimental effects on capsicum quality, increasing weight loss (shrivelling), pitting and the incidence of grey mould during a simulated postharvest handling chain. lists some examples of rates and conditions trialled for 1-MCP on capsicums.
Table 2. Examples of 1-methylcyclopropene (1-MCP) treatment regimes for capsicums.
If 1-MCP is effective on a particular cultivar, the concentration applied requires optimisation. Treatment of the red cultivar ‘Selika’ with either 27 or 40 pmol L−1 1-MCP for 24 h gave similar improvements in weight loss and decay, whereas for the green cultivar ‘H1530’, treatment with 27 pmol L−1 1-MCP reduced weight loss but treatment with 40 pmol L−1 actually increased it (Ilic et al. Citation2012). Cao et al. (Citation2012) found that treatment of ‘Sujiao 13’ with 45 pmol L−1 1-MCP gave slightly better retention of chlorophyll and weight than treatments with 22 or 67 pmol L−1.
Treatment of fruit with 1-MCP followed by storage at 20°C reduced respiration and ethylene biosynthesis, and produced small increases in the activities of the antioxidant enzymes superoxide dismutatase, catalase, peroxidase and ascorbate peroxidase, and increased contents of several polyamines, all of which would be expected to help delay senescence (Cao et al. Citation2012). In fruit stored at 4°C for 30 days, chilling injury was slightly reduced after 1-MCP treatment, with a much greater reduction occurring if 1-MCP treatment was combined with modified atmosphere (MA) packaging (Li et al. Citation2011). Peroxidase was the only antioxidant enzyme that showed increased activity under the 1-MCP plus MA packaging conditions.
Chilling injury
Capsicum is a subtropical crop that will suffer chilling injury when exposed to cold temperatures below a cultivar-specific threshold temperature for a sufficient period. The most common chilling injury symptoms in capsicums are surface pitting (that spreads into sheet pitting as symptoms become more pronounced), water soaking, calyx discolouration, poor surface colour development (especially in areas of peel pitting), failure to ripen, accelerated senescence, seed browning, increased microbial decay and shrivelling resulting from increased water loss. The manifestation of these symptoms frequently does not develop until after the cold-storage period, when the fruit are at warmer temperatures. Many biochemical processes are altered during chilling injury, resulting firstly in the appearance of the visible symptoms, and eventually cell death if cold exposure is sufficiently long. The early biochemical effects are reversible if fruit are removed from the chilling stress before irreversible damage occurs.
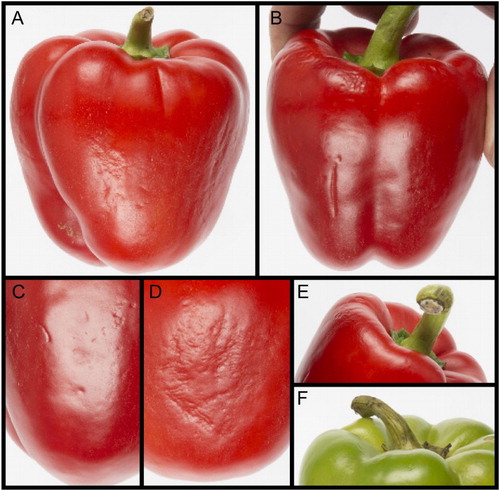
Chilling injury in capsicums first becomes visible as surface dot pitting (Lim et al. Citation2007), which as the disorder becomes more severe can develop into sheet pitting, sunken lesions or even areas of tissue collapse (). The primary cause appears to be cold-induced membrane damage that brings about water movement from inside the cell to outside, exacerbating water loss and resulting in cell collapse. Increased cell separation resulting from altered cell wall modification may also contribute to the symptoms. Areas affected by visible chilling injury show either a partial or complete failure to develop colour as the rest of the fruit ripens, and these areas are highly susceptible to colonisation by pathogenic microorganisms.
Figure 2. Chilling injury in capsicums. (A) Pitting on a fruit after storage at 2°C for 3 weeks. (B) Pitting developing into deeper lesions on the shoulder of a fruit after storage at 2°C for 3 weeks followed by 20°C for 3 days. (C) Close-up of dot pitting. (D) Close-up of pitting extending into sheet pitting. (E) Capsicum postharvest acceptability can also be limited by fungal infections of the pedicel, shown here beginning on the cut surface. (F) Necrotic infection spreading throughout the pedicel, which is turning black.
Chilling injury susceptibility in capsicums depends both on cultivar (Smith et al. Citation2006; Lim and Woolf Citation2010) and maturity (Lin et al. Citation1993). Capsicums are usually staged commercially by colour, as mature green (>95% of the surface green), or ripe (>95% of the surface red, yellow or orange). These two stages are separated in red capsicums by a brief period termed ‘breaker’ or ‘chocolate’, where (depending on cultivar) the fruit either develops redness in small patches that eventually spread to the entire fruit, or appears brown due to the presence of both green chlorophyll and the red carotenoid pigments that accumulate during ripening.
Capsicums at the ripe, coloured stage are relatively resistant to chilling injury. Ripe fruit of cultivars ‘Bison’ (red) and ‘Doria’ (yellow) showed no surface pitting symptoms after storage at 1°C for 2 weeks followed by 21°C for 7 days (Lin et al. Citation1993). In contrast, mature green fruit of both cultivars exhibited symptoms after as little as 3 days of storage at 1°C followed by 21°C for 7 days. Mature red ‘Lamuyo’ capsicums did not develop surface pitting after 6 weeks of storage at 2°C and ‘several’ days at ambient temperature, whereas mature green fruit developed surface pitting after 1 week at 2°C and 1 day at ambient (Serrano et al. Citation1997). Further study, using ‘Plenty’ stored at 1°C, found that fruit at the breaker stage were even more susceptible to chilling injury than mature green fruit (Lim et al. Citation2007). Breaker stage fruit exhibited severe surface pitting with more sheet pitting, deeper peel impressions and increased water loss. This study also confirmed the relative resistance of ripe fruit to chilling injury (Lim et al. Citation2007).
Chilling stress is thought to impair the electron transport pathway in the mitochondria leading to the formation of reactive oxygen species. These damaging ions diffuse through the cell and cause oxidative damage to membrane lipids, impairing membrane fluidity and function. These changes appear as increased malondialdehyde (a lipid breakdown product) and electrolyte leakage. Whitaker (Citation1995) showed membrane lipid changes and increased ion leakage in chilled capsicum only after fruit were transferred to a warmer temperature (∼20°C). The plant cell has a range of processes designed to counteract oxidative stress, including antioxidant metabolites (ascorbate, α-tocopherol, β-carotene, glutathione) and enzymes (superoxide dismutase, ascorbate peroxidase, catalase, glutathione reductase) (Sevillano et al. Citation2009). A robust antioxidant system and high activity of some or all of these enzymes in particular capsicum cultivars has been correlated with resistance to chilling injury, although how the chilling environment might have caused oxidative stress was not identified (Lim et al. Citation2009).
Lin et al. (Citation1993) showed a large rise in the rates of both respiration and ethylene production after cold-stored capsicum fruit were warmed to room temperature (20–22°C). CO2 production was similar in capsicums from 1°C storage that developed chilling injury (mature green) and those that did not (red ripe), suggesting that increased respiration is a response to the cold temperature and is not involved in chilling injury. The role of ethylene in chilling injury symptoms of capsicums is less clear. Lin et al. (Citation1993) showed a 14-fold increase in ethylene (at 20–22°C) in mature green fruit (chilling-sensitive) after storage at 1°C and a smaller increase (7-fold) in ripe fruit (chilling insensitive). Lim et al. (Citation2009) found that after storage the chilling-resistant variety ‘Buchon’ produced much more ethylene, but with no injury symptoms, than the chilling-sensitive ‘Nockgwang’, which did develop symptoms. In contrast, storage of ‘Plenty’ at 1°C or 5°C in MA bags showed that breaker stage fruit produced more ethylene and developed more injury symptoms compared with ripe fruit which had lower ethylene and no symptoms (Lim et al. Citation2007). These conflicting observations suggest that ethylene production in capsicum is probably a response to the low temperature rather than a causal factor in chilling injury symptoms.
Water loss
Water loss and rots are the major postharvest problems affecting capsicums. The extent of weight loss during storage is affected by the maintenance of storage RH, ventilation, packaging, storage duration and temperature, and fruit size (smaller fruit have a greater surface area relative to volume).
Capsicums are very susceptible to water loss (shrivel), since they are a hollow fruit with a high surface area to fresh weight ratio and lack the internal water reserves of solid fruit such as tomato, apple, etc. Relatively low amounts of water loss can result in reduced turgor and firmness that lower fruit quality, shelf life, market value and perceived freshness. Indeed, the postharvest quality of capsicums is frequently determined by the extent of water loss from the fruit since water loss and fruit firmness are directly related. In ‘Camelot’ stored at 20°C, fruit firmness did not change during the first 2 days when fruit lost ∼1.1% of weight, but after that both weight and firmness declined linearly (Díaz-Pérez et al. Citation2007). The lowest acceptable market firmness was reached when fruit had lost 4.5% of their fresh weight, although losses up to 7% have been considered acceptable elsewhere (Díaz-Pérez et al. Citation2007).
