?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Experiments were carried out to examine the oxido-reduction of manganese by extremely acidophilic Acidithiobacillus spp. grown with either elemental sulfur or molecular hydrogen as electron donor. While there was no evidence for manganese (II) oxidation, dissolution of solid phase manganese dioxide was observed in cultures grown aerobically on both electron donors, though this appeared not to involve reduction of the metal. Solubilization of MnO2 was much enhanced in cultures incubated anaerobically, even though biomass was smaller, and pH values increased significantly as a consequence of acid dissolution being accompanied by manganese (IV) reduction. Increases in cell numbers correlated with concentrations of soluble manganese in anaerobic cultures grown on hydrogen, demonstrating that iron-oxidizing/reducing Acidithiobacillus spp. can grow in the absence of oxygen using manganese (IV) as sole electron acceptor. Addition of ferric iron to anaerobic cultures further enhanced the reductive dissolution of MnO2 as a result of its reduction to ferrous iron which then reacted with the solid manganese phase, and confirming that Mn (IV) reduction by iron-reducing acidophiles can proceed both directly and indirectly, involving iron as a shuttle vector. The implications of these findings to developing technologies for bio-processing oxidized metal ore deposits are discussed.
Introduction
Manganese is the second most abundant transition metal in the lithosphere with an average abundance estimated at ca. 0.1% by weight, though its concentration in different terrestrial and aquatic environments can vary greatly (Ehrlich Citation2002). It occurs in a variety of minerals, including oxides, carbonates and silicates. While manganese can occur in a variety of oxidation states ranging from 0 to +7, only three (manganese (II), (III) and (IV)) are commonly encountered in nature. Manganese (III) is thermodynamically unstable and disproportionates to manganese (II) and (IV), though it can be stabilized when complexed with some ligands and may persist in oxygen-depleted water bodies (Johnson Citation2006). While manganese (IV) is highly insoluble, manganese (II) is soluble over a wide pH range and is the only oxidation state in which free (non-complexed) ions of this metal exist in aqueous systems (Ehrlich Citation2002).
Manganese is a ubiquitous micro-nutrient, acting as an activator of several enzymes and occasionally a replacement for magnesium. Its existance in different oxidation states allows it to be used as an electron donor or acceptor by some prokaryotic and eukaryotic microorganisms (Ehrlich Citation2002). Oxidation of manganese (II) requires a minimum pH of about 5, and can be catalyzed both by microorganisms that use the energy from this reaction to support their growth, and others that do not. A large variety of manganese (II)-oxidizing bacteria and fungi have been described (e.g., Akob et al. Citation2014; Bohu et al. Citation2016). Even though manganese (IV) oxides are generally considered to be insoluble, many phylogenetically-diverse bacteria have been shown to use manganese (IV) as an electron acceptor, in both aerobic and anaerobic environments. The redox potential of the MnO2(s)/Mn2+ couple is strongly electro-positive (+1.224 V at pH 7; Vanẏsek Citation2012) and on that basis is an attractive electron acceptor, though the insolubility of manganese (IV) oxides mitigates against this. The degree of crystallinity of MnO2 has a significant impact upon its amenability for micobial reduction (Ehrlich Citation2002). Bacteria reported to grow by respiring on manganese (IV) include Bacillus and Geobacter spp. (Ghiorse and Ehrlich Citation1976; Lovley Citation1991). Lin et al. (Citation2012) reported that microbial reduction of manganese (IV) proceeded via two successive one-electron transfer reactions, involving soluble manganese (III) as a transient intermediate. Manganese (IV) oxides are also known to scavenge various cationic metals, such as cobalt and copper, and can be solubilized by reaction with hydrogen sulfide, iron (II) and other reducing agents, with concommitant release of adsorbed metals.
Extremely acidophilic microorganisms are defined as those that have growth pH optima at or below 3 (Johnson and Quatrini Citation2020). The most widely studied of these are chemolitho-autotrophic bacteria of the genus Acidithiobacillus. All of these can use elemental (zero-valent) sulfur (ZVS), hydrogen sulfide and various sulfur oxy-anions as electron donors to support their growth in aerobic environments, and some species and strains can also use iron (II) and hydrogen. All iron-oxidizing Acidithiobacillus spp. have been found also to be able to grow in the absence of oxygen, using iron (III) as an electron acceptor and either sulfur or hydrogen as an electron donor (Johnson and Quatrini Citation2020). In their natural environments (e.g., streams draining metal mines and acid lakes) these bacteria frequently encounter relatively elevated concentration of soluble manganese (II), though no extreme acidophile has been reported to catalyze manganese oxidation, either directly or indirectly, as would be predicted by thermodynamic constraints (Ehrlich Citation2002). Conversely, while there have been a few reports of manganese (IV) being reduced by the iron/sulfur-oxidizer Acidithiobacillus ferrooxidans, whether this reaction is mediated directly or indirectly by this and related species, and whether the reduction of manganese (IV) can support the growth of acidophiles, has not been previously demonstated convincingly. Here we describe experiments that have shown that different Acidithiobacillus spp. are able to accelerate the dissolution of solid phase MnO2 in aerobic conditions. In the absence of oxygen the soluble, complexed manganese (IV) produced by acid dissolution of solid-phase MnO2 can be reduced, both directly and indirectly, and support the growth of those species of Acidithiobacillus that can also grow by reducing iron (III).
