ABSTRACT
Patients with esophageal cancer are at high risk of developing malnutrition during neoadjuvant chemoradiation therapy (CRT), which in turn is associated with postoperative morbidity. The aim of the study is to explore whether parameters of a complete pre-treatment nutritional status may predict deterioration of nutritional status during CRT in patients with esophageal cancer. In this prospective cohort study, 101 patients with esophageal cancer treated with CRT were included. Data of patient characteristics, tumor classification, performance score, %weight change, body mass index, fat (free) mass index, phase angle, handgrip strength, energy- and protein intake, and use of (additional) dietary supplements were collected. A prediction model was constructed to identify predictive parameters for deterioration in nutritional status (defined as weight loss of >5% and/or decline in fat free mass of ≥1.4 kg) during CRT. Nutritional status deteriorated in 49 patients (49%) during CRT. The only predictor for deterioration in nutritional status was fat free mass index (OR 1.21 (90% CI: 1.03 – 1.42)). Patients with a higher fat free mass index are at increased risk of deterioration in nutrition status during CRT. Results suggest that all patients should be carefully supervised during CRT, regardless of their nutritional status before start of CRT.
Introduction
The incidence of esophageal cancer is rapidly increasing in Western countries, mainly due to an increase in overweight, obesity and Barrett esophagus (Citation1). Currently, curative treatment for esophageal cancer consists of approximately 5.5 weeks concurrent neoadjuvant chemoradiation therapy (CRT), followed by a resection approximately 7 weeks after completing CRT (Citation2,Citation3). Weight loss, related to tumor location and side effects of the treatment is frequently reported both before and during treatment (Citation4,Citation5). Before the start of the treatment 34–74% of patients report weight loss (Citation6–10) and during treatment this is 40–57% (Citation6,Citation10,Citation11). As preoperative weight loss is associated with postoperative morbidity (Citation12–15) and increased healthcare costs (Citation4,Citation5), it is important to recognize which patients are at risk for deterioration of nutritional status during treatment. This creates opportunities for targeted interventions.
One previous study in patients with esophageal cancer identified the following factors to be associated with weight loss during CRT: no dietetic counseling, TNM (Tumor-Nodal-Metastasis) stages III and IV, total energy intake of <1441.3 kcal/day, depression, esophagitis, and loss of appetite (Citation11). Pre-treatment weight or pre-treatment weight loss were not associated with weight loss during treatment. Other parameters of nutritional status were not assessed.
In the present era, almost half of the patients with esophageal cancer are overweight at diagnosis (Citation9,Citation10,Citation16). Literature advises an assessment of nutritional status which, next to weight, body mass index (BMI) and weight loss, also comprises parameters of muscle mass or muscle strength (Citation17,Citation18) as overweight may mask a compromised nutritional status. Parameters such as low fat free mass (FFM) or handgrip strength (HGS), both reflecting a depletion of protein reserves, might be predictive of deterioration in nutritional status, even when weight and BMI (energy reserves) are within the normal or higher ranges.
Therefore, the aim of the study is to explore whether parameters of pre-treatment nutritional status, next to weight (loss) and BMI, may predict deterioration in nutritional status during CRT in patients with esophageal cancer.
Materials and Methods
Patients
The study population of the present study consisted of consecutive patients visiting the VU University Medical Center (Amsterdam) between August 2006 and January 2016 for curative treatment of esophageal cancer. Patients were treated with CRT, according to the CROSS trial protocol [concurrent Paclitaxel (50 mg/m2) and Carboplatin (AUC 2.0) (administered by intravenous infusion on days 1, 8, 15, 22, and 29) combined with external beam radiation with a total dose of 41.4 Gy (given in 23 fractions of 1.8 Gy, 5 fractions a week)] (Citation3).
During CRT all patients received protocolled dietetic care, consisting of an intensive nutritional support program with the aim to maintain or even improve their nutritional status. On average patients were counseled every other week.
As data collection was performed as part of routine patient care, the need for informed consent was waived by the Medical Ethic Review Committee of the VU University Medical Center.
Data Collection
Patient-, tumor- and treatment characteristics, American Society of Anaesthesiologists (ASA) score (Citation19), number of dietetic consultations and use of dietary supplements were extracted from medical and dietetic records. TNM stage was determined by the American Joint Committee on Cancer (Citation20). Malnutrition was assessed according to the recently published ESPEN consensus criteria (Citation18), which is defined as a low BMI or unintentional weight loss combined with either low BMI or low fat free mass index. Trained dieticians performed the measurements of the nutritional status 1–2 weeks before the start and on average 3 weeks after completion of CRT.