There are large differences in weight loss rate between capsicum cultivars, which can differ by 2- to 3-fold (Lownds et al. Citation1993, Citation1994; Kissinger et al. Citation2005). Most water loss occurs through the pericarp surface rather than through the cut calyx (Díaz-Pérez et al. Citation2007). Capsicum fruit lack stomata (Lownds et al. Citation1993) so the cuticle is the primary barrier to water loss, but there appears to be no simple relationship between the rate of water loss and the thickness of the cuticle or with the cutin or wax contents of the cuticle (Kissinger et al. Citation2005; Smith et al. Citation2006; Parsons et al. Citation2012, Citation2013). The proportions of cuticle constituents (Parsons et al. Citation2012, Citation2013), cuticle structure or the presence of cracks may be the most important determinant of permeability (Kissinger et al. Citation2005). The lipid content of the cellular membranes has also been suggested to be a key factor in controlling both water loss and ion leakage (Maalekuu et al. Citation2006).
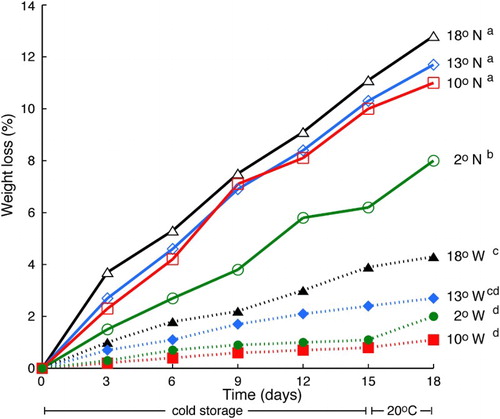
The rate of water loss in capsicums is affected by degree of ripeness. In ‘Maor’ fruit held at 17°C and 85% RH, the rate of weight loss was slightly higher in red ripe than in mature green fruit, but this caused a proportionally much larger decline in relative turgidity in red than in green fruit (Lurie et al. Citation1986). However, in ‘Camelot’, the water loss rate decreased with increasing ripeness (Díaz-Pérez et al. Citation2007). The rate of weight loss in ‘Camelot’ was high and constant for the first 6 days in storage at 20°C, and then declined linearly (Díaz-Pérez et al. Citation2007). This shows that another factor affecting the water loss rate is the initial fruit water content. Fruit with a high water content will lose water faster, and as the fruit water content declines, this rate will slow. However, while the initial water content may affect the rate of water loss, this is a relatively minor consideration and other factors such as cultivar differences are much more important (Lownds et al. Citation1994). In general, capsicums stored at 17–20°C will lose 5% of their fresh weight in approximately 6–9 days (Bojórquez Gálvez et al. Citation2010), with the rate of loss being less at lower temperature and much less if plastic film packaging is used (Moline and Hruschka Citation1977; ).
Figure 3. Effect of temperature and plastic packaging on weight loss in ‘California Wonder’ capsicums. Weight loss (as %) of wrapped (W) and non-wrapped (N) fruit was measured over 15 days of storage at the indicated temperature, followed by 3 days (days 15–18) at a shelf life temperature of 20°C. Letters show statistically different separation of groups at P < 0.05 using Duncan’s multiple range test. Figure redrawn from Moline and Hruschka (Citation1977).
Reducing water loss from capsicums is the priority during storage, since this prevents shrivel and softening, as well as the onset of senescence processes. Fruit water loss is also positively correlated with the severity of chilling injury (Smith et al. Citation2006; Lim et al. Citation2007). A hot water rinsing (55°C for 12 s) and disinfecting treatment combined with brushing has been shown to reduce the occurrence of rots and reduce water loss (Fallik et al. Citation1999). Presumably, the hot water and brushing distributes the cuticular wax more evenly and seals cracks and holes in the cuticle, reducing water permeability. The hot water treatment may also benefit quality retention by providing a heat shock response. A hot water rinsing and brushing treatment followed by storage of the fruit in individual shrink wrap packaging to reduce water loss was an even more effective regime for reducing chilling injury symptoms (Bar-Yosef et al. Citation2009).
Storing capsicums in a high humidity (close to 100% RH) atmosphere prevents water loss, a decreased water potential and decreased firmness (Lurie et al. Citation1986). Since water stress can hasten and/or trigger the onset of ripening in green fruit and senescence in red fruit, holding the fruit in a high-humidity atmosphere can delay these processes (Lurie et al. Citation1986; Kissinger et al. Citation2005). A high-humidity environment can be created using plastic film packaging (Ben-Yehoshua et al. Citation1983), which also reduces chilling injury since water stress is avoided (Meir et al. Citation1995). In the red ‘7158’ and yellow ‘Dinamo’ cultivars, bulk packaging with plastic box liners significantly reduced chilling injury after storage of fruit at 1.5°C or 4°C for 21 days to kill Mediterranean fruit fly (Fallik et al. Citation2012; Castro et al. Citation2016). However, high-humidity atmospheres promote the development of rots (Lownds et al. Citation1994) so fruit must be thoroughly cleaned before storage, for example by using an effective water washer at high pressure to remove microbes and their spores. High-pressure water washing technology to clean the fruit combined with high-humidity packaging has been shown to be successful in pilot studies (O’Donoghue et al. Citation2013). Red capsicums washed with high-pressure water, packaged in boxes with unsealed plastic liners, stored at 7°C for 14 days and then 20°C for 21 days maintained a high level of acceptability.
Storage temperature, humidity and atmosphere
Storing fruit at the lowest temperature at which the fruit is safe from chilling damage is the most important factor for maintaining their shelf life. Humidity is critical to minimise weight loss during storage and resultant shrivelling of the fruit. The storage temperature recommended for capsicum is 7–10°C and humidity recommendation is 95–98% (Kader Citation2002; González-Aguilar Citation2004). Cantwell (Citation2011) recommends storage at 7.5°C. At-harvest fruit temperature will usually be rather higher than this optimum so good practice is to remove heat in a timely and managed process using precooling technology (Kader Citation2002). Forced-air cooling is commonly used for capsicums. Humidity can be controlled through the coolstore technology, or by the use of plastic film packaging.
CA and MA storage involves holding produce in atmospheres different to that of air to delay deteriorative processes such as senescence and ripening. The evidence for CA storage prolonging storage and shelf life of capsicums is not convincing (Leshuk and Saltveit Citation1990). CA treatment does not appear to inhibit water loss or softening (Polderdijk et al. Citation1993). Despite this, the optimal atmosphere conditions for storage of capsicums have been suggested as 2–5% O2 and 2–5% CO2 (Saltveit Citation2001). Outside these ranges, the atmospheres can result in browning, breakdown, off-odours, calyx discolouration and internal softening. In a test on green capsicums stored at 10°C in a range of O2 atmospheres from 1% to 7% in N2, Luo and Mikitzel (Citation1996) reported lower decay, lower internal ethylene and slower ripening in 1% O2 when compared to higher O2 concentrations (3% and above). However, decay was still a major quality issue in these field-grown fruit. Decay reduction has been reported in capsicums stored in 2–3% O2, 3% CO2 at 8°C, an effect remaining after removal to 20°C (Polderdijk et al. Citation1993; Dogan et al. Citation2016).
MA packaging has proven beneficial for maintaining quality of stored capsicums although it has been suggested by a number of researchers that it is the in-package water vapour rather than the O2/CO2 concentration that was responsible for the extended shelf life (Ben-Yehoshua et al. Citation1983; Rodov et al. Citation1995; Saltveit Citation2001; Manolopoulou et al. Citation2010). However, the humidity in the package should not approach 100% as this facilitates condensation on the fruit, supporting the growth of decay microorganisms (Rodov et al. Citation1995).
MA packaging of capsicums in polymeric films is also beneficial because it enables storage at low temperatures that normally induce chilling injuries. Meir et al. (Citation1995) found that storing red ‘Maor’ in various kinds of polyethylene bags with different percentages of perforation (0.064–0.42%) was effective at reducing water loss in fruits stored for 2 weeks at 7–8°C and an additional 3 days at 17°C. They found that the packaging did not significantly increase rot development in the stored fruit during either storage or shelf life and enabled the capsicums to be stored at a reduced temperature (3°C) without the appearance of chilling injury.
Postharvest treatments: physical
Heat shock
Heat treatments can provide control of microbial rots that, for some markets, may be more acceptable than chemical treatment. Capsicums need to be stored at high humidity, which can promote microbial rots if fruit are not scrupulously clean before storage. Capsicum washing is therefore a common practice to aid microbial decontamination. Various chemical washes have been investigated, including 0.25% hydrogen peroxide (Fallik et al. Citation1994), 1% potassium bicarbonate (Fallik et al. Citation1997), 3 mg L−1 aqueous chlorine dioxide (Han et al. Citation2001), 1200 ppm acidified sodium chlorite (Yuk et al. Citation2006) and 2% lactic acid (Alvarado-Casillas et al. Citation2007), but the most simple, convenient and quick procedure is a hot water dip (Fallik et al. Citation1996). Red capsicums dipped in water at 50°C for 3 min exhibited complete prevention or significant reduction in decay caused by Alternaria alternata and Botrytis cinerea without any detrimental effects on quality (Fallik et al. Citation1996). However, heat damage was observed if the treatment was extended to 50°C for 5 min or increased to 55°C for 1 min.