Materials and methods
Bacterial strains and cultivation
Four species of mesophilic iron/sulfur-oxidizing Acidi-thiobacillus spp. were used in experimental work. These were the type strains of At. ferrooxidans (Temple and Colmer Citation1951), At. ferridurans (Hedrich and Johnson Citation2013a) and At. ferriphilus (Falagán and Johnson Citation2016), and At. ferrivorans strain Peru 6 (Hallberg et al. Citation2010). All of these bacteria, except At. ferriphilus, can also use hydrogen as an electron donor. The type strains of the moderately thermophilic sulfur-oxidizer Acidithiobacillus caldus (Hallberg and Lindstrom Citation1994) and the mesophilic sulfur-oxidizer Acidithiobacillus thiooxidans (Waksman and Joffe Citation1921) were used in some experiments. All of the bacteria were routinely cultivated in media containing 1% (v/w) ZVS (VWR, UK), basal salts and trace elements (Ňancucheo et al. Citation2016) and 50 µM ferrous iron, adjusted initially to pH 2.5 with sulfuric acid, in shake flasks incubated at either 30 °C (mesophilic strains) or 40 °C (At. caldus). At. ferrooxidans, At. ferridurans and At. ferrivorans were also grown aerobically using hydrogen as electron donor, using the technique described by Hedrich and Johnson (Citation2013b). In brief, this entailed placing inoculated flasks into an air-tight 2.5 L polycarbonate jar, together with a 20 mL universal bottle that contained 1.3 g of sodium bicarbonate, 300 mg sodium borohydride and 100 mg citric acid. Ten milliliters of water were added to the bottles immediately before sealing the jars, initiating the production of both hydrogen and carbon dioxide. The amount of oxygen present in the jars was calculated to be in excess of that required for complete microbial oxidation of hydrogen to water, ensuring that the atmosphere did not become anoxic during the incubation period.
Dissolution of MnO2 in aerobic cultures of Acidithiobacillus spp. grown on ZVS or hydrogen
Duplicate cultures of At. ferrooxidans, At. ferridurans, At. thiooxidans and At. caldus, containing 1% ZVS and 0.5% (w/v) solid manganese dioxide (MnO2, >99.99% pure; Sigma-Aldrich, USA) were incubated, shaken at 100 rpm, at either 30 °C or 40 °C, for up to 24 days. Samples were removed regularly to measure pH and redox potentials (as EH values) and concentrations of soluble manganese. Cultures of At. ferrooxidans and At. ferridurans in basal salts/trace elements medium (pH adjusted to 1.6) containing 0.1% MnO2 were prepared and incubated aerobically under H2/CO2-enriched atmospheres (as above), together with non-inoculated controls. Again, samples were withdrawn periodically to measure pH and EH values, concentrations of manganese, and also changes in culture optical densities (measured at 600 nm; the dense MnO2 particles settled immediately once flasks had stopped shaking).
Dissolution of MnO2 by At. ferrooxidans and At. ferridurans grown on hydrogen under anaerobic conditions, in the presence and absence of additional iron
At. ferrooxidans and At. ferridurans were grown on hydrogen as sole electron donor in sealed jars under H2/CO2-enhanced atmospheres, either with or without MnO2 (0.25%, w/v). These were then used to inoculate cultures that were incubated under the same conditions, except that the oxygen present was removed by placing a sachet of activated carbon (AnaeroGen sachets; Oxoid, UK) in each jar. A second anaerobic experiment used a similar set up, though in this case the bacteria were first grown to high cell densities (∼109 cells/mL) and iron (III) sulfate (5 mM, final concentration) was added to some of the cultures. Non-inoculated controls were prepared at the same time, and iron (II), rather than iron (III) sulfate, added to some of these. The sealed jars containing the cultures and controls were incubated at 30 °C, and samples analyzed periodically for pH and concentrations of iron (II) and soluble manganese.
