ABSTRACT
Objective: In the young generations with nitrous oxide abuse (N2O), featured electrophysiological response of the peripheral neuropathy caused by nitrous oxide remains to be defined.
Methods: Patients with nitrous oxide abuse (20 cases), two variants of Guillain-Barré syndrome (GBS), that is, acute inflammatory demyelinating polyradiculoneuropathy (GBS-AIDP, 19 cases) and acute motor axonal neuropathy (GBS-AMAN, 18 cases), as well as diabetic peripheral neuropathy (DPN, 20 cases) were enrolled into this study. Electrophysiological parameters including distal motor latency (DML), motor nerve conduction velocity (MNCV), sensory nerve conduction velocity (SNCV), amplitudes of compound muscle action potential (CMAP), and sensory nerve action potential (SNAP) were measured and analyzed by comparing the parameters between the aforementioned patients groups as well as normal control group (20 subjects).
Results: Compared to normal control subjects, patients with nitrous oxide abuse showed prolonged DML, slower MNCV and SNCV in the limbs, lower amplitudes of CMAP in the median, tibial and peroneal nerves, and lower SNAP in median and ulnar nerves. Abnormalities of MNCV and amplitudes of CMAP in the lower limbs were significantly higher than that in the upper limbs . Abnormal electrophysiological features of patients with nitrous oxide abuse were dramatically different from those in GBS-AIDP or DPN patients, but similar to those in GBS-AMAN patients.
Conclusions: Nitrous oxide abuse could cause abnormal electrophysiological response in the limbs. Some of the parameters (DML, MNCV, SNCV, CMAP and SNAP) appeared significantly different between the patients with nitrous oxide abuse, GBS with AIDP or AMAN, and DPN patients.
Significance: Electrophysiological examination could be considered as an important supporting factor in differential diagnosis for nitrous oxide abuse, GBS with AIDP or AMAN, and DPN.
Introduction
Nitrous oxide (N2O) gained popularity as a recreational drug. It was used for the first time as an anesthetic in dental practice in 1884, because of its euphoric, relaxing, and illusory properties [Citation1,Citation2]. Currently, it is one of the most popular drugs that are abused by young people worldwide, and particularly in China, an increasing number of young generations are using it for recreation and abusing it. The world’s first serious neurological complications from recreational use of nitrous oxide were reported in 1978 [Citation3]. In 2016, China published its first report of neurological disease caused by recreational use of nitrous oxide [Citation4] .
Several lines of evidence indicate that nitrous oxide abuse can cause neurological complications because of its neurotoxicity via the inactivation of vitamin B12 5−7. In this regard, the neurological complications caused by nitrous oxide have various clinical and neurophysiological manifestations, such as myelopathy, peripheral neuropathy, or subacute combined degeneration (SACD, a condition of combined myelopathy and peripheral neuropathy) [Citation5–8]. Previous studies suggested that the duration of nitrous oxide abuse was divided into acute phase less than 2 weeks, chronic phase more than 3 months, and subacute phase 2 weeks to 3 months [Citation9]. Besides, a case report suggested that daily doses over 80 g (10 N2O bulbs/day) were associated with an exponential increase in the risk of permanent neurological deficits [Citation10]. Recent studies had shown that nitrous oxide caused peripheral neuropathy in a dose-dependent manner, and was also influenced by gender and age [Citation11]. Regrettably, there was no clear definition or diagnosis of the duration, age, frequency, neurologic symptom and signs of nitrous oxide abuse in China [Citation4,Citation12]. In recent years, an increasing number of young patients with a history of nitrous oxide abuse were admitted to the department of neurology. Many of them presented with myelopathy or acute demyelinating neuropathy symptoms that were similar to those of Guillain–Barré syndrome (GBS) [Citation13]. However, GBS is an autoimmune-induced acute peripheral polyneuropathy that often follows an infectious process, which is significantly different from that of nitrous oxide abuse in terms of pathogenesis. GBS is usually characterized by numbness, paresthesia, and progressive weakness although various clinical variants may exist, including acute motor axonal neuropathy (AMAN), acute inflammatory demyelinating polyneuropathy (AIDP), acute motor and sensory axonal neuropathy (AMSAM), Miller–Fisher syndrome (MFS), a pharyngeal–cervical–brachial variant, and a paraparetic variant [Citation14].