The use of a hot water dip also provides a heat shock, which can induce plant stress response and defence response pathways that aid in maintaining postharvest quality and reduce chilling injury. González-Aguilar et al. (Citation2000) found that a hot water dip of 53°C for 4 min alleviated chilling injury in green bell capsicums stored at 8°C for up to 28 days. However, a simple hot water dip also slightly increased weight loss during storage, possibly due to removal of cuticular wax. Better results were obtained when the hot water washing was followed by packaging in low-density polyethylene plastic film, with weight loss, chilling injury and decay all significantly reduced (González-Aguilar et al. Citation2000) and no detrimental effects on compositional quality observed (Raffo et al. Citation2007).
A commercial-scale hot water rinsing combined with brushing technique has been developed and patented in Israel (Fallik et al. Citation1999). The technique was better than a hot water dip alone or dry brushing alone. Red capsicums treated in this way (a rinse at 55 ± 1°C for 12 ± 2 s was optimal) improved fruit appearance, removed dirt, dust and fungal spores, maintained firmness and reduced decay. Small cracks in the epidermis were sealed, presumably due to the heat melting the cuticular wax and the brushing redistributing it, which reduced water loss. A commercial-scale trial established that the fruit maintained quality, without development of rots, during 15 days shipping at 7°C followed by an additional 4 days at 16–18°C.
Although the biochemical mechanism underlying the effect of a heat treatment has not been established, the respiration rate of the fruit was significantly reduced (Fallik et al. Citation1999), and internal levels of O2 reduced and CO2 increased (González-Aguilar et al. Citation2000). A hot water dip at 50°C reduced electrolyte leakage during storage at 6–7°C (90–95% RH) for 45 days followed by a shelf life at 18–20°C for 3 days (Sakaldas and Kaynas Citation2010), suggesting that membrane integrity was improved. One effect of the heat treatment was that during subsequent storage at 8°C there was an increase or maintenance in the levels of the polyamines putrescine and spermine, natural plant compounds that appear to be involved in reducing chilling injury perhaps by preserving membrane fluidity and integrity at lower temperatures (González-Aguilar et al. Citation2000).
Intermittent warming
Interruption of cold storage with one or more periods of warm temperature has been shown to reduce chilling injury and hence increase storage life in tomato and other fruit. This procedure has not been well studied in capsicums. Early work found that for capsicums stored at 2°C for 15 days followed by 20°C for 3 days, two periods of warming to 20°C for 24 h (after day 6 and day 13 of storage) resulted in significantly less chilling injury compared with fruit stored continuously at 2°C (Moline and Hruschka Citation1977). However, condensation of moisture onto the fruit was a problem when moving to the higher temperature, particularly for wrapped fruit where the condensation did not evaporate quickly, and was associated with increased decay.
Storage of ‘Keystone Resistant Giant No. 3’ at 2.5°C for 13 days with warming to 20°C for 24 h after every third day substantially reduced chilling injury, but caused excessive softening relative to controls (Wang and Baker Citation1979). Frequent brief interruptions of low-temperature storage (at 0°C for 15 days) by warming intervals of 6 h at 18°C every second day were found to almost completely prevent chilling injury symptoms in a yellow capsicum (Geier and Weichmann Citation1985) without reported deleterious effects. Liu et al. (Citation2015) found that cycles of intermittent warming from 4°C to 20°C for 24 h every 7 days delayed the decline in unsaturated fatty acid content, preserving the integrity of cell membranes which could be damaged by low temperature storage. Nevertheless, these regimes would be difficult to implement in a commercial situation.
Low-temperature conditioning
Storage of fruit at intermediate temperature for a period before cold storage can condition the fruit to better withstand cold temperatures. This procedure (also known as step-down cooling) has not been studied much in capsicums. Han et al. (Citation2010) examined the effects of room cooling to 15°C or forced-air cooling to 8°C prior to storage in MA packaging at 0°C, 4°C and 8°C for 28 days followed by 7 days at 7°C. No difference between the cooling regimes was noted in respiration, firmness, weight loss and colouration, although storage at 0°C produced a lower quality in all these parameters than storage at 4°C and 8°C. The authors concluded precooling to 15°C followed by storage at 4°C or 8°C produced the most marketable fruit, although even with MA packaging, storage for 28 days plus 7 days of shelf life was too long for delivering an acceptable product.
Irradiation with ultraviolet light
Irradiation of capsicums with short-wave ultraviolet light (UV-C, wave length 100–280 nm) has been shown to provide a range of beneficial effects on storage quality. Part of the improvement in storage quality is caused by a sanitising effect on fungal conidia that reduces infections, but UV-C also appears to induce the fruits’ natural pathogen resistance pathways. Mercier et al. (Citation2001) found that irradiation of mature green ‘Bell Boy’ reduced the incidence of natural Botrytis cinerea infection by about half when assessed after storage for 28 days at 13°C. A dose of UV-C (peak emission 254 nm) of 0.88 kJ m–2 was optimal in reducing infections without causing tissue damage. UV-C treatment was effective on all maturity stages, although more mature fruit (turning/red) were more susceptible to infection than green fruit. In addition to the germicidal effect, UV-C also induced disease resistance mechanisms, since fruit challenged by pathogen infection 24 h after irradiation already showed increased resistance.
In ‘Zafiro’ at the 90% red stage, a 7 kJ m−2 dose of UV-C was found to reduce decay and maintain firmness for longer during subsequent storage for 18 days at 10°C (Vicente et al. Citation2005). A beneficial effect on chilling injury was also noted. Similar experiments using ‘Cornago’ irradiated at 10 kJ m–2 and stored at 0°C for 21 days showed reduced weight loss and chilling injury that was associated with temporary increases in superoxide dismutase and ascorbate peroxidase, and a more sustained increase in catalase (Cuvi et al. Citation2011). In experiments using the yellow cultivar ‘Golden Bell’, a UV-C treatment reduced chilling injury at 4°C and increased antioxidant capacity and catalase activity (Promyou and Supapvanich Citation2013). However, in a comparison of different treatments, UV-C was not as effective as a hot water dip in reducing decay and ion leakage (Sakaldas and Kaynas Citation2010).
Postharvest treatments: chemical
Gaseous ozone
Ozone (O3) is used as a sanitiser, either in aqueous solution as a dip to wash the fruit, or a gaseous treatment. An advantage over other chemical treatments used as antimicrobial agents is that O3 does not leave detectable residues on the fruit surface, and can even aid in lowering amounts of some pesticide and chemical residues in the skins since they are oxidised by the O3 (Horvitz and Cantalejo Citation2014; Glowacz et al. Citation2015a).
Low-level enrichment of the storage atmosphere with O3 is effective against a wide spectrum of bacterial and fungal contaminants, but effective treatment regimes are commodity-specific and the concentration and duration of treatment need to be optimised to avoid damage to the produce. O3 has been frequently reported to reduce bacterial counts, but its fungistatic effect can disappear with time and in some reports spore production resumed when the produce was removed from an O3-enriched atmosphere (Glowacz et al. Citation2015a). This may be because fungal structures inside wounds on the fruit surface or within rough surfaces (torn peduncle, stem scar) are protected from the oxidising effect of the O3 due to its limited penetration.
Gaseous O3 has been tested as both a pre-storage and a storage treatment for capsicums. A treatment of green capsicums with 7 mg L−1 O3 (3270 ppm v/v) at 22°C and 85% RH for 20 min reduced Escherichia coli O157:H7 counts by 5 log units (Han et al. Citation2002). In a study using fungal inoculations, green capsicums were treated with 3 ppm O3 for 3 days at 12°C and 95% RH, and then stored at 12°C and 95% RH for an additional 24 days (Alwi and Ali Citation2015). The incidence of anthracnose disease (usually due to Colletotrichum spp.) was reduced, without affecting respiration, colour, titratable acidity or firmness. Higher O3 concentrations (7 and 9 ppm) were detrimental, enhancing ripening and increasing membrane permeability, softening and weight loss.
In a study of red capsicums stored at 14°C for 14 days under continuous exposure to O3 at two low concentrations, rots on stem and peduncle were reduced from 25% in controls to 8% at 0.1 ppm O3, and no rots were observed at the higher O3 concentration of 0.3 ppm (Glowacz et al. Citation2015b). The O3 treatments had no significant effects on pH, soluble solids, colour, firmness or weight loss, suggesting that continuous exposure to O3 is a promising technology for maintaining quality in stored capsicums. However, consideration needs to be given to the packaging materials used and the treatment room. For example, the use of packaging such as cardboard and fibreboard provides competing oxidisable substrates for the O3 and reduces its effectiveness.
Methyl jasmonate and methyl salicylate
The jasmonates (notably jasmonic acid and its volatile ester methyl jasmonate) and the salicylates (notably salicylic acid and its volatile ester methyl salicylate) are natural plant hormones that are involved in activating plant disease resistance pathways and in responding to abiotic stresses such as cold (Aghdam and Bodbodak Citation2013). Salicylic acid and methyl jasmonate both activate a common set of genes involved in abiotic and biotic stress responses, in addition to sets of genes that are specific to each hormone (Lee and Choi Citation2013).
