Abstract
Excess energy dissipation pathways (heat and fluorescence) were monitored, at leaf level as indicators of plant physiological status, with field spectroscopy techniques on poplar clones subjected to ozone fumigation. Measurements of spectral radiance emerging from a leaf provide a fast, non‐destructive method for the assessment of excess energy dissipation: xanthophyll‐related heat dissipation was estimated with the photochemical reflectance index (PRI) calculated from a traditional field spectrometer, and steady‐state fluorescence (Fs) under natural illumination conditions was estimated by exploiting a variation of the Fraunhofer line‐depth principle, where the radiance collected with very high resolution spectrometers (FWHM = 0.13 nm) was spectrally modelled. Both remotely‐sensed dissipation pathways responded to fumigation. During a 26‐day fumigation experiment, four diurnal cycles of spectral measurements were collected in parallel to meteorological and key physiological variables (active fluorescence, net photosynthesis) and leaf sample collection for pigment extraction. We outline evidence of a link between the remotely‐sensed Fs and PRI and leaf physiological status. These results open up new possibilities for assessment of plant stress by means of hyperspectral remote sensing.
1. Introduction
Ozone (O3) is a widespread gaseous phytotoxic pollutant (Reich Citation1987) that is known to severely damage the physiological and biochemical processes of plants (Kangasjarvi et al. Citation1994). It is also known to affect photosynthetic performance through various mechanisms before symptoms of injury appear on the leaf surface. The high oxidative capacity of the pollutant determines the induction of reactive oxygen species that can impair, together with lipid peroxidation and changes in membrane permeability, the light and dark reactions of photosynthesis (Francini et al. Citation2007).
Physiological measurements, such as photosynthesis and excess energy dissipation pathways (chlorophyll (Chl) a fluorescence and heat dissipation), play a major role in the early detection of O3 stress (Guidi et al. Citation1997). The rationale for the use of such dissipation pathways is that once a fraction of the intercepted light is used to drive the photosynthesis (PQ, photochemical quenching of the fluorescence signal), the remaining energy may be detrimental to plant cells by causing oxidative damage. This damage is minimized by plants through dissipation of excess energy into fluorescence and heat (NPQ, non‐photochemical quenching). Thus, measurements of these two de‐excitation pathways are expected to provide an indirect assessment of photochemical efficiency.
Chl fluorescence parameters have often been used in the assessment of O3 injury (e.g. Owens Citation1994, Heath Citation1996) and are usually estimated by measuring the fluorescence yield in response to an artificial excitation pulse with a probe in contact with the sample. The detection of laser‐induced Chl fluorescence was proven possible in the near‐range distance as well (e.g. Flexas et al. Citation2000), but the requirement of a strong excitation laser pulse limits its remote sensing application in the far‐range distance. The retrieval of steady‐state Chl fluorescence (or solar induced Chl fluorescence under natural illumination, Fs) which can be measured passively (i.e. without artificial excitation source) is instead expected to be feasible in the far‐range distance (Stoll Citation2004).
Several studies attempted to measure Fs using remote sensing (RS) methods; these employed different devices, but all basically exploiting the Fraunhofer line‐depth (FLD) principle (for a review see Theisen (Citation2002)). The FLD principle was successfully exploited to compute the intensity of Fs and to gain insight on photosynthetic status (e.g. Plascyk Citation1975, McFarlane et al. Citation1980, Kebabian et al. Citation1999, Freedman et al. Citation2002, Rosema Citation2002, Moya et al. Citation2004, Louis et al. Citation2005, Pérez‐Priego et al. Citation2005, Meroni and Colombo Citation2006).
The remote assessment of the other de‐excitation pathway, NPQ, has recently been proposed for indirect assessment of photochemical efficiency (Gamon et al. Citation1992, Citation1997, Peñuelas et al. Citation1995a). According to Demmig‐Adams and Adams (Citation1996), heat dissipation is linked to the carotenoids that compose the xanthophyll de‐epoxidation cycle. Exploiting reflectance changes at 531 nm, Gamon et al. (Citation1990) demonstrated the feasibility of tracking the de‐epoxidation state by remote sensing. The photochemical reflectance index (PRI) was then introduced by Gamon et al. (Citation1992) and found to be correlated with heat dissipation (xanthophyll de‐epoxidation and trans‐thylakoid ΔpH).
Application of RS to ozone stress studies has limited examples in the literature. Carter (Citation1993, Citation1994) first identified the most sensitive spectral bands, Peñuelas et al. (Citation1995b) and Kraft et al. (Citation1996) studied the spectral effects and the possibility of identifying ozone damage at canopy level. Energy dissipation pathways (as measured remotely by Fs and PRI) have not been investigated up to now in relation to ozone stress.
In this paper, we investigate optical signals of oxidative stress linked to ozone exposure by focusing on the detection of steady‐state fluorescence and the xanthophyll de‐epoxidation state at leaf level, in the context of a chronic ozone fumigation experiment. We outline evidence for a link between photosynthetic status and energy dissipation pathways detected by passive hyperspectral remote sensing. We claim that these results may open up new possibilities for the assessment of plant stress with field spectroscopy.