In clinical practice, electrophysiological examination has been widely used as an important supporting method for the diagnosis of GBS [Citation15,Citation16]. Diabetic peripheral neuropathy (DPN) is a neurological disease characterized by painful neuropathy symptoms, paresthesia and loss of sensation [Citation17]. DPN is very common in neurology clinics, and electrophysiological examination is an important method to diagnose the disease. At present, electrophysiological studies for the patients with nitrous oxide abuse were rare in China [Citation4,Citation18]. There are few studies comparing the electrophysiological results of patients with nitrous acid abuse with those of GBS and DPN.
In this study, therefore, electrophysiological characteristics were analyzed in the patients who were diagnosed as nitrous oxide abuse and admitted to the department of neurology of our hospital from 2018 to 2020. Though all of them developed chronic and progressive limb paralysis rather than acute neuropathy, they had many neurophysiological manifestations that were similar to those observed in patients with acute GBS; e.g., progressive weakness, numbness in both hands and upper limbs, difficulty in moving, and a decrease in the disappearance of tendon reflexes. Patients with nitrous oxide abuse often hide their history of illegal use of nitrous oxide when they consult a physician. The similarity of clinical manifestations between nitrous oxide abuse and GBS hampers the establishment of accurate differential diagnosis in patients with nitrous oxide abuse. Therefore, in this study, we had retrospectively analyzed the data of electrophysiological examinations in the patients who were admitted to the same hospital with a diagnosis of GBS with AMAN or AIDP, and DPN between 2016 and 2020, and the findings were further compared with that of nitrous oxide abuse. Specifically, electrophysiological parameters including DML, MNCV, SNCV, CMAP, and SNAP in the limbs of normal controls, patients with nitrous oxide abuse, GBS (AMAN or AIDP), or DPN were analyzed and compared. Aim of this study was to allow clinicians to establish more accurate and differential diagnoses of nitrous oxide abuse based on electrophysiological examination.
Methods
Patients and study design
This study was approved by the Ethics Committee of the First Hospital of China Medical University. The data inclusion criteria of the nitrous oxide abuse were as follows: the nitrous oxide abuse duration was more than 2 weeks; presence of neurological disorders associated with recreational use of nitrous oxide and abnormal electrophysiological results; no diabetes, alcoholism, infection within 3 months or chronic digestive tract disease. The electrophysiological evaluations of 20 patients (14 males and 6 females) diagnosed with nitrous oxide abuse in our hospital from January 2018 to December 2020 were studied retrospectively. All patients had a history of subacute or chronic nitrous oxide use. Numbness and weakness in the limbs were the main complaints of these patients.
To compare the electrophysiological characteristics of the patients with nitrous oxide abuse with those of the patients with GBS or DPN, the electrophysiological data of 37 patients diagnosed with GBS as well as 20 cases of DPN who were hospitalized in our hospital from July 2016 to December 2020 were also analyzed retrospectively. In accordance with the criteria for the diagnosis of typical GBS and the criteria for electrophysiological classification [Citation19,Citation20], of the patients with GBS, 19 individuals (10 males and 9 females) were diagnosed as AIDP, and 18 patients (8 males and 10 females) were diagnosed as AMAN.
To record electrophysiological characteristics in normal controls, 20 age- and sex-matched normal people (14 males and 6 females) were included in this study. The data of the normal controls came from the people who had taken the electrophysiological exam in our hospital between 2018 and 2020. Inclusion criteria: (1) Age between 18 and 30; (2) Peripheral neuropathy, muscular dystrophy, metabolic myopathy, periodic paralysis, and myelopathy were excluded by electrophysiological examination due to various mild clinical symptoms, including myasthenia, muscle pain, and numbness of the limbs; (3) No history of diabetes, alcoholism, infection within 3 months or chronic digestive tract disease.