Body Weight and Body Mass Index
Body weight (kg) in the past was indicated by the patient. Body weight before and after CRT was measured within 0.1 kg on a calibrated digital scale (SECA 888) or patients' own scale at home. Repetitive measurements were performed on the same scale. Patients were preferably weighed wearing light indoor clothes and without shoes. Height was indicated by the patient or measured using a stadiometer (SECA 213) within 0.01m. BMI was calculated by dividing weight (kg) by height (m)2.
Bioelectrical Impedance Analysis (BIA) Measurements
Fat free mass (FFM) (kg), fat mass (FM) (kg), fat free mass index (FFMI) (kg/m2), fat mass index (FMI) (kg/m2) and phase angle (°) were calculated from bioelectrical impedance analysis (BIA) measurements. The measurements were performed using a bioelectrical impedance analyzer (Bodystat 1500, Euromedix) with patients in the supine position, preferably on the non-dominant body side.
FFM was calculated from resistance and reactance using the Kyle equation (Citation21). FM was derived from weight (kg) minus FFM. To calculate FFMI and FMI, FFM, and FM were divided by height (m)2, respectively. FFMI values <10th percentile of reference values were considered as low and FMI values >90th percentile were considered as high (Citation18). Phase angle was calculated by the following equation: (reactance/resistance) * (180 ° / π) (Citation22).
Handgrip Strength (HGS)
HGS was measured with a hydraulic hand dynamometer (JAMAR). Patients were instructed to perform three consecutive HGS tests, preferably with their non-dominant hand. The results were recorded to the nearest 1.0 kg and the mean of three HGSs was compared with reference values for HGS by Bohannon et al. (Citation23). HGS <10th percentile were considered as poor peripheral muscle strength.
Energy- and Protein Intake and Requirements
Current dietary intake was estimated using a one-day dietary history and rough estimates of energy (kcal) and protein (grams) were calculated. Energy requirements (kcal) were calculated using the FAO/WHO/UNU equation (Citation24) multiplied by 1.3 (for weight maintenance) to 1.5 (for weight gain). Protein requirements were calculated as at least 1.2 gram protein per kg bodyweight per day. Energy- and protein intakes were calculated as percentages of the requirements.
Outcome
The primary outcome of this study was deterioration in nutritional status during CRT. Based on reduced survival rate and increased postoperative morbidities reported in previous studies in patients with esophageal cancer, deterioration in nutritional status was defined as a decline in weight of >5% during CRT (Citation10) and/or a decline of FFM of ≥ 1.4 kg (Citation13).
Statistical Analyses
All variables were visually checked for normal distribution. Baseline data were used to describe the study population and were presented as number and proportion (%) or mean with standard deviation in case of normal distribution. Variables with skewed distributions were presented as median and interquartile ranges.
Logistic regression analysis was performed to create a prediction model for deterioration in nutritional status during CRT (yes/no). First, all possible predictors were tested in univariable analyses for single associations. To create the prediction model, a backward selection procedure was used including variables with a p-value <0.10. Variables that were not linearly related to the outcome were analyzed as categorical variables.
In addition, differences between in- and excluded patients and non-baseline differences for patients with and without deterioration in nutritional status were assessed by independent t-tests, chi-square tests or Mann-Whitney tests. P-values <0.05 were considered as statistical significant.
All statistical analyses were conducted with Statistical Package for the Social Sciences (SPSS) Statistics, version 22 (2013, IBM Corporation, New York, USA).
Results
Patients
A number of 213 patients with esophageal cancer were eligible for CRT in the period 2006–2015. Data were not available of 50 patients because they received no dietetic care due to logistical errors/barriers (in particular in the first start-up period of the protocolled dietetic care program) or received other dietetic care in their place of residence.
Data of 163 patients were available and assessed for eligibility to be included in this study. Sixty-two patients were excluded because of missing data of the outcome measurement. The remaining 101 patients were included. There were no statistically significant differences in baseline characteristics between included and excluded patients ().
