Abstract
The Prussian blue reaction (PB) detects ferric iron in histological sections but the nuclear fast red (NFR) counterstain does not selectively stain the surrounding tissue and cellular features very well. The PB/NFR stain has the advantage of detecting iron located in tissue sections, but a significant disadvantage of having poorly differentiated tissue components, as compared to a routine hematoxylin and eosin (H&E). We developed a combination of Gomori’s Prussian blue/H&E staining method (PB/H&E), and modified the technique for best performance and clarity, then assessed the ability of this new combination stain to differentiate histological features of the tissue and identify iron. Serial sections from seven formalin fixed paraffin-embedded liver samples previously diagnosed with the presence of ferric iron were subjected to our routine H&E, routine PB/NFR and three trials of the new Prussian blue/H&E combination (PB/H&E). The technique that best differentiated the histological components of tissues containing iron was further tested on liver sections from a variety of species to verify consistency i.e. equivalence in staining intensity, concentration, brightness between sections of the same sample and quality i.e. coloration, vividness, recognizable differentiation of tissue components, improved staining.
Introduction
The H&E takes advantage of the basic and acidic qualities of cellular components to stain nuclei purple or blue, and surrounding cytoplasm varying shades of pink [Citation1]. Hematoxylin, a basic dye, stains nucleic acids in the nucleoplasm, rough endoplasmic reticulum, and ribosomes in the cytoplasm [Citation2]. Eosin stains proteins without specificity and with a pink color [Citation3]. Eosin is the potassium salt of tetrabromofluorescein, an acidic dye with a negative charge, which binds to positively charged molecules in cellular cytoplasm and tissues such as connective tissue [Citation4]. Benefits of the H&E stain include the ability to identify mitotic activity, distinguish tissue types and cellular components, e.g. nuclei, which are essential for interpreting structural and cellular changes and making an accurate diagnosis [Citation1]. However, the H&E is unable to specifically identify most metal ions such as iron.
The PB reaction demonstrates the presence of ferric iron (Fe3+) of which is bound to hemoglobin, myoglobin, and hemosiderin. Overaccumulation of iron in cells can be toxic, and the unbound iron is stored weakly in the ferric state as hemosiderin [Citation5,6]. Under the acidic conditions of the Prussian blue reaction, iron is released, reacting with ferrocyanide ions provided by the potassium ferrocyanide solution, forming hexa-ferrocyanide Fe(CN)+6, an insoluble product. This yields a bright blue precipitate resulting in a blue granular deposit seen in a tissue section [Citation6].
The Prussian blue reaction is useful in estimating non-hemoglobin iron in both new and old smears of bone marrow or blood [Citation5]. The reaction is especially helpful in obtaining a diagnosis for people and animals with disorders of iron metabolism and the erythron (erythrocytes and their precursor cells) such as hemochromatosis, hemolytic anemia, abnormal erythropoiesis [Citation5]. However, due to the lack of differentiable staining of the surrounding tissues, the microanatomic location of iron can be difficult to pinpoint.
The hypothesis of this study was a combination of PB/H&E would demonstrate the presence of iron with PB and increase the histomorphological visibility of surrounding tissue with the H&E as compared to a PB stain utilizing the typical nuclear fast red counterstain. The combination staining method in this study was developed to reveal the presence of iron while also providing the histological differentiation of the H&E. Limited literature exists on combining these two stains, and our objectives were to evaluate the ability and usefulness of this PB/H&E combination.
Materials and methods
This study was performed at the Histology Laboratory, Connecticut Veterinary Medical Diagnostic Laboratory (CVMDL), University of Connecticut. In the original trial for this study, seven slides were prepared from a formalin fixed paraffin-embedded liver sample from a cat with hepatic hemosiderosis. Section thickness was 5 micrometers. Two controls were used, one stained with H&E, and one with PB i.e. Gomori’s iron reaction, according to our Standard Operating Procedure manual. There was a total of three experimental trials (Table ). Materials included a Leica ST5010 Autostainer model #XL, (Leica Biosystems, Buffalo Grove, IL, USA) distilled and tap water, and Shandon Instant Hematoxylin (cat#6765015), (Thermo Fisher Scientific, Waltham, MA, USA). The following laboratory made solutions came from Sheehan and Hrapchak protocols [Citation7] i.e. 1% alcoholic eosin Y, 20% hydrochloric acid (HCl), 10% aq. potassium ferrocyanide, nuclear fast red, 1% ammonium hydroxide, 1% HCl in 70% ethyl alcohol. Cover slips were mounted on the slide with Permount Mounting Medium (cat # 15-500), Thermo Fisher Scientific).
Table 1. Three experimental trials with the respective stain procedures used in each trial.