Electrophysiological evaluation
All electrophysiological examinations were performed by an experienced neurophysiologist using an electromyograph (MEB-9200 K, Nihon Kohden Corporation, Tokyo, Japan) following the standard protocols [Citation21]. Limb temperature was kept above 32 °C. The CMAPs of motor nerves were evoked from the median nerve (stimulating at the wrist and elbow; recording at the abductor pollicis brevis muscle), the ulnar nerve (stimulating at the wrist, below the elbow, and above the elbow; recording at the abductor digitus minimi muscle), the fibular nerve (stimulating at the ankle and below the fibular head; recording at the extensor digitorum brevis muscle), and the tibial nerve (stimulating at the ankle and the popliteal fossa; recording at the abductor hallucis muscle). The orthodromic conduction of sensory nerves was measured from the median nerve (stimulating at the digitus medius and palmar muscles; recording at the wrist), the ulnar nerve (stimulating at the little finger; recording at the wrist), and the sural nerve (stimulating at the lateral malleolus; recording at 11 cm proximal to the calf). The reference ranges used in our lab were shown in the supplementary material (Table S1-S5).
Electrophysiological parameters were recorded as distal motor latency (DML), amplitudes of CMAP after distal and proximal stimulation (dCMAP and pCMAP), motor nerve conduction velocity (MNCV), sensory nerve conduction velocity (SNCV), and sensory nerve action potential (SNAP). Abnormality was defined as the values beyond the mean ± 2 standard deviations of the aforementioned reference ranges. The percentage of extended DML, MNCV decrease, CMAP decrease, and SNCV decrease was calculated as (the actual measured value – the reference value of the lab)/the reference value of the lab × 100%.
F-waves were elicited in the ulnar nerve, using supramaximal stimuli at wrist, respectively, at 1 Hz stimulation rate for 20 consecutive trials. The conduction velocity and persistence of F wave were used for statistical analysis.
Statistical analysis
Statistical analysis was performed using the GraphPad Prism 7.04. Data were presented as means ± SD. Differences between groups were compared using one-way ANOVA (or Welch ANOVA when the data was not normally distributed), followed by Fisher’s LSD test. Abnormal rate of electrophysiological parameters was expressed as a percentage, and the chi-square test was used for comparative analyses. Gender differences among the five groups were also analyzed using the chi-square test. All tests were two-tailed, and statistical significance was set at P < 0.05.
Results
History of present illness and age of patients
The average time of nitrous oxide abuse in the 20 patients of this study was 15.8 ± 3.5 months (range: 1–36 months) with varying daily frequency and amount (1–20 cans daily). Average interval between electrophysiological examination and the first-time appearance of the symptoms was 32.1 ± 24.1 days (between 7 and 90 days) for nitrous oxide abuse, 17.4 ± 3.7 days (between 7 and 30 days) for GBS-AIDP, and 12.8 ± 3.9 days (between 4 and 45 days) for GBS-AMAN, and 4.6 ± 2.7 months (between 1 and 12 months) for DPN, respectively. Average age of the patients diagnosed with nitrous oxide abuse was 21.1 ± 4.1 years old (male: 14, female: 6), which was significantly younger than that of patients with GBS-AIDP (51.3 ± 16.6 years old, male: 10, female: 9), GBS-AMAN (56.5 ± 13.4 years old, male: 8, female: 10), or DPN (62.2 ± 9.9 years old, male: 13, female: 7, P < 0.001 in all comparison except for the normal controls, ). Chi-square test was conducted for gender differences among the five groups, and the results indicated no statistical difference (all P > 0.05).
Most patients with a history of chronic nitrous abuse presented with persistent symmetrical numbness and limitation of normal movement in their extremities. And the progressive weakness and glove-sock-like dysesthesia symptoms were more severe in the distal and lower limbs compared to that in the proximal and upper limbs. The detailed characteristic of 20 patients with nitrous oxide abuse were summarized in .
Table 1. Age and gender of patients
Table 2. Detailed characteristics of patients with nitrous oxide abuse
In addition, low serum vitamin B12 levels were noted in seven of the 20 nitrous oxide abuse patients, and with an average of 118.5 ± 22.9 pmol/L (normal value: 145–637 pmol/L). Four patients presented with high homocysteine levels (normal: <13.56 nmol/L), and two patients were associated low B12 levels and high homocysteine levels.
Spinal MRI was performed in 6 of the 20 patients and brain MRI was performed in 2 of the 20 patients. Three patients showed longitudinal high T2 signal in the dorsal cervical spinal cord and one patient in the thoracic spinal cord, suggesting the presence of myelopathy secondary to neuropathy due to N2O exposure. The brain MRI results of 2 patients were normal.