2. Materials and methods
2.1 Plant material and ozone exposure
Rooted cuttings of a poplar clone (Populus deltoides×maximowiczii Eridano), known for its O3 sensitivity, were grown for 2 months in plastic pots containing a steam‐sterilized soil:peat:perlite (1:1:1 volume) mix. Plants were grown in a cooled greenhouse during the summer, at a temperature ranging between 16 and 26 °C and relative humidity (RH) between 55 and 85%, and watered regularly.
Uniform‐sized plants were transferred to a growth chamber after complete expansion of the tenth leaf. Temperature was maintained at 20±1 °C, RH at 85±5%, and photosynthetic photon flux density (PPFD) at plant height of 500 µmol m−2 s−1 provided by incandescent lamps, during a 16 h photoperiod. Plants assigned to treatment were then placed in a controlled‐environment fumigation facility (Nali et al. Citation2005) under the same climatic conditions as the growth chamber. Fumigation was performed in two Perspex chambers, continuously ventilated with charcoal‐filtered air (two complete air changes/min). Ozone was generated by electrical discharge using a Fisher 500 air‐cooled apparatus (Zurich, Switzerland) supplied with pure oxygen, and mixed with the inlet air entering the fumigation chambers. Its concentration at plant height was continuously monitored with a photometric analyzer (Monitor Labs, mod. 8810, San Diego, CA, USA). Plants were exposed for 26 consecutive days (15 September to 10 October 2005) to 80 (±5.0) ppb O3 for 5 h d−1 in the form of a square wave, until visible symptoms appeared as sparse dark stipples. Control plants were exposed to charcoal‐filtered air only in two other Perspex chambers identical to those mentioned above.
2.2 Data collection
Intensive measurements were performed on the following four days: 0 (before the beginning of fumigation, which was started on day 1), 9, 16 and 26. During these days, diurnal cycles of measurements were collected outdoors under natural solar illumination: radiometry and gas exchanges measurements were performed on the same plant (three plants per thesis), on the fifth and fourth leaf, respectively, and active Chl a fluorescence was measured on the fifth leaf of different plants (one per thesis) exhibiting an average maximum assimilation response of the thesis. Measurements of active fluorescence parameters requiring dark adaptation of the plants were performed before dawn on four leaves per treatment. Diurnal cycles were started when weather forecasts were favourable (clear sky) and meteorological data were continuously logged.
For pigment extraction, leaf discs of known area (Ø = 1.12 cm) were collected at midday, frozen in liquid nitrogen and stored at −80 °C until use. Discs were sampled on the fifth leaf of different plants (three per thesis). Visual assessment of ozone injuries was carried out daily, in terms of percentage of leaves showing stipples.
2.2.1 Meteorological dataset
During the cycles, air temperature and RH (Rotronic, Germany), total and diffuse incident PPFD (BF3, Delta‐T, UK), were continuously logged (DL2, Delta‐T, UK). Ambient temperature ranged from 12 to 30 °C, vapour pressure deficit (VPD) from 0.2 to 2 kPa and maximum recorded incoming PPFD was 1600 µmol m−2 s−1. Day 0 was a sunny day while overcast conditions were reported on day 9 during the morning and the afternoon, with clear conditions only in the central hours of the day. Weather conditions were similar in days 16 and 26, and were characterised by the formation of clouds in the early afternoon. Incident solar PPFD at solar noon decreased during the 26 days of experiments from 1600 µmol m−2 s−1 (day 0, 15 September) to 1350 µmol m−2 s−1 (day 26, 10 October).
2.2.2 Photosynthetic parameters and pigment analysis
Active fluorescence measurements were performed with a pulse amplitude modulated fluorometer PAM‐2000 (Waltz, Effeltrich, Germany). The chlorophyll fluorescence parameters measured were maximum photochemical efficiency of dark‐adapted samples (F v / F m, where F m is the maximum fluorescence and F v is the difference between the maximum and the baseline fluorescence), Stern‐Volmer NPQ and fluorescence yield (F t). Procedures used for measuring F t and F v / F m were based on standard methodologies (PAM‐2000 manual, Heinz‐Walz‐GmbH 1993). The NPQ was calculated according to Bilger and Björkman (Citation1990) as F m / F m′−1 (where F m′ is the maximum fluorescence of the sample under environment illumination). The parameters F t and F m′ were measured every 20 s and 20 min, respectively, on a single leaf per treatment.
Leaf CO2 exchanges were measured with an open infrared gas exchange system (CIRAS‐1, PP‐Systems, Stotfold, UK) equipped with a Parkinson leaf chamber, able to clamp single leaves. Details are reported in Nali et al. (Citation2005). Measurements were performed at ambient CO2 concentrations and illumination. Net assimilation (A) of three leaves per treatment was measured every 15 min, approximately. Photosynthetic activity under maximum solar irradiance (i.e. around midday) is hereafter referred to as maximum assimilation (A max). Light‐use efficiency (LUE) was then estimated by forming the ratio of assimilation rate over incoming PPFD (measured with microsensors on the leaf chamber).
Chlorophyll (a and b) and xanthophyll (violaxanthin (V), antheraxanthin (A) and zeaxanthin (Z)) were extracted from leaf discs in 3 ml of 100% methanol at 4 °C in the dark for 24 h. The extract was filtered through a 0.2 µm Sartorius filter and immediately analysed by reverse‐phase high‐performance liquid chromatography (HPLC) following the method of Ciompi et al. (Citation1997). To quantify pigment concentration, known amounts of pure standard were injected into the HPLC system and an equation correlating peak area to pigment concentration was formulated.