Table 1. Patient characteristics differences of included and excluded patients with esophageal cancer before start of neoadjuvant chemoradiation therapy.
presents the baseline patient characteristics of the included patients. The age range was 40–83 years and 73% of the patients were men. Median BMI was 24.6 kg/m2 (interquartile range 22.2; 26.5 kg/m2). According to reference values, FFMI was below the 10th percentile in 27%, FMI was above the 90th percentile in 17%, and HGS was below the 10th percentile in 22% of patients. A poor nutritional status, according to the ESPEN definition 2015 (Citation18), before the start of CRT was present in 8% of the patients.
Table 2. Baseline patient characteristics of patients with esophageal cancer before start of neoadjuvant chemoradiation therapy.
During CRT the mean number of dietetic consultations was 6.5 (±2.7) (n = 89). The use of (additional) oral nutritional supplements was advised in 57 patients (56%) and the use of tube feeding in 12 patients (12%). Twelve patients (12%) were advised to use both enteral dietary supplementations and tube feeding and 19 patients (19%) were not advised any dietary supplementation.
Deterioration in Nutritional Status During CRT
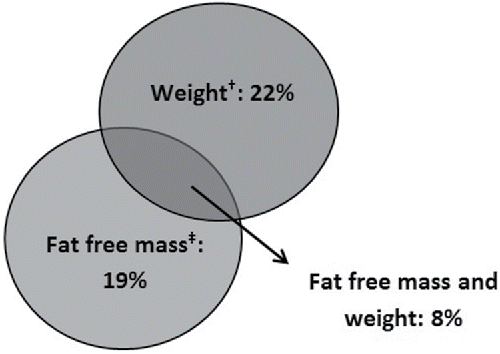
Forty-nine patients (49%) showed deterioration in nutritional status during CRT, of which 22 patients (22%) declined in weight > 5%, 19 patients (19%) declined in FFM ≥ 1.4 kg and 8 patients (8%) declined both in weight and FFM (). Malnutrition (Citation18) prevalence rates increased from 8% (pre CRT) to 17% (post CRT).
Figure 1. Percentage of patients with esophageal cancer and critical deterioration in nutritional status during neoadjuvant chemoradiation therapy (n=49).
†Loss of >5% in weight (Citation10)
‡Loss of 1.4 or more kg in fat free mass (Citation13)
No statistically significant differences were observed in the number of dietetic consultations (6.3 consultations versus 6.6 consultations; p = 0.52) and the use of (additional) oral nutritional supplements and/or tube feeding between patients with and without deterioration in nutritional status.
Predictors of Deterioration in Nutritional Status During CRT
In the univariable logistic regression analyses, FFMI and gender were associated with a deterioration of nutritional status and subsequently included in the multivariable analysis. After the backward selection procedure, only FFMI remained as an independent statistical significant predictor for deterioration in nutritional status (OR 1.21 (90% CI: 1.03 – 1.42)) (). The prediction equation was defined as: −3.522 + 0.188 * FFMI.
Table 3. Univariate logistic regression analyses for the prediction of deterioration in nutritional status during neoadjuvant chemoradiation therapy in patients with esophageal cancer.
Discussion
The aim of the study was to explore whether parameters of pre-treatment nutritional status, next to weight (loss) and BMI, may predict deterioration in nutritional status during CRT in patients with esophageal cancer. The results of this study showed that approximately half of the included patients deteriorated in nutritional status during CRT. The only parameter predicting deterioration was FFMI, suggesting that patients with a higher FFMI before start of CRT have a higher chance to deteriorate during CRT.
Baseline Nutritional Status
Compared to previous studies a relatively small number of patients had a poor nutritional status before CRT(6–10). Previous studies used different definitions of a poor nutritional status and therefore comparison between studies is difficult. The recently proposed ESPEN consensus definition of malnutrition requires a combination of weight loss and low BMI or FFMI to be defined malnourished which may be one explanation as to why the prevalence of malnutrition was relatively low. However, a recent published study found low prevalence rates as well, with 16% of patients at risk of malnutrition (Citation12). Perhaps, the nutritional profile of patients with esophageal cancer in diagnostic phase is possibly changing, as a result of an increasing incidence of overweight and obesity (Citation1).
The prevalence of critical pre-treatment weight loss >5% in the earlier studies was 40 – 70% (Citation6,Citation9,Citation10), which is in line with the data in the current study. Although almost half of the patients had declined in weight before the start of CRT, the median BMI was still relatively high (24.6 kg/m2) and corresponding with the range of BMIs reported in previous studies (Citation9,Citation10,Citation16). In addition, one in four to five patients in the current study had a FFMI and HGS below the 10th percentile of reference values. This illustrates that, despite high body weights, the nutritional status of these patients may be compromised and further deterioration should be prevented.