Characteristics and abnormal rates of nerve electrophysiology in patients with nitrous oxide abuse
As shown in , in the patients with chronic nitrous oxide abuse, majority of motor nerve abnormalities was found in the lower limbs in terms of DML (fibular: 55%; tibial: 55%), MNCV (fibular: 80%; tibial: 70%), and amplitude of CAMP (fibular: 65%; tibial: 70%), while majority of sensory nerve abnormalities was found in the upper limb (median: 55%; ulnar: 55%) nerves. Abnormal rate of MNCV and the amplitude of CMAP in the lower limbs were significantly higher than that of the upper limbs in the patients with chronic nitrous oxide abuse (; median vs. fibular, median vs. tibial, ulnar vs. fibular, and ulnar vs. tibial nerves; all P < 0.01). However, there was no significant difference in the abnormal rate in DML, SNCV, and amplitude of SNAP between the upper and lower limbs ().
Table 3. Abnormal rate of electrophysiological parameters in patients diagnosed with N2O abuse (n = 20)
Following the published criteria by the Joint Task Force of European Federation of Neurological Societies/Peripheral Nerve Society Guideline [Citation22], number of primary demyelinating and axonal neuropathies was classified in the patients with nitrous oxide abuse and diabetic peripheral neuropathy. It was found that six of the 20 cases of nitrous oxide abuse were primary demyelinating neuropathy, none of them was primary axonal neuropathy, 11 of them were equivocal, and 3 of them were normal; and that thirteen out of the 20 DPN patients were primary demyelinating neuropathy, three of them was primary axonal neuropathy, and 4 of them were equivocal.
Interestingly, comparison of the length of DML extension in the patients with nitrous oxide abuse found that it was greater in the lower limb nerves than that in the upper limbs (). However, there was no difference in the extent of MNCV decrease, SNCV decrease, and CMAP amplitude decrease between the upper and lower limb nerves in the patients with nitrous oxide abuse ().
Figure 1. Comparison of DML extension in the limbs of patients with nitrous oxide abuse. Percentage of the extended DML was calculated as described in the methods. Vertical axis: Extent of prolonged distal latency of motor nerve (%), horizontal axis: nerves examined. DML: distal motor latency. *P < 0.05, N = 20
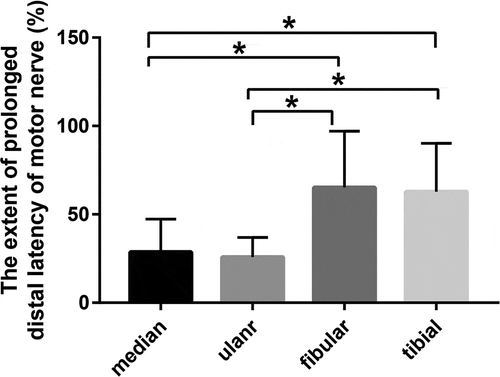
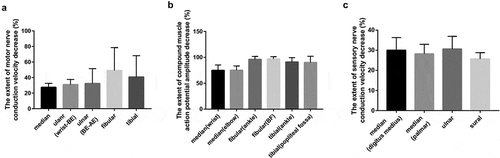
Figure 2. Comparison of MNCV decrease, CMAP amplitude decrease, and SNCV decrease in the limbs of patients diagnosed with nitrous oxide abuse. Percentage of the extended MNCV decrease (A), CMAP amplitude decrease (B), and SNCV decrease (C) was calculated as described in the methods. Vertical axes: Extent of MNCV decrease (A), CMAP amplitude decrease (B), and SNCV decrease (C); horizontal axes: nerves examined. MNCV: motor nerve conduction velocity; CMAP: compound muscle action potential; SNCV: sensory nerve conduction velocity; AE: above elbow; BE: below elbow; BF: below fibular head. N = 20. There was no difference in the extent of MNCV decrease, SNCV decrease, and CMAP amplitude decrease between the upper and lower limb nerves in the patients with nitrous oxide abuse
Ten of the 20 patients with nitrous oxide abuse underwent F wave detection in ulnar nerve, and the conduction velocity and persistence of F wave were both within the normal range. The average velocity of F-wave was 60.0 ± 5.8 m/s, and the persistence ranged from 69% to 100%. MRI of the cervical spine confirmed myelopathy in 3 of these 10 patients, but their F wave persistence was normal.