Anaerobic growth of iron/sulfur-oxidizing Acidithiobacillus spp. via hydrogen oxidation coupled to manganese reduction
To confirm that the reductive dissolution of MnO2 could support the growth of iron-oxidizing Acidithiobacillus spp. in the absence of oxygen, cultures were set up in shake flasks, and either hydrogen (At. ferrooxidans, At. ferridurans and At. ferrivorans) or 1% (w/v) ZVS (At. ferriphilus) was provided as the electron donor. Sterile MnO2 (1%, w/v) was added to 50% of the cultures. These were incubated under anaerobic conditions, as described above, for up to 42 days at 30 °C. Samples were withdrawn every 7 days, and new H2/CO2 gas-generating mixtures (hydrogen-grown cultures) and AnaeroGen sachets (all cultures) placed into each jar. In addition to measuring pH and concentrations of soluble manganese, changes in total and active biomass were estimated by enumerating planktonic cells and measuring concentrations of ATP, respectively.
Analytical techniques
Concentrations of soluble manganese in culture samples were determined using the formaldoxime colorimetric method (Goto et al. Citation1962), and ferrous iron using the ferrozine assay (Stookey Citation1970). Planktonic bacteria in culture samples were enumerated using a Thoma counting chamber and a Leitz Wetzlar 766200 phase contrast microscope, at ×400 magnification. Metabolically-active biomass was monitored by measuring the ATP contents of triplicate culture samples, using a commercial bioluminescence kit (CCK-2 kit, Hygiena, UK) and the protocol developed by Okibe and Johnson (Citation2011) for acidophilic microorganisms. pH values were measured using a pHase combination glass electrode, and redox potentials using a combination platinum electrode fitted with a silver/silver chloride reference cell, both coupled to an Accumet pH/redox meter 50. Measured redox potentials were adjusted to be relative to a hydrogen reference (EH values).
Results
Thermodynamic constraints predict that prokaryotes cannot, in theory, use the oxidation of manganese (II) to support their growth in acidic (pH < ∼4–5) environments (Ehrlich Citation2002). This was confirmed in the present study where cultures of extremely acidophilic Acidithiobacillus spp. oxidized iron (II) to iron (III) in low pH media whereas manganese (II) remained non-oxidized and in solution. Cell numbers increased in cultures containing both iron (II) and manganese (II), but not manganese (II) alone (data not shown). In contrast, the biologically-catalyzed reduction of manganese (IV) is thermodynamically feasible in acidic solutions, and evidence for this was obtained in cultures incubated under anaerobic conditions.
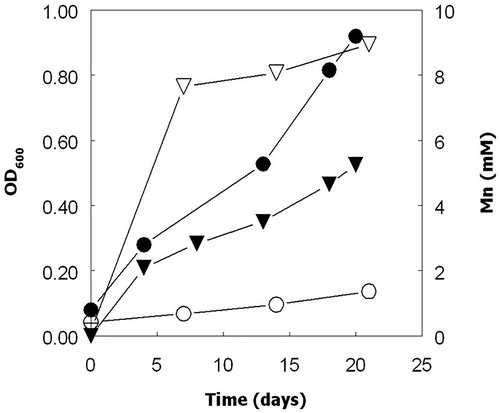
Dissolution of solid MnO2 (up to about 10% of that present) was observed in aerobic cultures of Acidithiobacillus spp. grown on ZVS (). The pH of all these cultures declined (sulfur oxidation is a proton-generating reaction) and there was a close correlation (r = 0.93) between culture pH values and manganese concentrations (). Redox potentials increased with time in all of these cultures from 600–650 at the start of the experiment to 770–810 mV at the end. Enhanced dissolution of MnO2 was also observed in aerobic cultures of Acidithiobacillus spp. grown at low pH using hydrogen as sole electron donor, as shown for At. ferridurans in . In contrast to cultures grown on ZVS, there were only minor fluctuations in pH values in these cultures. Increasing culture turbidities, which were correlated to cell numbers, during incubation provided evidence that these bacteria were growing under these conditions. The redox potentials of At. ferridurans cultures grown aerobically on hydrogen did not vary greatly during incubation, and remained about 100 mV more positive than those of the controls (data not shown). Concentrations of soluble manganese increased only slightly in sterile control cultures, where pH values also remained essentially unchanged ().