Deterioration in Nutritional Status
Previous studies showed a decline in weight in 40–45% of the patients during CRT(6, 11, 12). In the current study, 30% of the patients declined in weight. We assume that the intensive protocolled dietary care led to fewer patients with weight loss during treatment. A new finding of this study is that 19% of all patients declined in FFM, while preserving weight. This underlines the relevance of measuring FFM. Considering the results of our study, the incidence of deterioration in nutritional status reported in previous studies (Citation6,Citation11,Citation12), based on weight loss only, might be higher when FFM was included. In addition, previous studies (Citation11,Citation12,Citation25,Citation26) did not investigate FFMI as potential predictive parameter.
Possible Explanations for Deterioration in Nutritional Status
We can only hypothesize which factors may be responsible for the loss of weight and FFM in our study. A first explanation may be cancer cachexia. Cancer cachexia is characterized by inflammation and catabolism and is known to impact on FFM/weight and nutritional status (Citation27). Reversing loss of muscle mass is difficult in patients with cancer cachexia as long as the tumor activity is still present. However, recent studies suggest that it is possible to improve FFM in patients with progressive cancer by specific (nutritional) interventions (Citation28,Citation29).
In addition, it could be hypothesized that the patients with the highest FFM were the patients with the highest physical activity levels before CRT. Due to side effects of CRT, patients may have decreased their physical activity levels, leading to an accelerated loss of FFM. Indeed, a decrease in physical activity is frequently reported in patients receiving chemotherapy and/or radiation therapy (Citation30,Citation31). Unfortunately, physical activity was not measured in the present study.
A final explanation for the decline in FFM/weight could be that patients with more weight and/or higher FFM have higher nutritional needs to maintain their weight/FFM (Citation32,Citation33). A previous study has also reported that meeting nutritional requirements is more difficult for patients with higher bodyweight (Citation34).
Strengths and Limitations
Strength of this study is the homogeneous group of patients with esophageal cancer. Another strength is the complete nutritional assessment which was performed in all patients. A major limitation is the large number of excluded patients. However, no relevant differences were found between included and excluded patients. A second point of criticism may be the data collection. Data were collected as part of routine patient care, which may have had consequences for the quality of the collected data. Nevertheless, measurements like HGS or BIA measurements were performed according to standardized protocols and are thus thought to be subject to small errors. In addition, multiple sets of scales were used. However, each patient was weighed on the same scale for every measurement.
Our study included only 101 patients. However, this is one of the first studies based on data of a complete assessment of the nutritional status. Repetition in larger patient samples is necessary to confirm the findings. Nevertheless, these results contribute to knowledge in a hypothesis-generating way.
Finally it would be of interest to know whether the changes in FFM were associated with poorer clinical outcomes such as postoperative morbidity and mortality. This will be addressed in a future study.
In conclusion, this study shows that one out of two patients with esophageal cancer deteriorated in nutritional status during neo-adjuvant CRT. Patients with a higher FFMI, i.e., a better nutritional status, were at higher risk for deterioration. Therefore, our recommendation is to carefully supervise all patients receiving CRT, both well-nourished and malnourished. Perhaps, intensive nutritional counseling in combination with a physical activity program may prevent deterioration in nutritional status during therapy, and herewith reduce the risk of postoperative complications. Whether a multimodal intervention is feasible and effective needs further exploration.
Acknowledgments
This study was performed as a master thesis. Marian A.E. de van der Schueren, Jill E. Witvliet – van Nierop and Karen Ottens – Oussoren designed the study. Jill E. Witvliet – van Nierop, Gerdien C. Ligthart-Melis and Sofia C.M. Rietveld performed data collection. Sofia C.M. Rietveld performed the data analyses and wrote the manuscript as part of her internship. Marian A.E. de van der Schueren, Jill E. Witvliet – van Nierop, Karen Ottens – Oussoren and Donald L. van der Peet reviewed the manuscript.
The authors thank the registration team of the Netherlands Comprehensive Cancer Organisation (IKNL) for the collection of data for the Netherlands Cancer Registry as well as IKNL staff for scientific advice. We also would like to thank Gerdien C. Ligthart – Melis, Patty L.M. Lakenman and Nel de Vries for their contribution in data collection and data management. Finally we would like to thank Jos W. Twisk and Selmar K. Smit for their help and support in the statistical part of this study.