Postharvest treatments with methyl jasmonate or methyl salicylate have been used to reduce pathogen infections, ameliorate chilling injury and extend storage life in a range of tropical and subtropical fruit species. Early work showed that a 30 s dip in 25 µM methyl jasmonate reduced the incidence and severity of chilling injury in red ‘Maor’ capsicums that were stored at 2°C for 28 days and assessed after 2 days at 20°C (Meir et al. Citation1996). A treatment with methyl jasmonate vapour was also efficacious. For both dips and vapour treatments, amounts of methyl jasmonate above the optimal concentration were not effective in reducing chilling injury (assessed as surface pitting). Postharvest treatment with methyl jasmonate vapour has also been reported to reduce Botrytis spore production on green fruit of ‘Sammy’ (Tzortzakis et al. Citation2016).
For salicylates, a 15 min postharvest dip in 1 mM salicylic acid extended storage life from 26 days to 71 days for ‘Indra’ capsicums stored in polyethylene bags at 10°C (Rao et al. Citation2011). Part of the storage life extension was due to reduced weight loss from the fruit. In another study, capsicums were dipped for 15 min in 1 mM acetyl salicylic acid, an analogue of salicylic acid that is believed to be converted to salicylic acid in the plant. Fruit were then stored in plastic bags at 2°C or 10°C for 8 or 21 days (Sayyari and Ghanbari Citation2013). The treatment reduced weight loss from the fruit stored at 10°C and approximately halved the chilling injury symptoms on fruit stored at 2°C.
To investigate the mechanism responsible for methyl jasmonate or methyl salicylate reducing chilling injury, green ‘Century’ capsicums were exposed to 0.1 mM vapour for 24 h, then stored at 0°C for 14 days followed by 20°C for 9 days (Fung et al. Citation2004). Both treatments substantially reduced surface pitting over 10 days of cold storage. By 13 days of cold storage, however, pitting in treated fruit approached that in controls. The treatments were found to cause slight increases in the mRNA abundance of superoxide dismutase, catalase and ascorbate peroxidase, but a very substantial increase in alternative oxidase.
While superoxide dismutase, catalase and ascorbate peroxidase are enzymes involved in the scavenging of reactive oxygen species, alternative oxidase is an enzyme that reduces the production of these compounds (Maxwell et al. Citation1999). Interestingly, Fung et al. (Citation2004) found fruit that had been stored at 7°C for 7 days prior to methyl jasmonate treatment showed less chilling injury and the induction of alternative oxidase had already occurred even in controls, suggesting that storage at 7°C acts as a low-temperature conditioning treatment that induces alternative oxidase. The authors proposed that an increase in alternative oxidase brought about either by cold stress or by treatment with methyl jasmonate or methyl salicylate is a major factor in avoiding chilling injury.
Brassinolide
The brassinosteroids, such as brassinolide, are natural plant hormones involved in plant growth, development and stress responses. So far, there appears to be only a single study examining the effects of a postharvest treatment of capsicum fruit with brassinolide. Wang et al. (Citation2012) treated green capsicums (‘Zhongjiao 7’) with a dip for 20 min in 15 μM brassinolide, then packed the fruit in polyethylene film bags in plastic boxes. Fruit were stored at 3°C for up to 18 days. Chilling injury (surface pitting and/or calyx discolouration) was first detected in untreated controls at 3 days, but not until 6 days after a 15 µM brassinolide treatment. At 12 days, chilling injury in the brassinolide treatment was still minimal, and substantially less than in the control. At 18 days, chilling injury in brassinolide-treated fruit was approximately one third that of controls. Throughout cold storage, the reduced chilling injury in brassinolide-treated fruit was correlated with reduced ion leakage, reduced malondialdehyde content (indicating reduced membrane damage), and small increases in the antioxidant enzymes catalase, peroxidase, ascorbate peroxidase and glutathione reductase, all of which imply an improved antioxidant capacity. In addition, brassinolide treatment slowed the declines in chlorophyll and vitamin C content, hence improving quality.
Glycine betaine
Glycine betaine (trimethylglycine) is a naturally occurring amino acid derivative that acts as a compatible solute, accumulating in response to environmental stress such as water loss. A 20 min dip in 1 mM glycine betaine reduced chilling injury in green capsicums stored at 3°C for 16 days followed by 20°C for 3 days (Wang et al. Citation2016). This was attributed to reduced cellular leakage and reduced membrane damage, combined with increased gene expression and enzyme activity of peroxidase, catalase, ascorbate peroxidase and glutathione reductase.
Calcium chloride
A postharvest dip in calcium chloride has been used to maintain firmness and quality in a range of fruit species (including apple, strawberry and lemon). A 15 min dip in 1.5 mM calcium chloride approximately halved the weight loss of ‘Indra’ capsicums stored at 10°C for 18 days, and approximately doubled storage life (Rao et al. Citation2011). Calcium chloride is thought to act by stabilising both membranes and the middle lamella of the cell wall that is responsible for intercellular adhesion.
Conclusions and future prospects
Capsicums are a highly perishable commodity, and storage conditions require careful optimisation for each cultivar. Temperatures should be as low as possible to slow metabolism and development of decay, but can lead to chilling injury if temperatures are too low. Humidity should be high to prevent shrivel, but high humidity can support the growth of microbial pathogens and decay. However, careful cleaning protocols and high-humidity packaging have shown clear benefits in extending storage life.
Many treatments aimed at increasing the resistance of capsicums to chilling injury and extending storage life have been tried in laboratory settings, but few have been successfully translated into commercial practice. Issues with cost relative to effectiveness, convenience of application in the supply chain, restrictions on use or residue detection limits and risks to workers have been difficult to overcome. Nevertheless, the wide variation in ethylene physiology between different genotypes (Villavicencio et al. Citation1999) suggests that 1-MCP treatments and/or storage under CA may be effective in some cultivars. Furthermore, the wide variation in water loss and chilling injury between cultivars and accessions (Lownds et al. Citation1994; Maalekuu et al. Citation2006; Smith et al. Citation2006; Parsons et al. Citation2013; Elibox et al. Citation2015; Popovsky-Sarid et al. Citation2017) show the considerable potential for breeding lines with improved postharvest quality. For greenhouse production, an alternative for introducing stress resilience and better performance during postharvest cold storage is grafting existing cultivars onto different rootstocks (Shu et al. Citation2016).
Reducing wastage and improving the quality of fruit reaching the customer have long been an industry goal. For this, identifying measurable parameters that predict storage life are needed for inventory management, enabling rapid sale of less robust batches. Elibox et al. (Citation2015) determined that days to achieve 20% pedicel necrosis predicted marketability as early as eight days after harvest, and could be used to identify lines with long storage potential. Looking to the future, discerning customers are placing increasing emphasis on flavour and health attributes, suggesting that there is room in the market for premium capsicum products. In addition to breeding efforts, postharvest treatments and optimised supply chain protocols, this will require a better understanding of the role of preharvest factors that affect postharvest quality, and improved crop production practices. For fruit grown in the field, this can be as simple as the use of shade cloths since these can improve yield and decrease Phytophthora blight (Díaz-Pérez Citation2014). The use of different coloured shade netting for field production can even increase the content of pigments, ascorbate and other bioactives (Alkalai-Tuvia et al. Citation2014; Mashabela et al. Citation2015; Selahle et al. Citation2015).
Acknowledgements
We thank Tony Corbett for photography.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Erin M. O’Donoghue http://orcid.org/0000-0002-2840-6649
David A. Brummell http://orcid.org/0000-0002-2754-6340
Marian J. McKenzie http://orcid.org/0000-0002-0192-5799
Donald A. Hunter http://orcid.org/0000-0002-5570-6460
Ross E. Lill http://orcid.org/0000-0003-0400-663X
Additional information
Funding
References
- Ahmed SS, Gong Z-H, Khan MA, Yin Y-X, Guo W-L, Imran J. 2011. Activity and expression of polygalacturonase vary at different fruit ripening stages of sweet pepper cultivars. Genetics and Molecular Research. 10:3275–3290. doi: 10.4238/2011.November.22.10
- Aghdam MS, Bodbodak S. 2013. Physiological and biochemical mechanisms regulating chilling tolerance in fruits and vegetables under postharvest salicylates and jasmonates treatments. Scientia Horticulturae. 156:73–85. doi: 10.1016/j.scienta.2013.03.028
- Aizat WM, Able JA, Stangoulis JCR, Able AJ. 2013. Characterisation of ethylene pathway components in non-climacteric capsicum. BMC Plant Biology. 13:191. doi: 10.1186/1471-2229-13-191
- Ali AM, Kelly WC. 1993. Effect of preanthesis temperature on the size and shape of sweet-pepper (Capsicum annuum L.) fruit. Scientia Horticulturae. 54:97–105. doi: 10.1016/0304-4238(93)90058-X
- Alkalai-Tuvia S, Goren A, Perzelan Y, Weinberg T, Fallik E. 2014. The influence of coloured shade nets on pepper quality after harvest – a possible mode-of-action. Agriculture and Forestry. 60:7–18.
- Alvarado-Casillas S, Ibarra-Sánchez S, Rodríguez-García O, Martínez-González N, Castillo A. 2007. Comparison of rinsing and sanitizing procedures for reducing bacterial pathogens on fresh cantaloupes and bell peppers. Journal of Food Protection. 70:655–660. doi: 10.4315/0362-028X-70.3.655
- Alwi NA, Ali A. 2015. Dose-dependent effect of ozone fumigation on physiological characteristics, ascorbic acid content and disease development on bell pepper (Capsicum annuum L.) during storage. Food Bioprocessing Technology. 8:558–566. doi: 10.1007/s11947-014-1419-2
- Bar-Yosef A, Alkalai-Tuvia S, Perzelan Y, Aharon Z, Ilic Z, Lurie S, Fallik E. 2009. Effect of shrink packaging in combination with rinsing and brushing treatment on chilling injury and decay of sweet pepper during storage. Advances in Horticultural Science. 23:225–230.