Figure 1. Solubilization of manganese from MnO2 by cultures of (a) At. ferrooxidans (▲, Δ) and At. ferridurans (▼, ∇), and (b) At. thiooxidans (■, □) and At. caldus (●, ○), grown aerobically on ZVS. Closed symbols show concentrations of soluble manganese and open symbols pH. Data points are means of duplicate cultures.
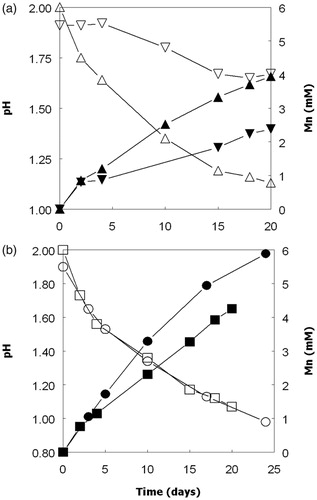
Figure 2. Correlation between pH and concentrations of soluble manganese in aerobic cultures of mesophilic Acidithiobacillus spp. grown aerobically of ZVS.
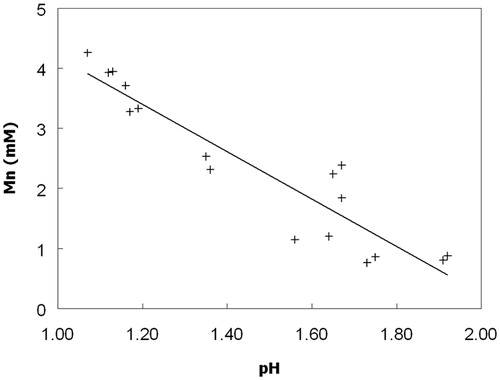
Figure 3. Solubilization of manganese from MnO2 by cultures of At. ferridurans (▼, ∇) grown aerobically on hydrogen, and non-inoculated controls (♦, ◊). Closed symbols show concentrations of soluble manganese and open symbols pH. Data points are means of duplicate cultures.
In contrast to cultures grown aerobically on either ZVS or hydrogen, the pH values of cultures of both At. ferrooxidans and At. ferridurans increased significantly (to above 4) when incubated under anaerobic conditions with hydrogen as electron donor and MnO2 as potential electron acceptor (). The pH values of cultures that did not contain MnO2 did not increase. Addition of iron (II) to non-inoculated controls containing MnO2 resulted in greater amounts of manganese being solubilized than in iron-free controls, and the iron (II) was oxidized to iron (III). Addition of iron (III) to inoculated anaerobic (hydrogen-grown) cultures also enhanced the dissolution of MnO2, and iron (II) was present in cultures measured after six days of incubation ().
Figure 4. Solubilization of manganese from MnO2 by cultures of At. ferrooxidans (▲, Δ) and At. ferridurans (▼, ∇) grown anaerobically on hydrogen. Closed symbols show concentrations of soluble manganese and open symbols pH. The broken lines show pH values of cultures that did not contain MnO2. Data points are means of duplicate cultures.
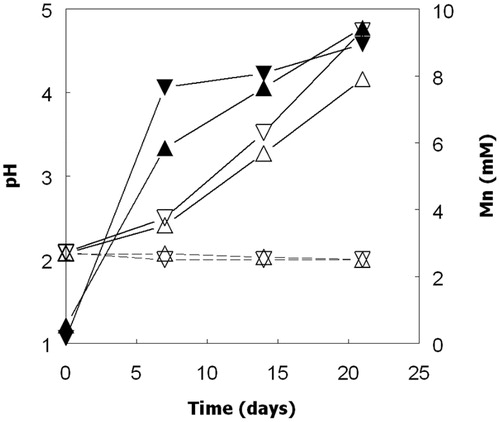
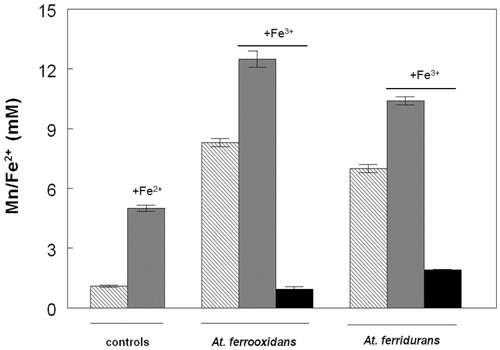
Figure 5. Concentrations of soluble manganese (shaded and hatched bars) and iron (II) (solid black bars) in cultures of At. ferrooxidans and At. ferridurans grown anaerobically on hydrogen, measured after a six day incubation period, with (shaded bars) or without (hatched bars) 5 mM iron (III) added at the start of the experiment. Manganese concentrations in non-inoculated controls either containing initially 5 mM iron (II) or no added iron are also shown (ferrous iron was not detected in controls by day 6). Error bars are range values of duplicate cultures.