Comparison of electrophysiological characteristics in patients diagnosed with nitrous oxide abuse, GBS-AIDP, GBS-AMAN, or DPN
The characteristics of electrophysiological parameters in the 20 patients with nitrous oxide abuse, 19 GBS with AIDP (GBS-AIDP), 18 GBS with AMAN (GBS-AMAN), and 20 DPN were analyzed and compared. As shown in , while DML of all tested nerves (median, ulnar, tibial, and fibular) was significantly longer in the patients with nitrous oxide abuse, GBS-AIDP, GBS-AMAN, or DPN than that of control (P < 0.05). However, it was significantly shorter in the nitrous oxide abuse patients than that of GBS-AIDP (P < 0.05). In contrast, MNCV of all tested nerves was significantly slower in the patients with chronic nitrous oxide abuse, GBS-AIDP, GBS-AMAN, or DPN than that of control (P < 0.05), but it was significantly faster in the patients with chronic nitrous oxide abuse than that of GBS-AIDP patients (P < 0.05).
Table 4. Electrophysiological parameters in controls and patients diagnosed with N2O, GBS-AIDP, GBS-AMAN and DPN
In the median, tibial and fibular nerves, the amplitude of dCMAP and pCMAP was significantly lower in the patients with nitrous oxide abuse than that of control (P < 0.05, ). In the ulnar nerve, however, the amplitude of dCMAP and pCMAP was not significantly different between the patients with chronic nitrous oxide abuse and control, but it was significantly higher in the patients with chronic nitrous oxide abuse compared to that of GBS-AIDP, GBS-AMAN, or DPN (P < 0.05, ).
In addition, patients with nitrous oxide abuse showed significantly slower SNCV and lower SNAP in the upper limbs (median and ulnar nerves) compared to the control, but they were significantly faster (SNCV) or higher (SNAP) compared to the GBS-AIDP or DPN (P < 0.05, ).
Discussion
The current study demonstrated that, compared to normal control, patients with nitrous oxide abuse presented prolonged DML, abnormal MNCV, CMAP, or SNCV, and a higher abnormal rate of MNCV and CMAP in the lower limbs compared to the upper limbs. Specifically, DML of all tested nerves (median, ulnar, fibular, tibial) was significantly shorter in the nitrous oxide abuse patients than that of the GBS patients with AIDP; while MNCV was significantly faster in the patients with chronic nitrous oxide abuse than that of the AIDP patients. In addition, amplitudes of CMAP in the ulnar nerve were greater in the patients with nitrous oxide abuse than that in the GBS patients with AIDP or AMAN, or the patients with diabetic peripheral neuropathy. Patients with nitrous oxide abuse also showed significantly faster SNCV and higher SNAP in the upper limbs (median and ulnar nerves) compared to the patients with GBS-AIDP or DPN. These findings suggested that electrophysiological alteration of DML, MNCV, SNCV and SNAP in the patients with nitrous oxide abuse were dramatically different from that in the GBS patients with AIDP or AMAN and the patients with DPN.
Nitrous oxide abuse is mainly seen in young generation (age range: 16–24 years) in England and Wales 23 In China, however, no clear evidence regarding to the age of the population abused with nitrous oxide has been reported. Here, we reported the average age of the 20 patients with chronic nitrous oxide abuse was 21 years old, which was consistent with the age range reported in other countries [Citation23].
In the current study, majority of the patients with a history of chronic nitrous oxide abuse complained of symmetrical numbness and limitation of normal movement in the limbs when they visited the hospital. Furthermore, most of them claimed that the progressive weakness and glove-sock-like dysesthesia symptoms were more severe in the distal and lower limbs compared to that in the proximal and upper limbs. Consistent with the symptoms, the electrophysiological evaluation of the patients indicated that the nerve damage was more severe in the lower limbs compared to the upper limbs, as evidenced by the findings that the abnormal rate of motor nerve (including MNCV and the amplitude of CMAP) was higher in the lower limbs than that in the upper limbs. However, difference of sensory nerve impairment was not found between the lower and upper extremities. These findings were partially consistent with those of previous clinical studies performed in other countries, which reported the presence of length-dependent sensorimotor axonal neuropathy in the lower extremities of patients with nitrous oxide abuse [Citation24–26].
