In the article “Experimental Measurement of Monovalent Cation Adsorption onto Bacillus subtilis Cells” by Daniel S. Alessi et al., published in Geomicrobiology Journal 27(5): 464–472, the incorrect was published. The correct figure and legend appear below.
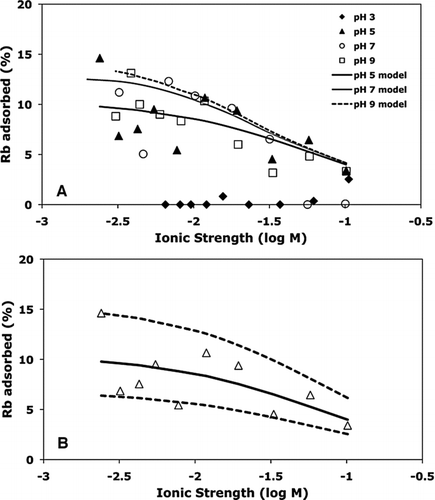
FIG. 2 (A) Rubidium adsorption to B. subtilis as a function of ionic strength and pH. Initial experimental conditions were 20 g l−1 B. subtilis cells and 2.34 × 10−5 M Rb. The pH 5 model curve represents the best-fit model that accounts for Rb adsorption onto Site 2 only. Curves for the pH 7 and pH 9 models show the extent of adsorption that would be predicted using the KNa and KRb values determined from modeling the pH 5 data, and assuming no additional adsorption of Rb onto Sites 3 or 4. (B) Best-fit model for Rb adsorption to Site 2 of B. subtilis at pH 5 (solid curve). Dashed curves are models resulting from a ± 0.2 variation in the best-fitting log stability constant value of K Rb.