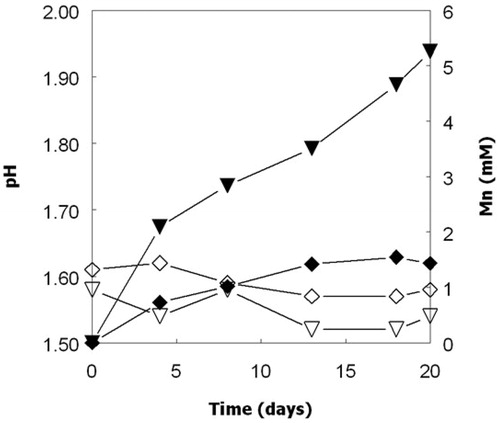
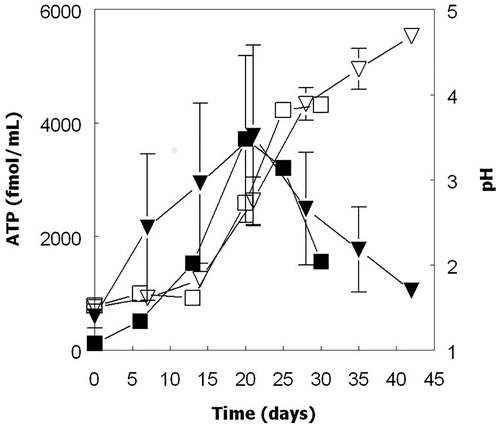
Confirmation that all four iron/sulfur-oxidizing Acidithiobacillus spp. could grow in the absence of oxygen via the dissimilatory reductive dissolution of MnO2 was obtained in cultures incubated anaerobically with either hydrogen (At. ferrooxidans, At. ferridurans and At. ferrivorans) or ZVS (At. ferriphilus) as sole electron donor (). Increased numbers of planktonic bacteria paralleled increasing concentrations of soluble manganese. Since some cells would be anticipated to be attached to residual MnO2 particles, their total numbers were probably under-estimated. Concentrations of ATP also increased, though only until day 20 with two of the four bacteria tested, and data showed large standard deviations for biological duplicates (). The pH of anaerobic cultures of ZVS/MnO2-grown At. ferriphilus were also found to increase during incubation (in contrast to aerobic cultures), as well as those of other Acidithiobacillus spp. grown on hydrogen. It was also noted that dissolution of MnO2 by At. ferridurans grown on H2 as electron-donor was much more extensive in cultures grown anaerobically (reductive acid dissolution) than aerobically (acid dissolution alone), even though growth was more limited in anaerobic cultures, and the increasing pH in these would be anticipated to impede acid dissolution of MnO2 ().
Figure 6. Changes in numbers of planktonic bacteria (closed symbols) and soluble manganese (open symbols) in cultures of (a) At. ferrooxidans (▲, Δ) and At. ferridurans (▼, ∇), and (b) At. ferriphilus (■, □) and At. ferrivorans (●, ○), grown anaerobically on ZVS (At. ferriphilus) or hydrogen (other species) in the presence of MnO2.
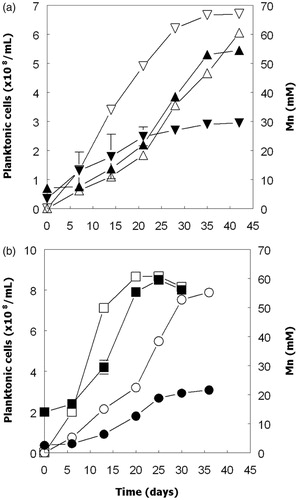
Figure 7. Changes in concentrations of ATP (closed symbols) and pH (open symbols) in cultures of At. ferridurans grown anaerobically on hydrogen (▼, ∇), and At. ferriphilus (■, □) grown anaerobically on ZVS with manganese (IV) as electron acceptor. The error bars indicate standard deviations for duplicate cultures (after averaging triplicate ATP analyses for each sample).
Discussion
Many transition metals can exist in different ionic forms, and oxido-reduction of complexed forms of some of these (e.g., iron and copper in cytochromes) are used to transfer electrons in prokaryotic and eukaryotic organisms. Some microorganisms are able also to exploit non-complexed transition metals as electron donors or acceptors to promote their growth. The most well-documented of these are iron and manganese, though there is increasing evidence that other metals (and metalloids, such as selenium) can also be utilized by a more limited diversity of life forms (Ehrlich Citation2002). In some cases, these redox transformations are catalyzed directly, though in others these are indirect, involving other metals or nonmetallic vectors. Johnson et al. (Citation2017) showed that reduction of both chromium (VI) and copper (II) by acidophilic bacteria was indirect and mediated by iron (II), generated under anaerobic conditions, and conversely, that oxidation of copper (I) was mediated by iron (III) produced by iron-oxidizing acidophiles grown aerobically. No oxido-reduction of these metals occurred in media containing only trace amounts of iron.