In clinical practice, DML and MNCV are widely used to evaluate function of the myelin sheath, while CMAP amplitude is considered as a response to the function of axons. Neuropathy induced by nitrous oxide abuse has been attributed to the effect of nitrous oxide on the inactivation of vitamin B12 through blocking methionine synthase, which converts homocysteine to methionine via a methylation process. The blockade of this enzyme could lead to the reduction of ‘functional’ vitamin B12 levels [Citation27]. Deficiency of ‘functional’ vitamin B12 prevents methylation of the myelin protein and leads to demyelination of within the central and peripheral nervous systems [Citation28–30]. These mechanisms may contribute to the abnormalities of DML and MNCV observed in patients with nitrous oxide abuse in this study. In addition, a recent study suggested that loss or conduction block of the largest nerve fibers caused by nitrous oxide toxicity to the paranodal region in the patients with nitrous oxide abuse may contribute to reduction in nerve conduction velocity [Citation31].
In the current study, patients with nitrous oxide abuse also presented CMAP abnormalities in limbs with a higher abnormal rate in the lower limbs than the upper limbs. CMAP amplitude reduction may be caused by both of blocking methionine metabolism or a toxicity that affects the paranodal region [Citation31]. In addition, most neuropathies associated with toxicity or vitamin deficiency were characterized predominantly by length-dependent axonal pathology with secondary demyeliantion in later period [Citation32]. This histopathologic features had been confirmed by sural nerve biopsy [Citation33]. Similar observations had been made in patients with B12 deficiency [Citation31] which was the putative mechanism of abnormal CMAP in patients with nitrous oxide abuse. Therefore, findings of electrophysiological assessment in the current study suggested functional abnormality, rather than anatomical integrity, could not identify which damage (myelin sheath or axonal damage) was more severe in the patients with nitrous oxide abuse. In this regard, while needle electromyography (EMG) examination could help to clarify this issue, it was not performed in this study and remains to be investigated in the future.
Guillain–Barré syndrome is an immune-mediated neuropathy that is characterized by weakness in the limbs or cranial-nerve-innervated muscles and areflexia [Citation34]. In this study, we compared the electrophysiological characteristics among patients with nitrous oxide abuse, GBS (AIDP or AMAN), and DPN. The electrophysiological parameters including DML, MNCV, CMAP, SNCV, and SNAP, in both upper and lower limbs of the patients with nitrous oxide abuse were dramatically different from those of patients with GBS-AIDP. These findings suggested that the electrophysiological evaluation of the limbs could be considered as a useful diagnostic method to differentiate nitrous oxide abuse from GBS-AIDP, and that nitrous oxide abuse may cause damage predominantly in myelin sheaths.
We also noted that electrophysiological features in the patients with nitrous oxide abuse were similar to those in the GBS patients with AMAN, with the exception that both the distal and proximal CMAP amplitudes were higher in the upper limbs of patients with nitrous oxide abuse compared to the GBS patients with AMAN. Furthermore, the axonal injury was even more severe in the GBS patients with AMAN than that in patients with nitrous oxide abuse. Therefore, the difference in the distal and proximal CMAP amplitudes of the upper limbs between the nitrous oxide abuse and AMAN groups can be considered as a parameter for the differential diagnosis of nitrous oxide abuse and AMAN. While it has been reported that axonal damage in patients with AMAN was caused by the deterioration of antibody-mediated motor axonal membranes upon by viral infection [Citation35], the difference in the extent of axonal damage between the nitrous oxide abuse and AMAN patients suggested that the axonal damage by nitrous oxide may be mediated by different pathological mechanisms.
Pathogenesis of DPN is complicated with involvement of vascular factors and metabolic interactions. Microvascular defects, such as basement membrane thickening, endothelial cell proliferation, hypertrophy, and decreased oxygen tension are the most common causes for the development of DPN [Citation36]. Patients with DPN first develop gradual and insidious damage to the distal sensory neurons followed by sensory loss of the nerves in the proximal limbs, and motor nerves damage at late phase [Citation37]. In DPN, sensory nerves are more preferably and severely damaged than motor nerves, which is consistent with the results of electrophysiological examination found in the DPN patients of the current study. In addition, sensory nerve damage was more severe in the patients with DPN than that in the patients with N2O abuse, which might be due to the complicated pathogenic mechanisms of DPN.