- Ben-Yehoshua S, Shapiro B, Chen ZE, Lurie S. 1983. Mode of action of plastic film in extending life of lemon and bell pepper fruits by alleviation of water stress. Plant Physiology. 73:87–93. doi: 10.1104/pp.73.1.87
- Bojórquez Gálvez A, Vega García M, Caro Corrales J, Carrillo López A, López Valenzuela JA. 2010. Effect of gradual cooling storage on chilling injury and phenylalanine ammonia-lyase activity in tomato fruit. Journal of Food Biochemistry. 34:295–307. doi: 10.1111/j.1745-4514.2009.00279.x
- Bonaccorsi I, Cacciola F, Utczas M, Inferrera V, Giuffrida D, Donato P, Dugo P, Mondello L. 2016. Characterization of the pigment fraction in sweet bell peppers (Capsicum annuum L.) harvested at green and overripe yellow and red stages by offline multidimensional convergence chromatography/liquid chromatography-mass spectrometry. Journal of Separation Science. 39:3281–3291. doi: 10.1002/jssc.201600220
- Borovsky Y, Oren-Shamir M, Ovadia R, de Jong W, Paran I. 2004. The A locus that controls anthocyanin accumulation in pepper encodes a MYB transcription factor homologous to Anthocyanin2 of Petunia. Theoretical and Applied Genetics. 109:23–29. doi: 10.1007/s00122-004-1625-9
- Borovsky Y, Paran I. 2008. Chlorophyll breakdown during pepper fruit ripening in the chlorophyll retainer mutation is impaired at the homolog of the senescence-inducible stay-green gene. Theoretical and Applied Genetics. 117:235–240. doi: 10.1007/s00122-008-0768-5
- Bosland PW, Votava EJ. 2012. Peppers: vegetable and spice capsicums. 2nd ed. Wallingford (UK): CAB International.
- Brand A, Borovsky Y, Hill T, Rahman KAA, Bellalou A, Van Deynze A, Paran I. 2014. CaGLK2 regulates natural variation of chlorophyll content and fruit colour in pepper fruit. Theoretical and Applied Genetics. 127:2139–2148. doi: 10.1007/s00122-014-2367-y
- Brummell DA. 2006. Cell wall disassembly in ripening fruit. Functional Plant Biology. 33:103–119. doi: 10.1071/FP05234
- Brummell DA, Pathirana R. 2007. Sweet and hot peppers. In: Pua MC, Davey MR, editors. Biotechnology in agriculture and forestry. Vol. 59: transgenic crops IV. Berlin: Springer-Verlag; p. 393–414.
- Buttery RG, Seifert RM, Guadagni DG, Ling LC. 1969. Characterization of some volatile constituents of bell peppers. Journal of Agricultural and Food Chemistry. 17:1322–1327. doi: 10.1021/jf60166a061
- Cantwell M. 2011. Bell pepper. Recommendations for maintaining postharvest quality. http://ucanr.org/sites/postharvest/pfvegetable/BellPepper/
- Cao S, Yang Z, Zheng Y. 2012. Effect of 1-methylcyclopene on senescence and quality maintenance of green bell pepper fruit during storage at 20°C. Postharvest Biology and Technology. 70:1–6. doi: 10.1016/j.postharvbio.2012.03.005
- Cappellini MC, Lachance PA, Hudson DE. 1984. Effect of temperature and carbon dioxide atmospheres on the market quality of green bell peppers. Journal of Food Quality. 7:17–25.
- Castro R, Fallik E, Nemny-Lavy E, Alkalai-Tuvia S, Rempoulakis P, Nestel D. 2016. Effects of cold post-harvest treatments of sweet bell peppers on the development of the Mediterranean fruit fly (Ceratitis capitata). Postharvest Biology and Technology. 120:16–22. doi: 10.1016/j.postharvbio.2016.05.006
- Cerqueira-Pereira EC, Pereira MA, Mello SdC, Jacomino AP, Trevisan MJ, Dias CTdS. 2007. Effect of the application of ethylene on the postharvest quality of red and yellow bell peppers fruits. Horticultura Brasileira. 25:590–593. doi: 10.1590/S0102-05362007000400019
- Cheng J, Shen H, Yang X, Yu S, Yuan L, Sun Z, Sun X. 2008. Changes in biochemical characteristics related to firmness during fruit development of pepper (Capsicum annuum L.) European Journal of Horticultural Science. 73:155–161.
- Cruz-Huerta N, Williamson JG, Darnell RL. 2011. Low night temperature increases ovary size in sweet pepper cultivars. HortScience. 46:396–401.
- Cuvi MJA, Vicente AR, Concellon A, Chaves AR. 2011. Changes in red pepper antioxidants as affected by UV-C treatments and storage at chilling temperatures. LWT-Food Science and Technology. 44:1666–1671. doi: 10.1016/j.lwt.2011.01.027
- Díaz-Pérez JC. 2014. Bell pepper (Capsicum annum L.) crop as affected by shade level: fruit yield, quality, and postharvest attributes, and incidence of Phytophthora blight (caused by Phytophthora capsici Leon.). HortScience. 49:891–900.
- Díaz-Pérez JC, Muy-Rangel M, Mascorro AG. 2007. Fruit size and stage of ripeness affect postharvest water loss in bell pepper fruit (Capsicum annuum L.). Journal of the Science of Food and Agriculture. 87:68–73. doi: 10.1002/jsfa.2672
- Dogan A, Selcuk N, Erkan M. 2016. Comparison of pesticide-free and conventional production systems on postharvest quality and nutritional parameters of peppers in different storage conditions. Scientia Horticulturae. 207:104–116. doi: 10.1016/j.scienta.2016.05.019
- Eggink PM, Maliepaard C, Tikunov Y, Haanstra JPW, Bovy AG, Visser RGF. 2012. A taste of sweet pepper: volatile and non-volatile chemical composition of fresh sweet pepper (Capsicum annuum) in relation to sensory evaluation of taste. Food Chemistry. 132:301–310. doi: 10.1016/j.foodchem.2011.10.081
- Elibox W, Meynard CP, Umaharan P. 2015. Morphological changes associated with postharvest fruit deterioration and physical parameters for early determination of shelf life in Capsicum chinense Jacq. HortScience. 50:1537–1541.
- Fallik E, Aharoni Y, Grinberg S, Copel A, Klein JD. 1994. Postharvest hydrogen peroxide treatment inhibits decay in eggplant and sweet red pepper. Crop Protection. 13:451–454. doi: 10.1016/0261-2194(94)90094-9
- Fallik E, Grinberg S, Alkalai S, Lurie S. 1996. The effectiveness of postharvest hot water dipping on the control of grey and black moulds in sweet red pepper (Capsicum annuum). Plant Pathology. 45:644–649. doi: 10.1046/j.1365-3059.1996.d01-175.x
- Fallik E, Grinberg S, Alkalai S, Yekutieli O, Wiseblum A, Regev R, Beres H, Bar-Lev E. 1999. A unique rapid hot water treatment to improve storage quality of sweet pepper. Postharvest Biology and Technology. 15:25–32. doi: 10.1016/S0925-5214(98)00066-0
- Fallik E, Grinberg S, Ziv O. 1997. Potassium bicarbonate reduces postharvest decay development on bell pepper fruits. Journal of Horticultural Science. 72:35–41. doi: 10.1080/14620316.1997.11515489
- Fallik E, Perzelan Y, Alkalai-Tuvia S, Nemny-Lavy E, Nestel D. 2012. Development of cold quarantine protocols to arrest the development of the Mediterranean fruit fly (Ceratitis capitata) in pepper (Capsicum annuum L.) fruit after harvest. Postharvest Biology and Technology. 70:7–12. doi: 10.1016/j.postharvbio.2012.03.004
- Fernández-Trujillo JP, Serrano JM, Martínez JA. 2009. Quality of red sweet pepper fruit treated with 1-MCP during a simulated post-harvest handling chain. Food Science and Technology International. 15:23–30. doi: 10.1177/1082013208100464
- Fox AJ, Del Pozo-Insfran D, Lee JH, Sargent SA, Talcott ST. 2005. Ripening-induced chemical and antioxidant changes in bell peppers as affected by harvest maturity and postharvest ethylene exposure. HortScience. 40:732–736.
- Frary A, Frary A. 2012. Physiology of metabolites. In: Russo VM, editor. Peppers: botany, production and uses. Cambridge (MA): CAB International; p. 176–188.
- Fung RWM, Wang CY, Smith DL, Gross KC, Tian M. 2004. MeSA and MeJA increase steady-state transcript levels of alternative oxidase and resistance against chilling injury in sweet peppers (Capsicum annuum L.). Plant Science. 166:711–719. doi: 10.1016/j.plantsci.2003.11.009
- Geier R, Weichmann J. 1985. Intermittent warming and chilling injury of stored sweet pepper (Capsicum annuum L.). Gartenbauwissenschaft. 50: 145–148.