Acidophilic microorganisms often encounter much more elevated concentrations of transition and some other metals in natural and anthropogenic environments, due to the enhanced solubilities of many cationic metals in low pH liquors. Different ionic forms of the same metal, such as iron and manganese, can display very different solubilities, the more oxidized forms being far less soluble than the more reduced forms. However, whereas both iron (II) and iron (III) (and manganese (II)) are soluble at very low pH (<2.5), manganese (IV) is not. This may help explain why very many phylogenetically-diverse acidophilic bacteria and archaea have been demonstrated to grow by respiring on iron (III) while there are few corresponding reports for manganese (IV).
Although generally considered to be insoluble even at low pH, some dissolution of MnO2 can occur in acidic liquors as noted by Swain et al. (Citation1975). Acid dissolution of MnO2 does not involve a corresponding change in the oxidation state of the metal, the soluble products generated being manganese (IV) ions complexed with hydroxyl ions (EquationEquation 1[1]
[1] ):
[1]
[1]
EquationEquation 1[1]
[1] predicts that dissolution of MnO2 should be more extensive as the pH of the bathing solution declines, as was found in the present study with cultures of different Acidithiobacillus spp. grown aerobically on ZVS. Microbial sulfur oxidation is strongly acid-producing reaction, generating proton acidity well in excess of that consumed in reaction [1], and there was a strong correlation between soluble manganese and the pH of cultures grown aerobically on ZVS in the presence of MnO2. Interestingly, although the aerobic oxidation of hydrogen is essentially a pH neutral reaction, as confirmed in the present study, more manganese was solubilized in cultures of At. ferridurans than in sterile controls that had similar values, suggesting that acidithiobacilli have a possible secondary role in addition to acid genesis.
In contrast to acid dissolution alone, the (bacterially-mediated) reductive dissolution of MnO2, coupled to the oxidation of hydrogen, is more highly consumptive of protons, so that, if this occurs, culture pH would be anticipated to increase (EquationEquation 2[2]
[2] ):
[2]
[2]
Acid consumption associated with manganese dissolution in anaerobic cultures was confirmed in a succession of experiments, not only involving hydrogen as electron donor, but also (and in marked contrast to aerobic cultures) ZVS (EquationEquation 3[3]
[3] ):
[3]
[3]
Since the formaldoxime colorimetric method used to determine soluble manganese does not differentiate between different oxidation states of the metal, whether culture pH increased or remained stable (or decreased, in aerobic ZVS cultures) was the main indicator of whether MnO2 dissolution involved reduction of the metal or not. The conclusion reached was that reductive dissolution only occurred under anaerobic conditions, and could be coupled to the oxidation of either hydrogen or ZVS. Whether the mechanism involved direct contact with the solid was not ascertained, but it was considered to be more likely to be a two-stage process, involving acid dissolution of the mineral phase (EquationEquation 1[1]
[1] ), followed by bacterial reduction of hydroxyl-complexed manganese (IV) (EquationEquation 4
[4]
[4] ):
[4]
[4]
This is analogous to the reductive dissolution of iron (III) minerals, such as goethite, by acidophilic bacteria (Bridge and Johnson Citation2000).
Regardless of the mechanism of dissolution, manganese reduction coupled to either the oxidation of hydrogen or ZVS was demonstrated to support the growth of all Acidithiobacillus spp. tested, as shown by increased cell numbers, culture turbidity and ATP contents, during culture incubation. This is the first time that Mn (IV) has been reported unequivocally to act as sole electron acceptor for acidophilic microorganisms. In many natural and anthropogenic environments (such as acidic lakes and biomines), acidophiles encounter much larger concentrations of iron than manganese. In these situations, it is likely that manganese reduction in anoxic sites is mediated, at least in part, by iron (II). The ability of acidophilic bacteria and archaea to reduce iron (III) to iron (II) in oxygen-depleted liquors is widespread (and more ubiquitous than iron (II) oxidation), and all of the iron-oxidizing Acidithiobacillus spp. used in the present study can also reduce iron (III). It was shown that dissolution of the solid phase MnO2 used could be catalyzed abiotically by iron (II), with the measured stoichiometry of iron oxidized to manganese reduced being close to the predicted value of 2:1 (EquationEquation 5[5]
[5] ):
[5]
[5]
Addition of ferric iron to inoculated cultures resulted in increased amounts of MnO2 being solubilized, and ferrous iron was also detected. This suggests that, in these cultures, iron (II) generated by reduction of the added iron (III) catalyzed the reductive dissolution of MnO2, regenerating iron (III) which was reduced again by the bacteria. Whether this was the exclusive mechanism of MnO2 reduction when iron was present is not known, though this work has shown that reduction of Mn (IV) by acidophiles can proceed both directly and indirectly, involving iron as a shuttle vector ().