The age of GBS cohort (19 patients with AIDP and 18 patients with AMAN) and DPN cohort were not matched with that of nitrous oxide abuse group, and the existence of selection bias was a limitation of this study. No difference in the impairment of sensory nerves was observed between the nitrous oxide abuse and GBS-AMAN groups. The failure to include the detailed clinical features of the patients was another limitation of this study. Electrophysiological assessment in a larger number of nitrous oxide abuse patients including clinical features remains to be conducted in the future in order to confirm the findings of the current study in the diagnosis and prognosis of nitrous oxide abuse.
Conclusion
This study indicated that chronic nitrous oxide abuse causes abnormal nerve conduction in both upper and lower limbs. However, characteristics of electrophysiological parameters (DML, MNCV, SNCV, and the amplitudes of CMAP and SNAP) in the patients with nitrous oxide abuse were dramatically different from those of the patients with GBS-AIDP and DPN, but they were similar to those observed in patients with GBS-AMAN except the amplitudes of CMAP in the upper limbs. These findings suggested electrophysiological examination on upper and lower limbs nerves could be used as supporting criteria for differential diagnosis of nitrous oxide abuse-induced neuropathy, AIDP or AMAN in GBS patients, and diabetic peripheral neuropathy. Retrospective data on 20 patients with N2O abuse nitrous oxide abuse showed the specificity in electrophysiological examination. However, further well-designed investigation are needed to verify our results.
Disclosure of potential conflicts of interest
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Yan Li
Yan Li M.D. Department of Neurology, The First Hospital of China Medical University.
Xiuchun Zhang
Xiuchun Zhang M.M. Department of Neurology, The First Hospital of China Medical University.
Chuansheng Zhao
Chuansheng Zhao M.D., Ph.D. Associate Editor of Restorative Neurology and Neuroscience Professor, Department of Neurology, The First Hospital of China Medical University.
References
- Kaar SJ, Ferris J, Waldron J, et al. Up: the rise of nitrous oxide abuse. An international survey of contemporary nitrous oxide use. J Psychopharmacol (Oxford, England). 2016;30:395–401.
- van Amsterdam J, Nabben T. Recreational nitrous oxide use: prevalence and risks. Regul Toxicol Pharmacol. 2015;73:790–796.
- Sahenk Z, Mendell JR, Couri D, et al. Polyneuropathy from inhalation of N2O cartridges through a whipped-cream dispenser. Neurology. 1978;28:485–487.
- Bao L, Li Q, Li Q, et al. Clinical, electrophysiological and radiological features of nitrous oxide-induced neurological disorders. Neuropsychiatr Dis Treat. 2020;16:977–984.
- Garakani A, Jaffe RJ, Savla D, et al. Neurologic, psychiatric, and other medical manifestations of nitrous oxide abuse: a systematic review of the case literature. Am J Addict. 2016;25:358–369.
- Dubrey S, Smith R. ‘Whippits’: nitrous oxide gas inhalation as recreational drug use. Br J Hosp Med (London, England : 2005). 2016;77:492.
- Chiang TT, Hung CT, Wang WM, et al. Recreational nitrous oxide abuse-induced vitamin B12 deficiency in a patient presenting with hyperpigmentation of the skin. Case Rep Dermatol. 2013;5:186–191.
- Lin RJ, Chen HF, Chang YC, et al. Subacute combined degeneration caused by nitrous oxide intoxication: case reports. Acta Neurol Taiwan. 2011;20:129–137.
- Alt RS, Morrissey RP, Gang MA, et al. Severe myeloneuropathy from acute high-dose nitrous oxide (N2O) abuse. J Emerg Med. 2011;41:378–380.
- Cheng HM, Park JH, Hernstadt D. Subacute combined degeneration of the spinal cord following recreational nitrous oxide use. BMJ Case Reports. 2013;2013.
- Winstock AR, Ferris JA. Nitrous oxide causes peripheral neuropathy in a dose dependent manner among recreational users. J Psychopharmacol. 2020;34:229–236.
- Zheng D, Ba F, Bi G, et al. The sharp rise of neurological disorders associated with recreational nitrous oxide use in China: a single-center experience and a brief review of Chinese literature. J Neurol. 2020;267:422–429.
- Dong X, Ba F, Wang R, et al. Imaging appearance of myelopathy secondary to nitrous oxide abuse: a case report and review of the literature. The International journal of neuroscience 2018:1–10.
- Hiew FL, Ramlan R, Viswanathan S, et al. Guillain-Barre Syndrome, variants & forms fruste: reclassification with new criteria. Clin Neurol Neurosurg. 2017;158:114–118.