- Gil MI, Tudela JA. 2012. Postharvest requirements of peppers. In: Russo VM, editor. Peppers: botany, production and uses. Cambridge (MA): CAB International; p. 241–254.
- Giuffrida D, Dugo P, Dugo G, Torre G, Mondello L. 2011. Analysis of native carotenoid composition of sweet bell peppers by serially coupled C30 columns. Natural Products Communications. 6:1817–1820.
- Glowacz M, Colgan R, Rees D. 2015a. The use of ozone to extend the shelf-life and maintain quality of fresh produce. Journal of the Science of Food and Agriculture. 95:662–671. doi: 10.1002/jsfa.6776
- Glowacz M, Colgan R, Rees D. 2015b. Influence of continuous exposure to gaseous ozone on the quality of red bell peppers, cucumbers and zucchini. Postharvest Biology and Technology. 99:1–8. doi: 10.1016/j.postharvbio.2014.06.015
- Gomez-Garcia MDR, Ochoa-Alejo N. 2013. Biochemistry and molecular biology of carotenoid biosynthesis in chili peppers (Capsicum spp.). International Journal of Molecular Sciences. 14:19025–19053. doi: 10.3390/ijms140919025
- Gonzalez G, Tiznado M. 1993. Postharvest physiology of bell peppers stored in low density polyethylene bags. Lebensmittel-Wissenschaft & Technologie. 26:450–455. doi: 10.1006/fstl.1993.1089
- González-Aguilar GA 2004. Pepper. In: Gross KS, Wang CY, Saltveit M, editors. The Commercial Storage of Fruits, Vegetables, and Florist and Nursery Stocks, USDA Agricultural Handbook Number 66, Beltsville. Available from: http://www.ba.ars.usda.gov/hb66/contents.html
- González-Aguilar GA, Gayosso L, Cruz R, Fortiz J, Baez R, Wang CY. 2000. Polyamines induced by hot water treatments reduce chilling injury and decay in pepper fruit. Postharvest Biology and Technology. 18:19–26. doi: 10.1016/S0925-5214(99)00054-X
- Gonzalez-Zamora A, Sierra-Campos E, Luna-Ortega JG, Perez-Morales R, Rodriguez-Ortiz JC, Garcia-Hernandez JL. 2013. Characterization of different capsicum varieties by evaluation of their capsaicinoids content by high performance liquid chromatography, determination of pungency and effect of high temperature. Molecules. 18:13471–13486. doi: 10.3390/molecules181113471
- Guzman I, Hamby S, Romero J, Bosland PW, O'Connell MA. 2010. Variability of carotenoid biosynthesis in orange coloured Capsicum spp. Plant Science. 179:49–59. doi: 10.1016/j.plantsci.2010.04.014
- Ha S-H, Kim J-B, Park J-S, Lee S-W, Cho K-J. 2007. A comparison of the carotenoid accumulation in Capsicum varieties that show different ripening colours: deletion of the capsanthin-capsorubin synthase gene is not a prerequisite for the formation of a yellow pepper. Journal of Experimental Botany. 58:3135–3144. doi: 10.1093/jxb/erm132
- Han HR, Park SW, Park YM. 2010. Effects of precooling and storage temperature on the quality of ‘Manna’ green peppers during export simulation. Horticulture Environment and Biotechnology. 51:403–408.
- Han Y, Floros JD, Linton RH, Nielsen SS, Nelson PE. 2002. Response surface modeling for the inactivation of Escherichia coli O157:H7 on green peppers (Capsicum annuum) by ozone gas treatment. Journal of Food Science. 67:1188–1193. doi: 10.1111/j.1365-2621.2002.tb09475.x
- Han Y, Linton RH, Nielsen SS, Nelson PE. 2001. Reduction of Listeria monocytogenes on green peppers (Capsicum annuum L.) by gaseous and aqueous chlorine dioxide and water washing and its growth at 7°C. Journal of Food Protection. 64:1730–1738. doi: 10.4315/0362-028X-64.11.1730
- Harpster MH, Brummell DA, Dunsmuir P. 2002. Suppression of a ripening-related endo-1,4-β-glucanase in transgenic pepper fruit does not prevent depolymerization of cell wall polysaccharides during ripening. Plant Molecular Biology. 50:345–355. doi: 10.1023/A:1019856929126
- Harpster MH, Lee KY, Dunsmuir P. 1997. Isolation and characterization of a gene encoding endo-β-1,4-glucanase from pepper (Capsicum annuum L.). Plant Molecular Biology. 33:47–59. doi: 10.1023/A:1005795028489
- Horvitz S, Cantalejo MJ. 2014. Application of ozone for the postharvest treatment of fruits and vegetables. Critical Reviews in Food Science and Nutrition. 54:312–339. doi: 10.1080/10408398.2011.584353
- Hwang D, Jeong H-J, Kwon J-K, Kim H, Kang S-Y, Kang B-C. 2014. Phenotypic variants among ethyl methanesulfonate M 2 mutant lines in Capsicum annuum. Plant Genetic Resources. 12:S141-S145. doi: 10.1017/S1479262114000434
- Ibiza VP, Cañizares J, Nuez F. 2010. EcoTILLING in Capsicum species: searching for new virus resistances. BMC Genomics. 11:631. doi: 10.1186/1471-2164-11-631
- Ilic ZS, Trajkovic R, Perzelan Y, Alkalai-Tuvia S, Fallik E. 2012. Influence of 1-methylcyclopropene (1-MCP) on postharvest storage quality in green bell pepper fruit. Food and Bioprocess Technology. 5:2758–2767. doi: 10.1007/s11947-011-0614-7
- Jen JJ, Robinson ML. 1984. Pectolytic enzymes in sweet bell peppers (Capsicum annuum L.). Journal of Food Science. 49:1085–1087. doi: 10.1111/j.1365-2621.1984.tb10398.x
- Kader AA. 2002. Postharvest technology of horticultural crops. Publication 3311. 3rd ed. Oakland: University of California.
- Kim S, Park M, Yeom S-I, Kim Y-M, Lee JM, Lee H-A, Seo E, Choi J, Cheong K, Kim K-T. 2014. Genome sequence of the hot pepper provides insights into the evolution of pungency in Capsicum species. Nature Genetics. 46:270–278. doi: 10.1038/ng.2877
- Kissinger M, Tuvia-Alkalai S, Shalom Y, Fallik E. 2005. Characterization of physiological and biochemical factors associated with postharvest water loss in ripe pepper fruit during storage. Journal of the American Society for Horticultural Science. 130:735–741.
- Krajayklang M, Klieber A, Dry PR. 2000. Colour at harvest and post-harvest behaviour influence paprika and chilli spice quality. Postharvest Biology and Technology. 20:269–278. doi: 10.1016/S0925-5214(00)00141-1
- Lama K, Alkalai-Tuvia S, Perzelan Y, Fallik E. 2016. Nutritional qualities and aroma volatiles of harvested red pepper fruits stored at suboptimal temperatures. Scientia Horticulturae. 213:42–48. doi: 10.1016/j.scienta.2016.10.015
- Lee SY, Choi D. 2013. Comparative transcriptome analysis of pepper (Capsicum annuum) revealed common regulons in multiple stress conditions and hormone treatments. Plant Cell Reports. 32:1351–1359. doi: 10.1007/s00299-013-1447-9
- Lefebvre V, Kuntz M, Camara B, Palloix A. 1998. The capsanthin-capsorubin synthase gene: a candidate gene for the y locus controlling the red fruit colour in pepper. Plant Molecular Biology. 36:785–789. doi: 10.1023/A:1005966313415
- Leshuk JA, Saltveit ME. 1990. Controlled atmosphere storage requirements and recommendations for vegetables. In: Calderon M, Barkai-Golan R, editors. Food preservation by modified atmospheres. Boca Raton (FL): CRC Press; p. 315–352.
- Li X, Yun J, Fan X, Xing Y, Tang Y. 2011. Effect of 1-methylcyclopropene and modified atmosphere packaging on chilling injury and antioxidative defensive mechanism of sweet pepper. African Journal of Biotechnology. 10:6581–6589.
- Lightbourn GJ, Griesbach RJ, Novotny JA, Clevidence BA, Rao DD, Stommel JR. 2008. Effects of anthocyanin and carotenoid combinations on foliage and immature fruit colour of Capsicum annuum L. Journal of Heredity. 99:105–111. doi: 10.1093/jhered/esm108
- Lim CS, Kang SM, Cho JL, Gross KC. 2009. Antioxidizing enzyme activities in chilling-sensitive and chilling-tolerant pepper fruit as affected by stage of ripeness and storage temperature. Journal of the American Society for Horticultural Science. 134:156–163.
- Lim CS, Kang SM, Cho JL, Gross KC, Woolf AB. 2007. Bell pepper (Capsicum annuum L.) fruits are susceptible to chilling injury at the breaker stage of ripeness. HortScience. 42:1659–1664.
- Lim CS, Woolf AB. 2010. Varietal differences of chilling-induced physiological responses and quality attributes in pepper (Capsicum annuum L.) cultivars during low temperature storage. Horticulture Environment and Biotechnology. 51:531–538.
- Lin WC, Hall JW, Saltveit ME. 1993. Ripening stage affects the chilling sensitivity of greenhouse-grown peppers. Journal of the American Society for Horticultural Science. 118:791–795.