The de‐epoxidation state of samples (DEPS) was calculated as (0.5A+Z) / (V+A+Z).
2.2.3 Radiometry
Spectral measurements were acquired contemporarily by three spectrometers: one FS HH Pro (Analytical Spectral Devices, Boulder, CO, USA) and two HR2000 spectrometers (OceanOptics, Dunedin, FL, USA). The FS HH Pro was used to calculate optical indices based on reflectance measurements, while the HR2000s were used to estimate fluorescence.
The FS HH Pro spectrometer shows spectral range of 325–1075 nm with a spectral resolution of 3.5 nm (full width at half maximum, FWHM) and a sampling interval of 1.6 nm (1 nm resampling). The HR2000 spectrometers display an elevated spectral resolution characterized by a FWHM of 0.13 nm, a sampling interval of 0.03 nm, and a spectral range of 635.5–802.5 nm (each spectrometer covers a range of 94 nm). These spectrometers are described in detail in Meroni and Colombo (Citation2006). Absolute radiance calibration was performed by the manufacturers (ASD and OceanOptics) on all spectrometers mounting bare fibre optics to allow the quantitative measurement of radiant fluxes. Spectrometers were housed in a Peltier thermally insulated box (model NT‐16, Magapor, Zaragoza, Spain) keeping the internal temperature at 20 °C in order to reduce dark current drift.
Six leaves from separate plants (three per treatment) were aligned on a custom‐designed sample holder that allows the spectrometers to view either the leaf sample or the reflectance standard panel within a few seconds, whilst carefully avoiding casting shadows over the samples. Leaves remained attached to the plants with leaf plane oriented horizontally during all measurements. An elliptical area with diameters of 13.6 and 13.3 mm (i.e. 142.5 mm2) of the leaves was observed by the spectrometers through three convergent fibre optics (with an angular field of view of 25°). Measurement operations required approximately 10 s per leaf. Sequential measurements of the full set of leaves were repeated approximately every 5 min.
Reflectance was calculated by rationing the radiance emerging from the leaf with the radiance of a calibrated reflectance standard panel (Zenith, Germany).
We employed the field spectroscopy technique referred to as ‘single beam’ (Milton and Rolling Citation2006), where target measurements are ‘sandwiched’ between two reflectance standard panel measurements carried out a few seconds apart. The radiance of the reference panel at the time of the target measurement is estimated by linear interpolation. This technique is based on the assumption that incident irradiance varies linearly between the two reference panel measurements. For every acquisition, four scans were averaged and stored as a single file. Additionally, a dark current measurement was collected for every set of acquisitions. Typical integration times for the fluorescence measurements were on the order of 5 s in the early morning (and late afternoon) and 100 ms at midday.
ASD FS HH Pro data were used to calculate the PRI according to the following equation:
For the sake of comparison, two optical indices commonly employed at leaf level were also calculated from the ASD FS HH:
The simple ratio index, SR, (Vogelmann et al. Citation1993, Zarco‐Tejada et al. Citation2001) and normalized difference index, NDI, (Gitelson and Merzlyak Citation1994, Gamon and Surfus Citation1999) are expected to be well suited for the assessment of chlorophyll concentration at leaf scale.
OceanOptics HR2000 spectrometers were used to estimate fluorescence parameters. Steady‐state fluorescence (Fs) was measured by exploiting a variation of the FLD principle applied in the 687 and 760 nm atmospheric oxygen absorption bands, as described in Meroni and Colombo (Citation2006). An index of fluorescence efficiency, normalized fluorescence (NFs), was calculated by rationing the estimated fluorescence with the radiation incident in a nearby restricted spectral range not including the Fraunhofer line, i.e. in the continuum. The passive red to far‐red fluorescence ratio (Fs687 / Fs760) was estimated by rationing fluorescence at 687 nm with the one at 760 nm. According to Gitelson et al. (Citation1999) and Buschmann (Citation2002), red fluorescence is strongly re‐absorbed by the chlorophylls of the internal leaf, whereas far‐red fluorescence is only slightly re‐absorbed. Thus, Fs687 / Fs760 is expected to decrease with increasing chlorophyll concentration.
3. Results and discussion
3.1 Time series of midday physiological and radiometric parameters
To analyse the overall evolution of measurements during the experiment, time series of the investigated parameters measured at maximum solar irradiance (i.e. at solar noon) were generated. Remote sensing and physiological measurements, monitored over the 26 days of the experiment, are shown in figure .
Figure 1 Time series of midday physiological and radiometric parameters: (a) net assimilation (A max), (b) xanthophyll de‐epoxidation state (DEPS), (c) normalized Fs at 760 nm (NFs760) and (d) photochemical reflectance index (PRI). Full and empty dots refer to control and treatment samples, respectively. Values represent means ± SE (standard error), n = 3 (number of samples). Comparison between means was performed according to Student's t‐test (ns: P>0.05, *: P⩽0.05, **: P⩽0.01, ***: P⩽0.001).