Figure 9. Schematic representation of the direct reductive dissolution of MnO2 by iron-oxidizing/reducing Acidithiobacillus spp. (top), and indirect reductive dissolution where iron is used as a shuttle vector (bottom).
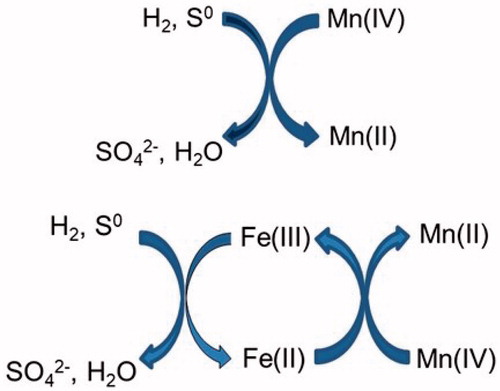
Early studies of MnO2 dissolution by At. thiooxidans and At. ferrooxidans grown on sulfur led the authors to propose that the mechanism involved the reduction of manganese (IV) by sulfur intermediates formed during sulfur oxidation (Ghosh and Imai Citation1985). Sulfide and sulfite were proposed, but not confirmed, as possible reactive intermediates. Sugio et al. (Citation1988) described work carried out with washed, intact cells of a strain of At. ferrooxidans, again using ZVS as electron donor. They proposed a two-stage mechanism in which ZVS was oxidized by iron (III) by a ‘sulfur:ferric iron oxidoreductase,’ generating iron (II) and sulfite, both of which rapidly reduced manganese (IV) to manganese (II), though the method used to determine soluble manganese (atomic absorption spectrophotometry) would not have differentiated between manganese (II) and soluble, complexed manganese (IV) (or manganese (III)). Sugio et al. (Citation1988) were not able to detect this proposed enzyme in cell-free extracts of iron-grown At. ferrooxidans. By cultivating Acidithiobacillus spp. on hydrogen rather than on ZVS under anaerobic conditions and in the presence of MnO2, we have demonstrated that reactive sulfur intermediates are not necessarily involved in manganese reduction. While iron (II) can effectively reduce MnO2 in acidic liquors, this also occurred in cultures that contained only very small (nutritional) amounts of iron (50 µM) so we conclude that a direct mechanism, probably involving soluble complexed manganese (IV) produced by acid dissolution of solid MnO2, also occurs. However, it seems likely that a more proficient mechanism for the reductive dissolution of MnO2 is likely to be mediated by the dissimilatory reduction of iron by acidithiobacilli and other acidophiles, using iron reductase systems that have been postulated elsewhere (e.g., Osorio et al. Citation2013). Whether direct or indirect via an iron shuttle, our data provide convincing evidence that the dissimilatory reduction of manganese (IV) can serve to support the growth of those Acidithiobacillus spp. that can also grow by iron respiration. This has important implications, not only for the geochemistry of low pH environments, but also to potential industrial applications where valuable metals, notably cobalt, are often associated with manganese (IV) minerals, such as asbolane (Johnson and du Plessis Citation2015).
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Akob DM, Bohu T, Beyer A, Schäffner F, Händel M, Johnson CA, Merten D, Büchel G, Totsche KU, Küsel K. 2014. Identification of Mn(II)-oxidizing bacteria from a low-pH contaminated former uranium mine. Appl Environ Microbiol 80(16):5086–5097.
- Bohu T, Akob DM, Abratis M, Lazar CS, Kusel K. 2016. Biological low-pH Mn(II) oxidation in a manganese deposit influenced by metal-rich groundwater. Appl Environ Microbiol 82(10):3009–3021.
- Bridge TAM, Johnson DB. 2000. Reductive dissolution of ferric iron minerals by Acidiphilium SJH. Geomicrobiol J 17:193–206.
- Ehrlich HL. 2002. Geomicrobiology. 4th ed. New York: Taylor and Francis.