- Liu L, Wei Y, Shi F, Liu C, Liu X, Ji S. 2015. Intermittent warming improves postharvest quality of bell peppers and reduces chilling injury. Postharvest Biology and Technology. 101:18–25. doi: 10.1016/j.postharvbio.2014.11.006
- Lownds NK, Banaras M, Bosland PW. 1993. Relationships between postharvest water loss and physical properties of pepper fruit (Capsicum annuum L.). HortScience. 28:1182–1184.
- Lownds NK, Banaras M, Bosland PW. 1994. Postharvest water loss and storage quality of nine pepper (Capsicum) cultivars. HortScience. 29:191–193.
- Luning PA, Derijk T, Wichers HJ, Roozen JP. 1994a. Gas-chromatography, mass-spectrometry, and sniffing port analyses of volatile compounds of fresh bell peppers (Capsicum annuum) at different ripening stages. Journal of Agricultural and Food Chemistry. 42:977–983. doi: 10.1021/jf00040a027
- Luning PA, Devries RV, Yuksel D, Ebbenhorstseller T, Wichers HJ, Roozen JP. 1994b. Combined instrumental and sensory evaluation of flavor of fresh bell peppers (Capsicum annuum) harvested at 3 maturation stages. Journal of Agricultural and Food Chemistry. 42:2855–2861. doi: 10.1021/jf00048a038
- Luo YG, Mikitzel LJ. 1996. Extension of postharvest life of bell peppers with low oxygen. Journal of the Science of Food and Agriculture. 70:115–119. doi: 10.1002/(SICI)1097-0010(199601)70:1<115::AID-JSFA477>3.0.CO;2-8
- Lurie S, Shapiro B, Ben-Yehoshua S. 1986. Effects of water stress and degree of ripeness on rate of senescence of harvested bell pepper fruit. Journal of the American Society for Horticultural Science. 111:880–885.
- Maalekuu K, Elkind Y, Leikin-Frenkel A, Lurie S, Fallik E. 2006. The relationship between water loss, lipid content, membrane integrity and LOX activity in ripe pepper fruit after storage. Postharvest Biology and Technology. 42:248–255. doi: 10.1016/j.postharvbio.2006.06.012
- Manolopoulou H, Xanthopoulos G, Douros N, Lambrinos G. 2010. Modified atmosphere packaging storage of green bell peppers: quality criteria. Biosystems Engineering. 106:535–543. doi: 10.1016/j.biosystemseng.2010.06.003
- Mashabela MN, Selahle KM, Soundy P, Crosby KM, Sivakumar D. 2015. Bioactive compounds and fruit quality of green sweet pepper grown under different colored shade netting during postharvest storage. Journal of Food Science. 80:H2612–H2618. doi: 10.1111/1750-3841.13103
- Maxwell, DP, Wang Y, McIntosh L. 1999. The alternative oxidase lowers mitochondrial reactive oxygen production in plant cells. Proceedings of the National Academy of Sciences USA. 96:8271–8276. doi: 10.1073/pnas.96.14.8271
- Meir S, Philosoph-Hadas S, Lurie S, Droby S, Akerman M, Zauberman G, Shapiro B, Cohen E, Fuchs Y. 1996. Reduction of chilling injury in stored avocado, grapefruit, and bell pepper by methyl jasmonate. Canadian Journal of Botany. 74:870–874. doi: 10.1139/b96-108
- Meir S, Rosenberger I, Aharon Z, Grinberg S, Fallik E. 1995. Improvement of the postharvest keeping quality and colour development of bell pepper (cultivar ‘Maor’) by packaging with polyethylene bags at a reduced temperature. Postharvest Biology and Technology. 5:303–309. doi: 10.1016/0925-5214(94)00035-Q
- Mercier J, Baka M, Reddy B, Corcuff R, Arul J. 2001. Shortwave ultraviolet irradiation for control of decay caused by Botrytis cinerea in bell pepper: induced resistance and germicidal effects. Journal of the American Society for Horticultural Science. 126:128–133.
- Minguez-Mosquera MI, Honero-Mendez D. 1994. Formation and transformation of pigments during the fruit ripening of Capsicum annuum cv. Bola and Agridulce. Journal of Agricultural and Food Chemistry. 42:38–44. doi: 10.1021/jf00037a005
- Moline HE, Hruschka HW. 1977. Storage and handling of ‘California Wonder’ sweet peppers (Capsicum annuum L.). Acta Horticulturae. 62:257–266. doi: 10.17660/ActaHortic.1977.62.27
- Monforte AJ, Diaz AI, Caño-Delgado A, van der Knaap E. 2014. The genetic basis of fruit morphology in horticultural crops: lessons from tomato and melon. Journal of Experimental Botany. 16:4625–4637.
- Murray KE, Whitfield FB. 1975. The occurrence of 3-alkyl-2-methoxypyrazines in raw vegetables. Journal of the Science of Food and Agriculture. 26:973–986. doi: 10.1002/jsfa.2740260714
- O'Donoghue EM, Somerfield S, McLachlan A, Olsson S, Woolf AB. 2013. High-pressure water washing and continuous high humidity during storage and shelf conditions prolongs quality of red capsicums (Capsicum annuum L.). Postharvest Biology and Technology. 81:73–80. doi: 10.1016/j.postharvbio.2013.02.012
- Ogasawara S, Abe K, Nakajima T. 2007. Pepper beta-galactosidase 1 (PBG1) plays a significant role in fruit ripening in bell pepper (Capsicum annuum). Bioscience Biotechnology and Biochemistry. 71:309–322. doi: 10.1271/bbb.60179
- Osorio S, Alba R, Nikoloski Z, Kochevenko A, Fernie AR, Giovannoni JJ. 2012. Integrative comparative analyses of transcript and metabolite profiles from pepper and tomato ripening and development stages uncovers species-specific patterns of network regulatory behavior. Plant Physiology. 159:1713–1729. doi: 10.1104/pp.112.199711
- Paran I, van der Knaap E. 2007. Genetic and molecular regulation of fruit and plant domestication traits in tomato and pepper. Journal of Experimental Botany. 58:3841–3852. doi: 10.1093/jxb/erm257
- Park SY, Yu JW, Park JS, Li J, Yoo SC, Lee NY, Lee SK, Jeong SW, Seo HS, Koh HJ, Jeon JS, Park YI, Paek NC. 2007. The senescence-induced staygreen protein regulates chlorophyll degradation. Plant Cell. 19:1649–1664. doi: 10.1105/tpc.106.044891
- Parr WV, Green JA, White KG, Sherlock RR. 2007. The distinctive flavour of New Zealand sauvignon blanc: sensory characterisation by wine professionals. Food Quality and Preference. 18:849–861. doi: 10.1016/j.foodqual.2007.02.001
- Parsons EP, Popopvsky S, Lohrey GT, Alkalai-Tuvia S, Perzelan Y, Bosland P, Bebeli PJ, Paran I, Fallik E, Jenks MA. 2013. Fruit cuticle lipid composition and water loss in a diverse collection of pepper (Capsicum). Physiologia Plantarum. 149:160–174. doi: 10.1111/ppl.12035
- Parsons EP, Popopvsky S, Lohrey GT, Lu S, Alkalai-Tuvia S, Perzelan Y, Paran I, Fallik E, Jenks MA. 2012. Fruit cuticle lipid composition and fruit post-harvest water loss in an advanced backcross generation of pepper (Capsicum sp.). Physiologia Plantarum. 146:15–25. doi: 10.1111/j.1399-3054.2012.01592.x
- Polderdijk J, Boerrigter H, Wilkinson E, Meijer J, Janssens M. 1993. The effects of controlled atmosphere storage at varying levels of relative humidity on weight loss, softening and decay of red bell peppers. Scientia Horticulturae. 55:315–321. doi: 10.1016/0304-4238(93)90042-O
- Popovsky S, Paran I. 2000. Molecular genetics of the y locus in pepper: its relation to capsanthin-capsorubin synthase and to fruit colour. Theoretical and Applied Genetics. 101:86–89. doi: 10.1007/s001220051453
- Popovsky-Sarid S, Borovsky Y, Faigenboim A, Parsons EP, Lohrey GT, Alkalai-Tuvia S, Fallik E, Jenks MA, Paran I. 2017. Genetic and biochemical analysis reveals linked QTLs determining natural variation for fruit post-harvest water loss in pepper (Capsicum). Theoretical and Applied Genetics. 130:445–459. doi: 10.1007/s00122-016-2825-9
- Pretel MT, Serrano M, Amoros A, Requelme F, Romojaro F. 1995. Non-involvement of ACC and ACC oxidase activity in pepper fruit ripening. Postharvest Biology and Technology. 5:295–302. doi: 10.1016/0925-5214(94)00025-N
- Priya Sethu KM, Prabha TN, Tharanathan RN. 1996. Post-harvest biochemical changes associated with the softening phenomenon in Capsicum annuum fruits. Phytochemistry. 42:961–966. doi: 10.1016/0031-9422(96)00057-X
- Promyou S, Supapvanich S. 2013. Chilling injury alleviation in ‘Golden Bell’ sweet pepper caused by UV-C treatment. Acta Horticulturae. 1011:357–362. doi: 10.17660/ActaHortic.2013.1011.45
- Raffo A, Baiamonte I, Nardo N, Paoletti F. 2007. Internal quality and antioxidants content of cold-stored red sweet peppers as affected by polyethylene bag packaging and hot water treatment. European Food Research and Technology. 225:395–405. doi: 10.1007/s00217-006-0430-x
- Ramana Rao TV, Gol NB, Shah KK. 2011. Effect of postharvest treatments and storage temperatures on the quality and shelf life of sweet pepper (Capsicum annuum L.). Scientia Horticulturae. 132:18–26. doi: 10.1016/j.scienta.2011.09.032
- Rao GU, Paran I. 2003. Polygalacturonase: a candidate gene for the soft flesh and deciduous fruit mutation in Capsicum. Plant Molecular Biology. 51:135–141.