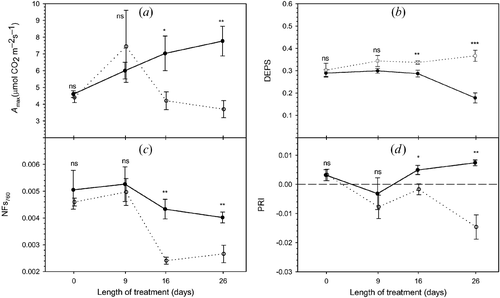
On day 0, both physiological and RS measurements indicated that control and treatment plants were in similar vigour (no statistically significant difference for any parameter) before fumigation. Afterwards, treated plants experienced increasing stress due to chronic ozone fumigation. In fact, photosynthetic activity at maximum solar irradiance, A max, was significantly reduced by O3 treatment from day 16 on (figure ). The ratio F v / F m (data not shown) was able to detect a difference between control and ozonated leaves on day 26 only. Visible symptoms appeared as sparse dark stipples on day 26 on 30% of treated leaves.
The t‐test indicated that fluorescence and PRI could be separated by O3 treatment from day 16 on. NFs at 760 nm (fluorescence per unit incident radiance) (figure ) was similar in control and treated leaves after 9 days of exposure, while a decrease in treated leaves, as compared to the controls, was observed from day 16 on. These results are in agreement with other studies (e.g. Dobrowski et al. Citation2005) that observed a reduction in Fs and NFs due to prolonged stress. The PRI of treated samples was significantly lower on day 16, and the difference in PRI values became larger on day 26 (figure ). Lower values of treated samples reflected greater investment in photoprotective xanthophyll‐cycle pigments for the ozonated leaves, as suggested by Gamon et al. (Citation1997). This result was confirmed by xanthophyll extractions: DEPS was significantly higher in treated samples from day 16 on (figure ).
In this experiment, total Chl concentration did not decrease as a result of the 26 days of fumigation. Only on day 26 was an increase for the control samples observed, probably due to leaf growth (figure ). The passive red to far‐red fluorescence ratio (Fs687 /Fs760) detected the different Chl concentration on day 26 (figure ). Moreover, Fs687 / Fs760 and measured Chl concentration were negatively correlated (R 2 = 0.60, P<0.05).
Figure 2 (a) Time series of total chlorophyll concentration, (b) midday radiometric measurements: Fs687/Fs760, red/far‐red fluorescence ratio and (c) normalized difference index (NDI). Full and empty dots refer to control and treatment samples, respectively. Values represent means ± SE (n = 3). Comparison between means was performed according to Student's t‐test (ns: P>0.05, *: P⩽0.05, **: P⩽0.01, ***: P⩽0.001).
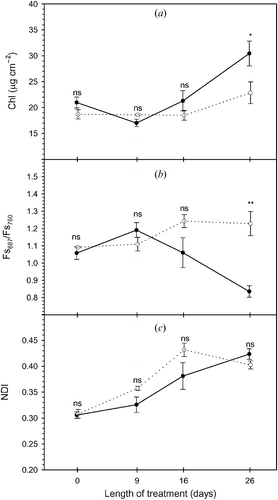
The reduction of Fs760 and NFs760 may thus be explained by an increase in NPQ (as indicated by PRI), rather than a decrease in Chl concentration (as observed by Louis et al. Citation2005). In fact, neither pigment extraction nor remote sensing measurements (Fs687 / Fs760) indicated a reduction in Chl concentration.
Reflectance‐based optical indices NDI (figure ) and SR (not shown) were not affected by O3 exposure and failed to detect a difference in Chl concentration between treatments (R 2<0.3 for both optical indices). The increasing trend of these indices may be related to changes in leaf internal structure associated with leaf development that probably mask the relationship to Chl concentration (Sims and Gamon Citation2002).
3.2 Daily course of physiological and radiometric parameters
The link between physiological parameters and remotely‐sensed energy dissipation pathways is further proven by the analysis of diurnal patterns. A different diurnal evolution for control and treated plants appeared on day 16 and was enhanced on day 26 (figure ).
Figure 3 Diurnal evolution(day 26) of meteorological, spectral and physiological variables: (a) incident photosynthetic photon flux density (PPFD, continuous line) and vapour pressure deficit (VPD, dotted line), (b) photochemical reflectance index (PRI), (c) Fs760, fluorescence measured at the 760 nm, (d) normalized fluorescence at 760 nm (NFs760), (e) net assimilation rate under natural illumination (A) and (f) light use efficiency (LUE). Full and empty dots refer to control and treatment samples, respectively. Values correspond to the means ± SE (n = 3).
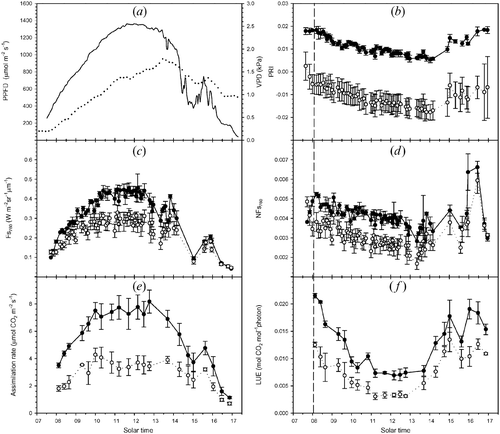
On day 26, the different physiological status between healthy and fumigated samples was clearly separated in terms of A and LUE, passive fluorescence and PRI.