- Uncini A, Ippoliti L, Shahrizaila N, et al. Optimizing the electrodiagnostic accuracy in Guillain-Barré syndrome subtypes: criteria sets and sparse linear discriminant analysis. Clin Neurophysiol. 2017;128:1176–1183.
- Uncini A, Kuwabara S. Electrodiagnostic criteria for Guillain-Barrè syndrome: a critical revision and the need for an update. Clin Neurophysiol. 2012;123:1487–1495.
- Tesfaye S, Boulton AJ, Dyck PJ, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33:2285–2293.
- Zheng R, Wang Q, Li M, et al. Reversible neuropsychiatric disturbances caused by nitrous oxide toxicity: clinical, imaging and electrophysiological profiles of 21 patients with 6-12 months follow-up. Neuropsychiatr Dis Treat. 2020;16:2817–2825.
- van Doorn PA. Diagnosis, treatment and prognosis of Guillain-Barre syndrome (GBS). Presse Med (Paris, France : 1983). 2013;42:e193–201.
- Hadden RD, Cornblath DR, Hughes RA, et al. Electrophysiological classification of Guillain-Barre syndrome: clinical associations and outcome. Plasma exchange/sandoglobulin Guillain-Barre syndrome trial group. Ann Neurol. 1998;44:780–788.
- Wang TY, Chen SC, Peng CW, et al. Relevance of nerve conduction velocity in the assessment of balance performance in older adults with diabetes mellitus. Disabil Rehabil. 2017;39:419–427.
- JTFotEat PNS. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on the use of skin biopgy in the dagnosis of small fiber neuropathy. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society. Journal of the Peripheral Nervous System 2010;15:79–92.
- Randhawa G, Bodenham A. The increasing recreational use of nitrous oxide: history revisited. Br J Anaesth. 2016;116:321–324.
- Hew A, Lai E, Radford E. Nitrous oxide abuse presenting with acute psychosis and peripheral neuropathy. Aust N Z J Psychiatry. 2018;52:388.
- Thompson AG, Leite MI, Lunn MP, et al. Whippits, nitrous oxide and the dangers of legal highs. Pract Neurol. 2015;15:207–209.
- Richardson PG. Peripheral neuropathy following nitrous oxide abuse. Emerg Med Australas. 2010;22:88–90.
- Savage S, Ma D. The neurotoxicity of nitrous oxide: the facts and “putative” mechanisms. Brain Sci. 2014;4:73–90.
- Kumar N. Neurologic aspects of cobalamin (B12) deficiency. Handb Clin Neurol. 2014;120:915–926.
- Patel KK, Mejia Munne JC, Gunness VRN, et al. Subacute combined degeneration of the spinal cord following nitrous oxide anesthesia: a systematic review of cases. Clin Neurol Neurosurg. 2018;173:163–168.
- Franques J, Chiche L, De Paula AM, et al. Characteristics of patients with vitamin B12-responsive neuropathy: a case series with systematic repeated electrophysiological assessment. Neurol Res. 2019;41:569–576.
- Tani J, Weng HY, Chen HJ, et al. Elucidating unique axonal dysfunction between nitrous oxide abuse and Vitamin B12 deficiency. Front Neurol. 2019;10:704.
- Staff NP, Windebank AJ. Peripheral neuropathy due to vitamin deficiency, toxins, and medications. Continuum (Minneap Minn). 2014;20:1293–1306.
- Koike H, Takahashi M, Ohyama K, et al. Clinicopathologic features of folate-deficiency neuropathy. Neurology. 2015;84:1026–1033.
- Wakerley BR, Uncini A, Yuki N. Guillain-Barré and Miller Fisher syndromes--new diagnostic classification. Nat Rev Neurol. 2014;10:537–544.
- Kuwabara S, Yuki N. Axonal Guillain-Barre syndrome: concepts and controversies. Lancet Neurol. 2013;12:1180–1188.
- Tesfaye S, Selvarajah D. Advances in the epidemiology, pathogenesis and management of diabetic peripheral neuropathy. Diabetes Metab Res Rev. 2012;28(Suppl 1):8–14.
- Kobayashi M, Zochodne DW. Diabetic neuropathy and the sensory neuron: new aspects of pathogenesis and their treatment implications. J Diabetes Investig. 2018;9:1239–1254.