- Rao TVR, Gol NB, Shah KK. 2011. Effect of postharvest treatments and storage temperatures on the quality and shelf life of sweet pepper (Capsicum annuum L.). Scientia Horticulturae. 132:18–26. doi: 10.1016/j.scienta.2011.09.032
- Rodov V, Ben-Yehoshua S, Fierman T, Fang D. 1995. Modified-humidity packaging reduces decay of harvested red bell pepper fruit. HortScience. 30:299–302.
- Rodriguez-Burruezo A, Kollmannsberger H, Carmen Gonzalez-Mas M, Nitz S, Nuez F. 2010. HS-SPME comparative analysis of genotypic diversity in the volatile fraction and aroma-contributing compounds of Capsicum fruits from the annuum-chinense-frutescens complex. Journal of Agricultural and Food Chemistry. 58:4388–4400. doi: 10.1021/jf903931t
- Rodriguez-Uribe L, Guzman I, Rajapakse W, Richins RD, O'Connell MA. 2012. Carotenoid accumulation in orange-pigmented Capsicum annuum fruit, regulated at multiple levels. Journal of Experimental Botany. 63:517–526. doi: 10.1093/jxb/err302
- Russo VM. 2012. Peppers: botany, production and uses. Cambridge (MA): CAB International.
- Ryona I, Leclerc S, Sacks GL. 2010. Correlation of 3-isobutyl-2-methoxypyrazine to 3-isobutyl-2-hydroxypyrazine during maturation of bell pepper (Capsicum annuum) and wine grapes (Vitis vinifera). Journal of Agricultural and Food Chemistry. 58:9723–9730. doi: 10.1021/jf102072w
- Sakaldas M, Kaynas K. 2010. Biochemical and quality parameters changes of green sweet bell peppers as affected by different postharvest treatments. African Journal of Biotechnology. 9:8174–8181. doi: 10.5897/AJB10.1021
- Saltveit ME. 2001. A summary of CA requirements and recommendations for vegetables. Acta Horticulturae. 600:723–727.
- Sayyari M, Ghanbari F. 2013. Effect of acetyl salicylic acid on quality and chilling resistance of sweet pepper (Capsicum annuum L.) at different storage temperatures. Acta Horticulturae. 1012:559–568. doi: 10.17660/ActaHortic.2013.1012.75
- Selahle KM, Sivakumar D, Jifon J, Soundy P. 2015. Postharvest responses of red and yellow sweet peppers grown under photo-selective nets. Food Chemistry. 173:951–956. doi: 10.1016/j.foodchem.2014.10.034
- Serrano M, Martinez-Madrid MC, Pretel MT, Riquelme F, Romojaro F. 1997. Modified atmosphere packaging minimizes increases in putrescine and abscisic acid levels caused by chilling injury in pepper fruit. Journal of Agricultural and Food Chemistry. 45:1668–1672. doi: 10.1021/jf960866h
- Sevillano L, Sanchez-Ballesta MT, Romojaro F, Flores FB. 2009. Physiological, hormonal and molecular mechanisms regulating chilling injury in horticultural species. Postharvest technologies applied to reduce its impact. Journal of the Science of Food and Agriculture. 89:555–573. doi: 10.1002/jsfa.3468
- Shu C, Yang R, Yin LK, Ai XZ, Wang SH, Zhao WC. 2016. Selection of rootstocks for better morphological characters and resistance to low-temperature stress in the sweet pepper cultivar ‘Hongxing No. 2’. Horticulture Environment and Biotechnology. 57:348–354. doi: 10.1007/s13580-016-0065-1
- Smith DL, Stommel JR, Fung RWM, Wang CY, Whitaker BD. 2006. Influence of cultivar and harvest method on postharvest storage quality of pepper (Capsicum annuum L.) fruit. Postharvest Biology and Technology. 42:243–247. doi: 10.1016/j.postharvbio.2006.06.013
- Stewart C, Kang BC, Liu K, Mazourek M, Moore SL, Yoo EY, Kim BD, Paran I, Jahn MM. 2005. The Pun1 gene for pungency in pepper encodes a putative acyltransferase. Plant Journal. 42:675–688. doi: 10.1111/j.1365-313X.2005.02410.x
- Stommel JR, Lightbourn GJ, Winkel BS, Briesbach RJ. 2009. Transcription factor families regulate the anthocyanin biosynthetic pathway in Capsicum annuum. Journal of the American Society for Horticultural Science. 134:244–251.
- Stommel JR, Pushko M, Haynes KG, Whitaker BD. 2014. Differential inheritance of pepper (Capsicum annuum) fruit pigments results in black to violet fruit colour. Plant Breeding. 133:788–793. doi: 10.1111/pbr.12209
- Tan CK, Ali ZM, Zainal Z. 2012. Changes in ethylene production, carbohydrase activity and antioxidant status in pepper fruits during ripening. Scientia Horticulturae. 142:23–31. doi: 10.1016/j.scienta.2012.04.030
- Tanaka Y, Sasaki N, Ohmiya A. 2008. Biosynthesis of plant pigments: anthocyanins, betalains and carotenoids. Plant Journal. 54:733–749. doi: 10.1111/j.1365-313X.2008.03447.x
- Tian M, Gupta D, Lei XY, Prakash S, Xu C, Fung RWM. 2004. Effects of low temperature and ethylene on alternative oxidase in green pepper (Capsicum annuum L.). Journal of Horticultural Science and Biotechnology. 79:493–499. doi: 10.1080/14620316.2004.11511795
- Tian S-L, Li L, Shah SNM, Gong Z-H. 2015. The relationship between red fruit colour formation and key genes of capsanthin biosynthesis pathway in Capsicum annuum. Biologia Plantarum. 59:507–513. doi: 10.1007/s10535-015-0529-7
- Tzortzakis N, Chrysargyris A, Sivakumar D, Loulakakis K. 2016. Vapour or dipping applications of methyl jasmonate, vinegar and sage oil for pepper fruit sanitation towards grey mould. Postharvest Biology and Technology. 118:120–127. doi: 10.1016/j.postharvbio.2016.04.004
- Vicente AR, Pineda C, Lemoine L, Civello PM, Martinez GA, Chavez AR. 2005. UV-C treatments reduce decay, retain quality and alleviate chilling injury in pepper. Postharvest Biology and Technology. 35:69–78. doi: 10.1016/j.postharvbio.2004.06.001
- Villavicencio L, Blankenship SM, Sanders DC, Swallow WH. 1999. Ethylene and carbon dioxide production in detached fruit of selected pepper cultivars. Journal of the American Society for Horticultural Science. 124:402–406.
- Wang CY, Baker JE. 1979. Effects of two free radical scavengers and intermittent warming on chilling injury and polar lipid composition of cucumber and sweet pepper fruits. Plant and Cell Physiology. 20:243–251.
- Wang Q, Ding T, Gao L, Pang J, Yang N. 2012. Effect of brassinolide on chilling injury of green bell pepper in storage. Scientia Horticulturae. 144:195–200. doi: 10.1016/j.scienta.2012.07.018
- Wang Q, Ding T, Zuo J, Gao L, Fan L. 2016. Amelioration of postharvest chilling injury in sweet pepper by glycine betaine. Postharvest Biology and Technology. 112:114–120 (and Correction, 116:129–130). doi: 10.1016/j.postharvbio.2015.07.008
- Weryszko-Chmielewska E, Michalojc Z. 2011. Anatomical traits of sweet pepper (Capsicum annuum L.) fruit. Acta Agrobotanica. 64:181–188. doi: 10.5586/aa.2011.059
- Whitaker BD. 1995. Lipid changes in mature green bell pepper fruit during chilling at 2°C and after transfer to 20°C subsequent to chilling. Physiologia Plantarum. 93:683–688. doi: 10.1111/j.1399-3054.1995.tb05117.x
- Yoshida C, Takahashi M, Iwasaki Y, Furuno S, Matsunaga H, Nagata M. 2014. Factors affecting color development in sweet pepper (Capsicum annuum L.) fruit harvested at breaker stage of mature-green fruit. Horticulture Research (Japan). 13:155–160. doi: 10.2503/hrj.13.155
- Yuk HG, Bartz JA, Schneider KR. 2006. The effectiveness of sanitizer treatments in inactivation of Salmonella spp. from bell pepper, cucumber, and strawberry. Journal of Food Science. 71:M95–M99. doi: 10.1111/j.1365-2621.2006.tb15638.x
- Zhigila DA, AbdulRahaman AA, Kolwole OS, Oladele FA. 2014. Fruit morphology as taxonomic features in five varieties of Capsicum annuum L. Solanaceae. Journal of Botany. 540868. doi:10.1155/2014/540868.