- Falagán C, Johnson DB. 2016. Acidithiobacillus ferriphilus sp. nov., a facultatively anaerobic iron- and sulfur-metabolizing extreme acidophile. Int J Syst Evol Microbiol 66(1):206–211.
- Ghiorse WC, Ehrlich HL. 1976. Electron transport components of the MnO2 reductase system and the location of the terminal reductase in a marine Bacillus. Appl Environ Microbiol 31(6):977–985.
- Ghosh J, Imai K. 1985. Leaching of manganese dioxide by Thiobacillus ferrooxidans growing on elemental sulfur. J Ferment Technol 63:259–262.
- Goto K, Komatsu T, Furukawa T. 1962. Rapid colorimetric determination of manganese in waters containing iron. Anal Chim Acta 27:331–334.
- Hallberg KB, González-Toril E, Johnson DB. 2010. Acidithiobacillus ferrivorans, sp. nov.; facultatively anaerobic, psychrotolerant iron-, and sulfur-oxidizing acidophiles isolated from metal mine-impacted environments. Extremophiles 14(1):9–19.
- Hallberg KB, Lindstrom EB. 1994. Characterization of Thiobacillus caldus sp. nov., a moderately thermophilic acidophile. Microbiol 140(12):3451–3456.
- Hedrich S, Johnson DB. 2013a. Acidithiobacillus ferridurans, sp. nov.; an acidophilic iron-, sulfur- and hydrogen-metabolizing chemolithotrophic Gammaproteobacterium. Int J System Evol Microbiol 63(Pt_11):4018–4025.
- Hedrich S, Johnson DB. 2013b. Aerobic and anaerobic oxidation of hydrogen by acidophilic bacteria. FEMS Microbiol Lett 349(1):40–45.
- Johnson DB, Du Plessis C. 2015. Biomining in reverse gear: using bacteria to extract metals from oxidized ores. Miner Eng 75:2–5.
- Johnson DB, Hedrich S, Pakostova E. 2017. Indirect redox transformations of iron, copper, and chromium catalyzed by extremely acidophilic bacteria. Front Microbiol 8:211
- Johnson DB, Quatrini R. 2020. Acidophile microbiology in space and time. Curr Issues Mol Biol 39:63–76.
- Johnson KS. 2006. Manganese redox chemistry revisited. Science 313(5795):1896–1897.
- Lin H, Szeinbaum NH, DiChristina TJ, Taillefert M. 2012. Microbial Mn(IV) reduction requires an initial one-electron reductive solubilization step. Geochim Cosmochim Acta 99:179–192.
- Lovley DR. 1991. Dissimilatory Fe(III) and Mn(IV) reduction. Microbiol Rev 55(2):259–287.
- Ňancucheo I, Rowe OF, Hedrich S, Johnson DB. 2016. Solid and liquid media for isolating and cultivating acidophilic and acid-tolerant sulfate-reducing bacteria. FEMS Microbiol Lett 363(10):fnw083.
- Okibe N, Johnson DB. 2011. A rapid ATP-based method for determining active microbial populations in mineral leach liquors. Hydrometallurgy 108(3–4):195–198.
- Osorio H, Mangold S, Denis Y, Ñancucheo I, Esparza M, Johnson DB, Bonnefoy V, Dopson M, Holmes DS. 2013. Anaerobic sulfur metabolism coupled to dissimilatory iron reduction in the extremophile Acidithiobacillus ferrooxidans. Appl Environ Microbiol 79(7):2172–2181.
- Stookey L. 1970. Ferrozine – a new spectrophotometric reagent for iron. Anal Chem 42(7):779–781.
- Sugio T, Tsujita Y, Hirayama K, Inagaki K, Tano T. 1988. Mechanism of tetrathionate manganese reduction with elemental sulfur by Thiobacillus ferrooxidans. Agri Biol Chem 52(1):185–190.
- Swain HA, Lee C, Rozelle RB. 1975. Determination of the solubility of manganese hydroxide and manganese dioxide at 25.deg. by atomic absorption spectrometry. Anal Chem 47(7):1135–1137.
- Temple KL, Colmer AR. 1951. The autotrophic oxidation of iron by a new bacterium, Thiobacillus ferrooxidans. J Bacteriol 62(5):605–611.
- Vanẏsek P. 2012. Electrochemical series. In: Haynes, WM, editor. Handbook of Chemistry and Physics. 93rd ed. Boca Raton, FL: CRC Press, p5–80.
- Waksman SA, Joffe JS. 1921. Acid production by a new sulfur-oxidizing bacterium. Science 53(1366):216.