The Fs760 (figure ) of treated samples was lower (30% on average) than that of controls and the daily course of PRI (figure ) of treated samples was shifted towards lower values compared to controls (40% on average), indicating greater recourse to non‐photochemical quenching.
The evolution of Fs760 approximately followed the incoming PPFD (figure ) in both the theses. However, similarly to assimilation, Fs760 reached a plateau around 10:30, while PPFD kept increasing until midday.
The NFs760 (figure ) and PRI showed a diurnal evolution that mirrored that of PPFD and was similar to that of LUE. The decrease in PPFD after noon did not correspond to an increase in PRI due to the slow reversion of NPQ (Evain et al. Citation2004); with treated samples it incompletely reversed in the afternoon.
In the early morning with moderate light intensities (to the left of the vertical dashed lines in figure ), treated samples activated the xanthophyll de‐epoxidation cycle, while such activation was delayed in controls, as indicated by PRI. A contrasting pattern appears also in NFs760, and control and treated samples responded differently to increasing PPFD in the early morning. Controls initially increased their fluorescence efficiency while treated samples decreased it. A similar pattern was observed by Flexas et al. (Citation2000) on well‐watered and water‐stressed plants.
Around 14:00, the sky condition became variable due to intermittent clouds. It is interesting to note the response of NFs760 and PRI to different illumination conditions (varying direct and diffuse components of incoming PPFD). The rapid transitions in LUE in response to different illumination conditions measured between 15:00 and 16:30 were well captured by NFs760 and not by PRI. This underlines the different response time of these two dissipation pathways.
Fluorescence measured at 687 nm (not shown) exhibited a greater range of variation, but essentially followed, with greater scatter, the one measured at 760 nm.
As expected, reflectance‐based indices NDI and SR showed no significant daily pattern (data not shown) for any diurnal cycle performed. In fact, with the exception of PRI, the majority of vegetation indices were not sensitive to rapid changes in plant photosynthetic status brought on by common environmental stressors such as diurnal fluxes of irradiance and heat (Dobrowski et al. Citation2005).
3.3 Comparison of active and passive measurements
Diurnal time courses for day 26 of PAM‐2000 F t and NPQ are shown in figure . F t decreased in the morning, reached a minimum around 14:00 and increased afterwards, while the NPQ trend approximately mirrored that of F t.
Figure 4 Diurnal evolution(day 26) of active fluorometer measurements: (a) fluorescence yield (F t) in relative units, (b) non‐photochemical quenching (NPQ) in relative units. Full and empty dots refer to control and treatment samples, respectively.
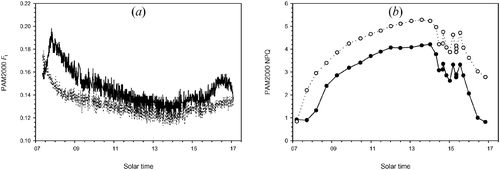
Although fluorescence flux per incident radiation unit was gathered with different methods (i.e. the active pulse method used for F t and the passive FLD principle exploited for NFs760), the daily evolution of F t in figure was tracked by that of NFs760 in figure in clear sky conditions. This result confirms those of Moya et al. (Citation2004) obtained at canopy level. In the afternoon, the performances of the passive measurements are affected by the rapid changes of the incoming irradiance due to overcast sky conditions.
This correspondence also holds true for the comparison between NPQ (active, figure ) and PRI (passive, figure ), which are inversely correlated because an increasing recourse to NPQ corresponds to a decreasing PRI. In particular, in the early morning (until 8:00), the control samples exhibited an increase in F t (NFs760) associated with delayed activation of NPQ (PRI), while for treated samples F t (NFs760) decreased and NPQ (PRI) increased in the same time range.
A single significant linear relationship (R 2 = 0.59) was found between NFs760 and F t, while two linear regressions with similar slopes and statistically different intercepts were needed to explain the relationship between PRI and NPQ (R 2 = 0.71 and 0.72 for controls and treatments respectively). This may indicate that PRI is correlated with the contents of the xanthophyll‐cycle pigments as well as their activity (e.g. Stylinsky et al. 2002).
Analogous results were obtained by Peñuelas et al. (Citation1995a), who found varying coefficients in different species in the linear relationship between PRI and (F m′−F t) / F m′, an indicator of photosystem II (PSII) light use efficiency (Genty et al. Citation1989), which has a close inverse relationship with NPQ (Evain et al. Citation2004).
3.4 Comparison of LUE and passive measurements
The relationships between LUE and remotely‐sensed energy dissipation pathways are shown in figure (data from day 26).
Figure 5 Scatter plots of light use efficiency versus physiologically meaningful RS variables for day 26: (a) LUE versus NFs760 of all samples and (b) LUE versus PRI of controls (full dots) and PRI of treated samples (empty dots). Horizontal and vertical error bars correspond to ± SE on x‐ and y‐axis, respectively.