Trial 1: Routine Prussian Blue/Routine Hematoxylin and Eosin Combination (PB/H&E): the Prussian blue reaction (PB) was completed first. We used the Leica autostainer to deparaffinize and rehydrate the sections, then performed Gomori’s Prussian blue using equal parts, 20% aq. HCl and 10% aq. potassium ferrocyanide. The nuclear fast red counterstain was omitted and the H&E was used as the counterstain. The PB-stained sections were returned to the Leica autostainer with a modified H&E setting, which omitted the deparaffinization and rehydration steps [Citation8]. The sections were stained in Shandon Instant Hematoxylin for 6 min followed by counterstaining with the eosin solution according to Leica Autostainer XL Manual [Citation8].
Trial 2: Hematoxylin and Eosin/Prussian Blue Combination (H&E/PB): our routine H&E stain was completed first. Sections went through the entire Leica XL autostainer H&E procedure referenced in the Leica Autostainer Manual[Citation8], spending 6 min in hematoxylin and stopping in distilled water. The sections were then put through our routine PB reaction but without the nuclear fast red counterstain.
Trial 3: New Combination Prussian Blue/Modified Hematoxylin and Eosin (PB/H&E):the routine Prussian blue stain was completed first but the H&E was modified further to reduce exposure to hematoxylin by shortening the staining time from 6 to 2 min, as described in the procedure provided in Table .
Table 2. Staining procedure for the modified combination of PB/H&E stain.
To assess the outcome for the most successful staining technique, a database was created from all cases diagnosed as PB positive at CVMDL from January 2012 to September 2014. A total of 57 cases were categorized on basis of species i.e. mammalian (canine, equine, feline); fish, bird, amphibian, reptile, number of tissues tested, number of sections compiled in Table . All cases were stained with H&E, PB/NFR, and Trial 3 modified PB/H&E technique (Table ) with a total of 100 tissues tested for iron in which one sample from each species was iron positive (Table ). Liver represented 53.6% of all tissues tested and was one of the tissues in 88.1% of these cases. Therefore, only those cases with iron-positive liver sections were chosen for analysis. Any sections found not to have iron after staining with PB/NFR were eliminated from the study. The final selection process for evaluation and scoring resulted in total of 34 liver sections from 21 different species for final evaluation (Table ), all stained with PB/NFR and H&E according to our original procedures, and the Trial 3 PB/H&E method.
Table 3. Initial PB/H&E-stained section evaluation from cases of various species diagnosed having iron positive tissues.
Table 4. Species and number of cases and iron positive liver sections for final modified PB/H&E stain evaluation.
The modified PB/H&E-stained sections were then evaluated using a semi-quantitative scoring system created for stain assessment. These stained sections were evaluated for consistency, i.e. equivalent staining of nuclei, iron, and other tissue components between these sections, and for staining qualities i.e. pigmentation, background coloration, vividness. Three evaluators, including a histology technician and two veterinary pathologists i.e. E1, E2, E3, blindly evaluated the sections according to the following scoring system. Each new modified PB/H&E-stained liver section was scored as −1, 0, or 1 as compared to our routine PB/NFR and H&E stains from the same liver case. Example: if the new PB/H&E section was comparable in quality and was consistent in the iron staining in the PB/NFR section, it was given a 0; if the PB/H&E section had lower quality and inconsistent iron staining, it received −1; if the PB/H&E section had higher quality and consistent iron staining it received a +1. This same scoring was used when comparing a new PB/H&E-stained section to the H&E section. An example of the scoring chart and calculations are seen in Table . A standard deviation (SD) was calculated from evaluators’ scores and calculations, and a two-tailed t test was performed for each comparison condition previously described (Table ). The actual staining results are provided in Table .
Table 5. Example of scoring table comparing Trial 3 modified PB/H&E to PB/NFR and H&E-stained sections.
Table 6. Statistics for the semi-qualitative evaluation of the modified PB/H&E combination technique using a two-tailed t test.
Results
Trial 1: Prussian blue/routine hematoxylin and eosin (PB/H&E) sequence appeared to show appropriate results. The iron was easily seen in the sample sections after the Prussian blue reaction, as well as the pink and purple staining of the cellular cytoplasm and nuclei, respectively by the H&E stain. However, this PB/H&E section was very intense in color, which made tissues bluish and less decipherable, compared to either stain done separately. In Trial 2, with the routine hematoxylin and eosin/PB sequence, it was observed that all the color from the H&E stain had washed out after placing this section into the potassium ferrocyanide/HCl solution.