Funding
This study was not funded.
References
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, et al.: Global cancer statistics, 2012. CA Cancer J Clin 65, 87–108, 2015. doi:10.3322/caac.21262.
- Duan X-F, Tang P, and Yu Z-T: Neoadjuvant chemoradiotherapy for resectable esophageal cancer: an in-depth study of randomized controlled trials and literature review. Cancer Biol Med 11, 191–201, 2014.
- Heijl M, Lanschot J, Koppert L, Henegouwen MB, Muller K, et al.: Neoadjuvant chemoradiation followed by surgery versus surgery alone for patients with adenocarcinoma or squamous cell carcinoma of the esophagus (CROSS). BMC Surg 8, 1, 2008.
- Mariette C, De Botton M-L, and Piessen G: Surgery in esophageal and gastric cancer patients: what is the role for nutrition support in your daily practice? Ann Surg Oncol 19, 2128–2134, 2012. doi:10.1245/s10434-012-2225-6.
- Van Cutsem E, and Arends J: The causes and consequences of cancer-associated malnutrition. Eur J Oncol Nurs 9, S51–S63, 2005. doi:10.1016/j.ejon.2005.09.007.
- Adenis A, Tresch E, Dewas S, Romano O, Messager M, et al.: Clinical complete responders to definite chemoradiation or radiation therapy for esophageal cancer: predictors of outcome. BMC Cancer 13, 1, 2013. doi:10.1186/1471-2407-13-413.
- Andreyev H, Norman A, Oates J, and Cunningham D: Why do patients with weight loss have a worse outcome when undergoing chemotherapy for gastrointestinal malignancies? Eur J Cancer 34, 503–509, 1998. doi:10.1016/S0959-8049(97)10090-9.
- Bozzetti F: Nutritional support in patients with esophageal cancer. Support Care Cancer 18, 41–50, 2010. doi:10.1007/s00520-009-0664-9.
- Clavier JB, Antoni D, Atlani D, Ben Abdelghani M, Schumacher C, et al.: Baseline nutritional status is prognostic factor after definitive radiochemotherapy for esophageal cancer. Dis Esophagus 27, 560–567, 2014. doi:10.1111/j.1442-2050.2012.01441.x.
- Zemanova M, Novak F, Vitek P, Pazdro A, Smejkal M, et al.: Outcomes of patients with esophageal cancer treated with preoperative chemoradiotherapy, followed by tumor resection: influence of nutritional factors. J BUON 17, 310–316, 2012.
- Jiang N, Zhao J-Z, Chen X-C, Li L-Y, Zhang L-J, et al.: Clinical determinants of weight loss in patients with esophageal carcinoma during radiotherapy: a prospective longitudinal view. Asian Pac J Cancer Prev 15, 1943–1948, 2013. doi:10.7314/APJCP.2014.15.5.1943.
- Cox S, Powell C, Carter B, Hurt C, Mukherjee S, et al.: Role of nutritional status and intervention in esophageal cancer treated with definitive chemoradiotherapy: outcomes from SCOPE1. Br J Cancer 115, 172–177, 2016. doi:10.1038/bjc.2016.129.
- Ida S, Watanabe M, Karashima R, Imamura Y, Ishimoto T, et al.: Changes in body composition secondary to neoadjuvant chemotherapy for advanced esophageal cancer are related to the occurrence of postoperative complications after esophagectomy. Ann Surg Oncol 21, 3675–3679, 2014. doi:10.1245/s10434-014-3737-z.
- Lee JLC, Leong LP, Lim SL: Nutrition intervention approaches to reduce malnutrition in oncology patients: a systematic review. Support Care Cancer 24, 469–480, 2016. doi:10.1007/s00520-015-2958-4.
- Clavier JB, Antoni D, Atlani D, Ben Abdelghani M, Schumacher C, et al.: Baseline nutritional status is prognostic factor after definitive radiochemotherapy for esophageal cancer. Dis Esophagus 27, 560–567, 2014. doi:10.1111/j.1442-2050.2012.01441.x.
- Tamandl D, Paireder M, Asari R, Baltzer PA, Schoppmann SF, et al.: Markers of sarcopenia quantified by computed tomography predict adverse long-term outcome in patients with resected esophageal or gastro-esophageal junction cancer. Eur Radiol 26(5), 1–9, 2015.