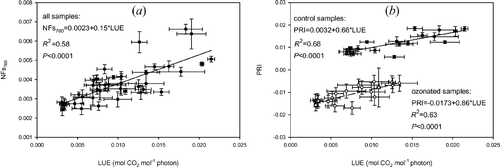
A unique significant linear regression with LUE was found with NFs760 for both theses (figure ), while this is not the case for the regression with PRI, where a unique regression did not hold. Two regression lines (figure ) with the same slopes (PRI sensitivity to LUE variations) and statistically different intercepts (minimum value of PRI), describe the relationships of LUE with controls and treated samples respectively. This underlines the difference between NFs760 and PRI: the former appear to be directly linked to photosynthesis, while the latter may be sensitive to both photosynthesis and the photo‐protection level that is activated to withstand the given irradiance level. Analogous results were presented by Nakaji et al. (Citation2006) who, by monitoring seasonal changes of LUE–PRI relationships on larch needles, found a seasonal drift in both the intercept and slope of the regression line.
The correlation between LUE and PRI of treated samples (R 2 = 0.63) was weaker than that of the control (R 2 = 0.68), possibly due to ozone‐induced uncoupling between PSII activity and down‐regulation of photosynthesis (i.e. reversible reduction in assimilation), as found by Peñuelas et al. (Citation1997) in the presence of water stress.
Part of the scatter present in the relationships shown in figure may be due to the fact that spectral and gas exchange measurements were carried out nearly contemporarily, but on a different leaf belonging to the same plant. Furthermore, a discrepancy between assimilation and energy dissipation of the photosystems may arise from an imbalance between assimilation and electron transport rate due to photorespiration and the Mehler reaction (Harbison et al. Citation1990, Krall and Edwards Citation1992).
4. Conclusions
This study shows that solar‐induced fluorescence and xanthophyll de‐epoxidation state can be detected from the near‐range distance and can be linked to plant physiological status. We conducted a 26‐day fumigation experiment in which four diurnal cycles of spectral measurements at leaf level were collected parallel to meteorological and key physiological variable measurements.
Poplar clones subjected to O3 exposure over a period of 26 days demonstrated lower fluorescence and higher use of the xanthophyll cycle than their counterparts provided with filtered air. A separation of unstressed and stressed samples using midday measurements was possible, even in the absence of visible symptoms (day 16). These results are in agreement with others (e.g. Kebabian et al. Citation1999, Freedman et al. Citation2002) that indicate that Fs provides pre‐visual stress detection. Measurements of net assimilation at solar noon were able to separate the treatments on the same date, while significant differences in active fluorescence F v / F m appeared only on the last day of measurements.
Since plants were usually maintained in a controlled environment (growth chambers), we cannot exclude that the exposure to natural conditions (e.g. high irradiance at noon) has given rise to additional light stress for both control and treatment plants. Nevertheless, this possible side effect of the experimental setup did not prevent the detection of a differential response due to ozone fumigation
Analysis of the results showed that PAM‐2000 active measurements (F t and NPQ) are well tracked by passive spectroscopy measurements (NFs760 and PRI). These results, obtained at the near‐range distance, add support to the identification of fluorescence from passive sensors at the far‐range distance.
LUE showed a significant linear correlation with NFs760 for both theses, thus indicating a link between photochemistry and passive fluorescence, regardless of the stress experienced. On the contrary, a single relationship with PRI was not found. In fact, treated samples showed greater exploitation of the xanthophyll de‐epoxidation cycle with respect to controls to attain analogous assimilation levels. These observations provide support for the use of NFs760 and PRI in monitoring plant physiological status, and a reduction in fluorescence may be regarded as an indicator of plant stress if it is accompanied by a higher level of NPQ (i.e. lower PRI).
Acknowledgements
E. Pellegrini and A. Francini are acknowledged for their help at the San Piero a Grado (Pisa) fumigation facility. We also thank F. Fava for his support during the field campaign. This research was funded by ERSAF‐Lombardia and by the Italian MIUR‐PRIN project.
References
- Bilger , W. and Björkman , O. 1990 . Role of the xanthophyll cycle in photoprotection elucidated by measurements of light‐induced absorbance changes, fluorescence and photosynthesis in leaves of Hedera canariensis. . Photosynthesis Research , 25 : 173 – 185 .
- Buschmann , C. 2002 . “ Interpretation of the fluorescence signatures from vegetation. ” . In Proceedings of the FLEX Workshop (ESA SP‐527) Remote Sensing of Solar‐Induced Vegetation Edited by: Harris , R. A . 19–20 June 2002, ESTEC, Noordwijk, The Netherlands published on CDROM
- Carter , G. A. 1993 . Responses of leaf spectral reflectance to plant stress. . American Journal of Botany , 80 : 239 – 243 .
- Carter , G. A. 1994 . Ratios of leaf reflectances in narrow wavebands as indicators of plant stress. . International Journal of Remote Sensing , 15 : 697 – 703 .
- Ciompi , S. , Castagna , A. , Ranieri , A. , Nali , C. , Lorenzini , G. and Soldatini , G. F. 1997 . CO2 assimilation, xanthophyll cycle pigments and PSII efficiency in pumpkin plants as affected by ozone fumigation. . Physiologia Plantarum , 101 : 881 – 889 .
- Demmig‐Adams , B. and Adams , W. W. 1996 . The role of xanthophyll cycle carotenoids in the protection of photosynthesis. . Trends in Plant Science , 1 : 21 – 26 .
- Dobrowski , S. Z. , Pushnik , J. C. , Zarco‐Tejada , P. J. and Ustin , S. L. 2005 . Simple reflectance indices track heat and water stress‐induced changes in steady‐state chlorophyll fluorescence at the canopy scale. . Remote Sensing of Environment , 97 : 403 – 414 .