In Trial 3, the new combination of PB/H&E stain with the reduced exposure to hematoxylin demonstrated iron as clearly as our routine PB/NFR stain. In addition, the surrounding tissues not differentially stained by the PB/NFR were now stained as clearly as with a routine H&E. The histological features of the tissues were well differentiated with distinct, well-stained nuclei. The bluish hue seen in Trial 1 (PB/H&E) was less intense and the pink cellular features were easier to see microscopically in Trial 3 (PB/H&E)-stained sections
Statistically, the new combined PB/H&E technique was considered better than the PB/NFR, with an average above 0 and p-value that was <0.05. Statistical difference encountered when comparing the new PB/H&E to the H&E was also significant (p < 0.05). Thus, this modified combination of PB/H&E technique was assessed to be a higher quality stain for the identification of iron compared to the PB/NFR technique. However, the Trial 3PB/H&E was assessed to be a lower quality stain than the H&E. Overall, comments from the evaluators were positive. Comments with regards to lower quality H&E staining when combined with the PB were noted as an overall blue background (hue) and pale eosinophilic tissues. Images from the results of the final combination of PB/H&E protocol in comparison to the routine H&E and PB/NFR are provided in Figure . Control sections for both the H&E and PB/NFR were effective.
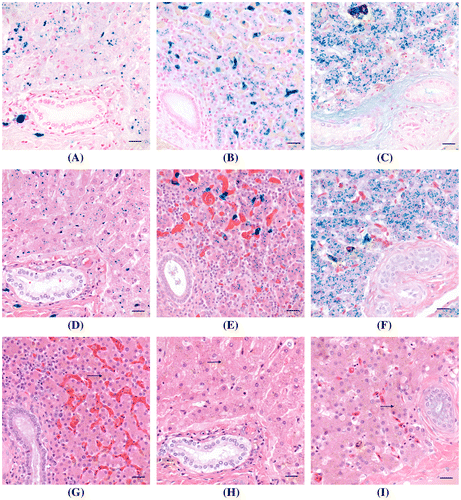
Figure 1. A comparison of the modified PB/H&E combination stain to H&E and PB/NFR stains on liver samples containing iron from three species. (A) Liver, mammal; (B) liver, bird; (C) liver, reptile. Iron is the granular blue material in cytoplasm of hepatocytes and sinusoidal macrophages. Cytoplasm of hepatocytes is pale pink with a light blue background. Collagen is pale pink. Nuclei are stained red. PB/NFR. Bar = 20 μm. (D) Liver, mammal; (E) liver, bird; (F) liver, reptile. Iron is the granular blue material in cytoplasm of hepatocytes. Cytoplasm of hepatocytes and collagen stain various shades of pink, and nuclei are stained deep blue, comparable to the H&E-stained section. Modified PB/H&E. Bar = 20 μm. (G) Liver, mammal; (H) liver, bird; (I) liver, reptile. Iron i.e. hemosiderin (arrows) is the granular brown material in cytoplasm of hepatocytes and sinusoidal macrophages. Cytoplasm of hepatocytes and collagen stain various shades of pink. Nuclei are deep blue. H&E. Bar = 20 μm.
Discussion
The hypothesis of this study was to determine if a combination of PB/H&E would allow for detection of iron and differential staining of surrounding tissues and cellular components, i.e. nuclei. This was based on the premise it is possible to combine the H&E and Prussian blue techniques and our results indicate this was indeed possible. In Trial 2, it was observed that all color from H&E staining washed out upon placing the section into the potassium ferrocyanide/HCl solution. It is theorized bleaching of the H&E can be attributed to the strong HCl in potassium ferrocyanide solution [Citation9,10]. The best method was the modified PB/H&E, which applied the Prussian blue reaction prior to staining with H&E, along with modifications to both staining procedures. This modification included elimination of the NFR counterstain and reduction of time sections were stained in hematoxylin from 6 to 2 min. The modified PB/H&E technique allowed the most comprehensive histopathological assessment of sections. The bright blue precipitation of iron and differential staining of tissues were comparable to either the PB/NFR or H&E stains used alone (Figure ). The modified PB/H&E technique detected ferric iron in tissue sections to the same degree as the PB/NFR reaction. However, there was some variation in quality across species, primarily observed in fish, and seen as slightly dimmer staining of the tissues. It was postulated this was due to the acidic decalcification method used to prepare fish tissues for histological sectioning and the potential effects of acid on the availability of ferric iron and quality of the H&E stain. Decalcification was accomplished using a HCl decalcifying solution (Cal – Ex™ Decalcifer, Cat. No. CS-510, Fisher Scientific, Hampton, NH, USA). Acidic decalcification changes the pH and causes protein hydrolysis, subsequently having adverse affects on the quality of both the nuclear and cytoplasmic staining. Despite this, the new PB/H&E method still fulfilled its purpose in fish by staining iron and seeing differentiated tissue components. These adverse effects could