- Gibson RS. Principles of Nutritional Assessment. USA: Oxford University Press, 2005.
- Cederholm T, Bosaeus I, Barazzoni R, Bauer J, Van Gossum A, et al.: Diagnostic criteria for malnutrition–an ESPEN consensus statement. Clin Nutr 34, 335–340, 2015. doi:10.1016/j.clnu.2015.03.001.
- Daabiss M: American society of anaesthesiologists physical status classification. Indian J Anaesth 55, 111, 2011. doi:10.4103/0019-5049.79879.
- Rice TW, Blackstone EH, and Rusch VW: of the AJCC Cancer Staging Manual: esophagus and esophagogastric junction. Ann Surg Oncol 17, 1721–1724, 2010. doi:10.1245/s10434-010-1024-1.
- Kyle UG, Genton L, Karsegard L, Slosman DO, Pichard C: Single prediction equation for bioelectrical impedance analysis in adults aged 20–94 years. Nutrition 17, 248–253, 2001. doi:10.1016/S0899-9007(00)00553-0.
- Norman K, Stobäus N, Zocher D, Bosy-Westphal A, Szramek A, et al.: Cutoff percentiles of bioelectrical phase angle predict functionality, quality of life, and mortality in patients with cancer. Am J Clin Nutr 92, 612–619, 2010. doi:10.3945/ajcn.2010.29215.
- Bohannon RW, Peolsson A, Massy-Westropp N, Desrosiers J, and Bear-Lehman J: Reference values for adult grip strength measured with a Jamar dynamometer: a descriptive meta-analysis. Physiotherapy 92, 11–15, 2006. doi:10.1016/j.physio.2005.05.003.
- FAO. Energy and Protein Requirements: Report of a Joint FAO/WHO/UNU Expert Consultation. World Health Organization, 1985.
- Buskermolen S, Langius JA, Kruizenga HM, Ligthart-Melis GC, Heymans MW, et al.: Weight loss of 5% or more predicts loss of fat-free mass during palliative chemotherapy in patients with advanced cancer: a pilot study. Nutr Cancer 64, 826–832, 2012. doi:10.1080/01635581.2012.690062.
- Halpern-Silveira D, Susin LRO, Borges LR, Paiva SI, Assunção MCF, et al.: Body weight and fat-free mass changes in a cohort of patients receiving chemotherapy. Support Care Cancer 18, 617–625, 2010. doi:10.1007/s00520-009-0703-6.
- Mendes MCS, Pimentel GD, Costa FO, and Carvalheira JB: Molecular and neuroendocrine mechanisms of cancer cachexia. J Endocrinol 226, R29–R43, 2015. doi:10.1530/JOE-15-0170.
- Engelen M, Safar A, Bartter T, Koeman F, and Deutz N: High anabolic potential of essential amino acid mixtures in advanced non-small cell lung cancer. Ann Oncol 26(9), mdv271, 2015.
- Prado CM, Sawyer MB, Ghosh S, Lieffers JR, Esfandiari N, et al.: Central tenet of cancer cachexia therapy: do patients with advanced cancer have exploitable anabolic potential? Am J Clin Nutr 98, 1012–1019, 2013. doi:10.3945/ajcn.113.060228.
- Al-Majid S, and McCarthy DO: Cancer-induced fatigue and skeletal muscle wasting: the role of exercise. Biol Res Nurs 2, 186–197, 2001. doi:10.1177/109980040100200304.
- Ahlberg K, Ekman T, Gaston-Johansson F, and Mock V: Assessment and management of cancer-related fatigue in adults. Lancet 362, 640–650, 2003. doi:10.1016/S0140-6736(03)14186-4.
- Frankenfield DC, and Ashcraft CM: Estimating energy needs in nutrition support patients. JPEN J Parenter Enteral Nutr 35, 563–570, 2011. doi:10.1177/0148607111415859.
- Weijs PJ, Cynober L, DeLegge M, Kreymann G, Wernerman J, et al.: Proteins and amino acids are fundamental to optimal nutrition support in critically ill patients. Crit Care 18, 1–13, 2014. doi:10.1186/s13054-014-0591-0.
- Leistra E, Willeboordse F, Visser M, Weijs PJ, Haans-van den Oord A, et al.: Predictors for achieving protein and energy requirements in undernourished hospital patients. Clin Nutr 30, 484–489, 2011. doi:10.1016/j.clnu.2011.01.008.