- Evain , S. , Flexas , J. and Moya , I. 2004 . A new instrument for passive remote sensing: 2. Measurement of leaf and canopy reflectance changes at 531 nm and their relationship with photosynthesis and chlorophyll fluorescence. . Remote Sensing of Environment , 91 : 175 – 185 .
- Flexas , J. , Briantais , J. M. , Cerovic , Z. , Medrano , H. and Moya , I. 2000 . Steady‐state and maximum chlorophyll fluorescence responses to water stress in grapevine leaves: a new remote sensing system. . Remote Sensing of Environment , 73 : 283 – 297 .
- Francini , A. , Nali , C. , Picchi , V. and Lorenzini , G. 2007 . Metabolic changes in white clover clones exposed to ozone. . Environmental and Experimental Botany , 60 : 11 – 19 .
- Freedman , A. , Cavender‐Bares , J. , Kebabian , P. L. , Bhaskar , R. , Scott , H. and Bazzaz , F. A. 2002 . Remote sensing of solar‐excited plant fluorescence as a measure of photosynthetic rate. . Photosynthetica , 40 : 127 – 132 .
- Gamon , J. A. and Surfus , J. S. 1999 . Assessing leaf pigment content and activity with a reflectometer. . New Phytologist , 143 : 105 – 117 .
- Gamon , J. A. , Peñuelas , J. and Field , C. B. 1992 . A narrow‐waveband spectral index that tracks diurnal changes in photosynthetic efficiency. . Remote Sensing of Environment , 41 : 35 – 44 .
- Gamon , J. A. , Serrano , L. and Surfus , J. S. 1997 . The photochemical reflectance index: an optical indicator of photosynthetic radiation use efficiency across species, functional types, and nutrient levels. . Oecologia , 112 : 492 – 501 .
- Gamon , J. A. , Field , C. B. , Bilger , W. , Björkman , O. , Fredeen , A. L. and Peñuelas , J. 1990 . Remote‐Sensing of the xanthophyll cycle and chlorophyll fluorescence in sunflower leaves and canopies. . Oecologia , 85 : 1 – 7 .
- Genty , B. , Briantais , J. M. and Baker , N. R. 1989 . The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. . Biochimica et Biophysica Acta , 990 : 87 – 92 .
- Gitelson , A. A. and Merzlyak , M. N. 1994 . Spectral reflectance changes associated with autumn senescence of aesculus‐hippocastanum L and acer‐platanoides L leaves – spectral features and relation to chlorophyll estimation. . Journal of Plant Physiology , 143 : 286 – 292 .
- Gitelson , A. A. , Buschmann , C. and Lichtenthaler , H. K. 1999 . The chlorophyll fluorescence ratio F‐735/F‐700 as an accurate measure of the chlorophyll content in plants. . Remote Sensing of Environment , 69 : 296 – 302 .
- Guidi , L. , Nali , C. , Ciompi , S. , Lorenzini , G. and Franco , G. 1997 . The use of chlorophyll fluorescence and leaf gas exchange as methods for studying the different responses to ozone of two bean cultivars. . Journal of Experimental Botany , 48 : 173 – 179 .
- Harbison , J. , Genty , B. and Baker , N. R. 1990 . The relationship between CO2 assimilation and electron transport in leaves. . Photosynthesis Research , 25 : 213 – 224 .
- Heath , R. L. 1996 . “ The modification of photosynthetic capacity induced by ozone exposure. ” . In Photosynthesis and the Environment , Edited by: Baker , N. R . 409 – 433 . Dordrecht : Kluwer Academic Publishers .
- Heinz‐Walz‐GmbH . 1993 . “ Portable fluorometer PAM‐2000 and data acquisition software DA‐2000 ” . Effeltrich, , Germany : Heinz‐Walz‐GmbH .
- Kangasjarvi , J. , Talvinen , J. , Utriainen , M. and Karjalainen , R. 1994 . Plant defense systems induced by ozone. . Plant Cell and Environment , 17 : 783 – 794 .
- Kebabian , P. L. , Theisen , A. F. , Kallelis , S. and Freedman , A. 1999 . A passive two‐band sensor of sunlight‐excited plant fluorescence. . Review of Scientific Instruments , 70 : 4386 – 4393 .
- Kraft , M. , Weigel , H. J. , Mejer , G. J. and Brandes , F. 1996 . Reflectance measurements of leaves for detecting visible and non‐visible ozone damage to crops. . Journal of Plant Physiology , 148 : 148 – 154 .
- Krall , J. P. and Edwards , G. E. 1992 . Relationship between photosystem II and activity and CO2 fixation in leaves. . Physiologia Plantarum , 86 : 180 – 187 .
- Louis , J. , Ounis , A. , Ducruet , J. M. , Evain , S. , Laurila , T. , Thum , T. , Aurela , M. , Wingsle , G. , Alonso , L. , Pedros , R. and Moya , I. 2005 . Remote sensing of sunlight‐induced chlorophyll fluorescence and reflectance of Scots pine in the boreal forest during spring recovery. . Remote Sensing of Environment , 96 : 37 – 48 .
- McFarlane , J. C. , Watson , R. D. , Theisen , A. F. , Jackson , R. D. , Ehrler , W. L. , Pinter Jr. , P. J. , Idso , S. B. and Reginato , R. J. 1980 . Plant stress detection by remote measurement of fluorescence. . Applied Optics , 19 : 3287 – 3289 .