potentially be averted by decalcification using EDTA, a chelating agent, decreasing the HCl concentration, or using higher pH 2.0 buffered formic acid [Citation11,12]. Overall, the modified PB/H&E combination allows more extensive analysis for the presence of iron in tissues given its enhanced ability to highlight various morphological components of surrounding tissues. This provides an investigator with a greater appreciation of the pathology resulting from iron accumulation in a tissue. Our modified PB/H&E combination stain is not to be used in lieu of a H&E due to the overall blue background hue and paler eosinophilic tissues, which makes the PB/H&E a lower quality stain than the H&E. The PB/H&E is rather a replacement of the PB/NFR. This would provide a more comprehensive diagnoses of suspected iron-related disorders performed in research and pathology laboratories. In clinical and research applications, the H&E is more commonly used because it demonstrates fine cellular structures, i.e. nuclei and varying shades of red color in the cytoplasm to reveal information about properties of tissues [Citation2]. With our new PB/H&E method, enhanced coloration using H&E as a counterstain can provide diagnostic value beyond the NFR counterstain by staining cellular structures that may be associated with lesions containing mitochondria-rich oncogenic cells or inclusion bodies [Citation2]. While the H&E reveals some iron accumulation in tissues as brown hemosiderin, the PB reaction provides a definitive diagnosis for iron. The PB reaction with H&E as a counterstain is enhanced, since iron in low quantities can be visualized more definitively at the same time as seeing H&E-stained tissue components. This means an additional comprehensive diagnosis can be provided using a combination of PB/H&E for iron and other pathological changes in surrounding tissues.
Conclusion
The combination modified PB/H&E stain was considered overall to be successful but with some variation in staining quality between histological sections from different species. For most of the species we studied, this new staining method produced differential tissue staining and iron detection comparable to the H&E and PB/NFR, respectively. However, tissue sections from fish tended to be slightly duller in color, although the new method still stained iron and other tissue components. The modified PB/H&E combination stain provides a clear demonstration of the histologic architecture of tissue and allows a more comprehensive analysis for iron in the context of a differentially stained tissue section.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Avwioro G. Histochemical uses of haematoxylin – a review. JPCS. 2011;1:24–34.
- Chan JKC. The wonderful colors of the hematoxylin-eosin stain in diagnostic surgical pathology. Int J Surg Pathol. 2014;22(1):12–32.10.1177/1066896913517939
- Fischer AH, Jacobson KA, Rose J, et al. Hematoxylin and eosin staining of tissue and cell sections. Cold Spring Harbor Protocols; Cold Spring Harbor, NY, USA: Cold Spring Harbor Press; 2008.
- King DF, King LA. A brief historical note on staining by hematoxylin and eosin. Am Dermatopathol. 1986;8:168.10.1097/00000372-198604000-00013
- Andrews NC. Disorders of iron metabolism. N Engl J Med. 1999;341:1986–1995.10.1056/NEJM199912233412607
- Humason GL. Animal tissue techniques. San Francisco (CA): W.H. Freeman and Company; 1962. Chapter 17, Pigments and minerals; p. 232–233.
- Sheehan DC, Hrapchak BB. Theory and practice of histotechnology. 2nd ed. Columbus (OH): Battelle Press; 1987. Chapter 12, Pigments and minerals; p. 217.
- Leica Biosystems. Leica Autostainer XL. Appendix 3: compatible staining programs. V2.5, 2017; p. 51. Available from: http://drp8p5tqcb2p5.cloudfront.net/fileadmin/downloads_lbs/Leica%20ST5010/User%20Manuals/Leica_ST5010_IFU_2v5H_en.pdf
- Gamble M. The hematoxylins and eosin. Chapter 9. In: Bancroft JD, Gamble M, editors. Theory and practice of histological techniques. 6th ed. Nottingham: Churchill Livingstone; 2008. pp. 121–127.
- Guidelines for Hematoxylin and Eosin Staining. Bowie (MD): National Society for Histotechnology; 2001. Available from: http://nsh.org/sites/default/files/Guidelines_For_Hematoxylin_and_Eosin_Staining.pdf
- Sterchi DL, Chapter 16, Bone. In: Suvarna SK, Bancroft J, Lyton C, editors. Bancroft’s theory and practice of histological techniques. 7th ed. Sheffield: Churchill Livingston Elsevier; 2013. pp. 323–326.
- Sheehan DC, Hrapchak BB. Theory and practice of histotechnology. 2nd ed. Columbus (OH): Battelle Press; 1987. Chapter 6, Bone; p. 90.