- Meroni , M. and Colombo , R. 2006 . Leaf level detection of solar induced chlorophyll fluorescence by means of a subnanometer resolution spectroradiometer. . Remote Sensing of Environment , 103 : 438 – 448 .
- Milton , E. J. and Rolling , E. M. 2006 . Estimating the irradiance spectrum from measurements in a limited number of spectral bands. . Remote Sensing of Environment , 100 : 348 – 355 .
- Moya , I. , Camenen , L. , Evain , S. , Goulas , Y. , Cerovic , Z. G. , Latouche , G. , Flexas , J. and Ounis , A. 2004 . A new instrument for passive remote sensing: 1. Measurements of sunlight‐induced chlorophyll fluorescence. . Remote Sensing of Environment , 91 : 186 – 197 .
- Nakaji , T. , Oguma , H. and Fujinuma , Y. 2006 . Seasonal changes in the relationship between photochemical reflectance index and photosynthetic light use efficiency of Japanese larch needles. . International Journal of Remote Sensing , 27 : 493 – 509 .
- Nali , C. , Pucciariello , C. , Mills , G. and Lorenzini , G. 2005 . On the different sensitivity of white clover clones to ozone: Physiological and biochemical parameters in a multivariate approach. . Water Air and Soil Pollution , 164 : 137 – 153 .
- Owens , T. G. 1994 . “ In vivo chlorophyll fluorescence as a probe of photosynthetic physiology. ” . In Plant Responses to the Gaseous Environment , Edited by: Alscher , R. G and Wellburn , A. R . 195 – 217 . London : Chapman and Hall .
- Peñuelas , J. , Filella , I. and Gamon , J. A. 1995a . Assessment of photosynthetic radiation‐use efficiency with spectral reflectance. . New Phytologist , 131 : 291 – 296 .
- Peñuelas , J. , Filella , I. , Elvira , S. and Inclan , R. 1995b . Reflectance assessment of summer ozone fumigated Mediterranean white pine seedlings. . Environmental and Experimental Botany , 35 : 299 – 307 .
- Peñuelas , J. , Llusia , J. , Pinol , J. and Filella , I. 1997 . Photochemical reflectance index and leaf photosynthetic radiation‐use‐efficiency assessment in Mediterranean trees. . International Journal of Remote Sensing , 18 : 2863 – 2868 .
- Pérez‐Priego , O. , Zarco‐Tejada , P. J. , Miller , J. R. , Sepulcre‐Cantó , G. and Fereres , E. 2005 . Detection of water stress in orchard trees with a high‐resolution spectrometer through chlorophyll fluorescence in‐filling of the O‐2‐A band. . IEEE Transactions on Geoscience and Remote Sensing , 43 : 2860 – 2869 .
- Plascyk , J. A. 1975 . The MK II Fraunhofer line discriminator (FLD‐II) for airborne and orbital remote sensing of solar‐stimulated luminescence. . Optical Engineering , 14 : 339 – 346 .
- Reich , P. B. 1987 . Quantifying plant response to ozone: a unifying theory. . Tree Physiology , 3 : 63 – 91 .
- Rosema , A. 2002 . “ Chlorophyll fluorescence and photosynthesis: prospect for remote sensing. ” . In Proceedings of the FLEX Workshop (ESA SP‐527) Remote Sensing of Solar‐Induced Vegetation Edited by: Harris , R. A . 19–20 June 2002, ESTEC, Noordwijk, The Netherlands CDROM
- Sims , D. A. and Gamon , J. A. 2002 . Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. . Remote Sensing of Environment , 81 : 337 – 354 .
- Stoll , M.‐P. 2004 . “ The fluorescence explorer (FLEX) space mission project: endeavour to making an old idea real. ” . In Proceedings of the FLEX Workshop (ESA WPP‐242) 2nd International Workshop on Remote Sensing of Vegetation Fluorescence 17–19 November 2004, John H. Chapman Space Centre‐CSA, Saint‐Hubert, Canada, CDROM
- Stylinski , C. D. , Gamon , J. A. and Oechel , W. C. 2002 . Seasonal patterns of reflectance indices, carotenoid pigments and photosynthesis of evergreen chaparral species. . Oecologia , 131 : 366 – 374 .
- Theisen , A. F. 2002 . “ Detecting chlorophyll fluorescence from orbit: the Fraunhofer line depth model. ” . In From Laboratory Spectroscopy to Remotely Sensed Spectra of Terrestrial Ecosystems , Edited by: Muttiah , R. S . 203 – 232 . Dordrecht : Kluwer Academic Publishers .
- Vogelmann , J. E. , Rock , B. N. and Moss , D. M. 1993 . Red edge spectral measurements from sugar maple leaves. . International Journal of Remote Sensing , 14 : 1563 – 1575 .
- Zarco‐Tejada , P. J. , Miller , J. R. , Noland , T. L. , Mohammed , G. H. and Sampson , P. H. 2001 . Scaling‐up and model inversion methods with narrowband optical indices for chlorophyll content estimation in closed forest canopies with hyperspectral data. . IEEE Transactions on Geoscience and Remote Sensing , 39 : 1491 – 1507 .