Abstract
Cancer treatments, toxicities and their effects on lifestyle, may impact levels of vitamin D. The aim of this study was to determine serum 25-hydroxyvitamin D3 (25(OH)D3) levels before, directly after and 6 months after chemotherapy in breast cancer patients (n = 95), and a comparison group of women (n = 52) not diagnosed with cancer. Changes in 25(OH)D3 levels over time were compared using linear mixed models adjusted for age and season of blood sampling. Before start of chemotherapy, 25(OH)D3 levels were lower in patients (estimated marginal mean 55.8 nmol/L, 95% confidence interval (95%CI) 51.2–60.4) compared to the comparison group (67.2 nmol/L, 95%CI 61.1–73.3, P = 0.003). Directly after chemotherapy, 25(OH)D3 levels were slightly decreased (–5.1 nmol/L, 95%CI –10.7–0.5, P = 0.082), but ended up higher 6 months after chemotherapy (10.9 nmol/L, 95%CI 5.5–16.4, P < 0.001) compared to pre-chemotherapy values. In women without cancer, 25(OH)D3 levels remained stable throughout the study. Use of dietary supplements did not explain recovery of 25(OH)D3 levels after chemotherapy. We reported lower 25(OH)D3 levels in breast cancer patients, which decreased during chemotherapy, but recovered to levels observed in women without cancer within 6 months after chemotherapy. Suboptimal 25(OH)D3 levels in the majority of the participants highlight the relevance of monitoring in this vulnerable population.
Introduction
Vitamin D, together with calcium, plays a critical role in the regulation of bone health (Citation1,Citation2). Hence, suboptimal levels of vitamin D are associated with low bone mineral density, osteoporosis and bone fractures, which are commonly observed in breast cancer patients (Citation3–6). Moreover, emerging evidence suggests that low levels of circulating vitamin D at the time of breast cancer diagnosis are associated with poor clinical outcomes, such as an increased risk of cancer recurrence and mortality (Citation7–11). These findings highlight the relevance of a sufficient vitamin D status in women with breast cancer.
Circulating levels of 25-hydroxyvitamin D (25OHD) are considered a reliable biomarker for vitamin D status (Citation12). Although consensus about an optimal vitamin D status has not been reached yet, vitamin D deficiencies and insufficiencies are commonly defined as plasma or serum levels <50 nmol/L (or <20 ng/mL) and 50–75 nmol/L (20–30 ng/mL), respectively, whereas levels of ≥75 nmol/L (or ≥30 ng/mL) are considered sufficient (Citation13). Epidemiological and clinical studies demonstrated that up to 96% of newly diagnosed breast cancer patients had a vitamin D deficiency (Citation10,Citation14–21). Most studies focusing on clinical outcomes in breast cancer patients used a single measurement of 25OHD at or around cancer diagnosis. It has, however, been hypothesized that common systemic cancer treatments may interfere with 25OHD levels, possibly through altered activities of drug-metabolizing enzymes, therapy-induced toxicities, avoidance of sunlight and other personal or clinical factors (Citation22). Circulating levels of 25OHD have been shown to decline after chemotherapy in breast cancer patients in some (Citation17,Citation22–24), but not all studies (Citation25). To what extent these effects are persistent over time is still unclear. Kim et al. demonstrated that 25OHD levels first declined, but recovered after the end of chemotherapy in women with breast cancer (Citation24), whereas another study showed that 25OHD levels were still lower 8 months after chemotherapy as compared to pretreatment values (Citation23). How potential therapy-related changes in 25OHD levels in breast cancer patients relate to fluctuations of 25OHD levels in women without cancer remains unclear.
The aim of this study was to determine changes in vitamin D levels by measuring serum 25-hydroxyvitamin D3 25(OH)D3 levels before, directly after, and 6 months after chemotherapy in patients with stage I–III breast cancer, and within a similar time frame in a comparison group of women without cancer.
Subjects and Methods
Study Participants
This study was conducted as part of the COBRA study, which is an observational multicenter study among breast cancer patients receiving chemotherapy and a comparison group of women not diagnosed with cancer. Design of the study and recruitment of the participants have been previously described in detail (Citation26). Briefly, 181 eligible patients with incident stage I–IIIB breast cancer were recruited between May 2013 and November 2016 from 12 academic and peripheral hospitals in the Netherlands prior to start of neoadjuvant or adjuvant chemotherapy. The comparison group consisted of 180 women who had a similar age (± 2 years) and who were recruited via the breast cancer patients. All participants needed to be at least 18 years old and be able to communicate in Dutch. Exclusion criteria were: a history of cancer except basal cell carcinoma, previous treatment with chemotherapy, being pregnant or the intention to get pregnant during the study period, dementia or other mental conditions that made it impossible to comply with the study procedures. This study was approved by the Medical Ethical Committee of Wageningen University & Research (Protocol no. NL40666.081.12) and all participants provided written informed consent before enrollment. For the current study, circulating 25(OH)D3 levels were analyzed in all participants who completed the study by the end of March 2016 (i.e., recruited up to April 2015) and who had blood samples available, resulting in a total of 441 samples from 147 participants (n = 95 patients and n = 52 women from the comparison group). Recruitment of the larger cohort continued after March 2016 (for other research objectives) and therefore a proportion of the original cohort has not been considered.
Blood Sampling and Vitamin D Measurements
Sample collection took place at three moments during the study period, namely before start of chemotherapy (T1), 1–3 weeks after the last cycle of chemotherapy (T2), and 6 months after chemotherapy (T3). For women in the comparison group, blood samples were collected at similar time points namely at baseline (T1), 6 months after baseline (T2), and 12 months after baseline (T3). Nonfasted blood samples were collected in 8.5-mL serum tubes (Becton Dickinson B.V.). Serum was collected after centrifugation and stored at –80 °C until analyses. All samples were shipped at the same time and analyzed in the same laboratory (department of Clinical Chemistry, Canisius Wilhelmina Hospital, Nijmegen, the Netherlands). Levels of 25(OH)D3 were measured using isotope-dilution liquid chromatography (LC) tandem-mass spectrometry (MS/MS) (Citation27). The interassay coefficients of variation were as follows: 5.3%, 3.1% and 2.9% at 25(OH)D3 concentrations of 39.0, 92.5 and 127.0 nmol/L, respectively. The lower limit of quantification (LLOQ) was 1 nmol/L. No samples showed 25(OH)D3 levels below the LLOQ. The current method was also able to quantify levels of 25(OH)D2; however, since 25(OH)D2 levels were below the LLOQ (2 nmol/L) for all except one patients, these values were not considered for the current analyses. The vitamin D status was defined as deficient (<50 nmol/L), insufficient (50–75 nmol/L) or sufficient (≥75 nmol/L) (Citation13).
Data Collection
Demographic and general characteristics, including age, height, smoking status (current, former, never), educational level and menopausal status (pre- or post-menopausal), were collected from a general questionnaire provided at baseline (T1). Educational level was defined as low (primary school and lower vocational education), medium (vocational education), or high (higher professional education and university). The validated Short QUestionnaire to ASsess Health enhancing physical activity (SQUASH) was used to calculate adherence to the Dutch physical activity guideline (i.e., at least 30 min moderate intense physical activity for a minimum of 5 days per week) as described previously (Citation28,Citation29). Body weight was determined by use of a dual-energy X-ray absorptiometry (DEXA)-scanner in the hospitals at baseline (T1). Body mass index (BMI) was calculated as body weight divided by squared height (kg/m2). Use of dietary supplements during the previous month was reported as part of a food frequency questionnaire provided at baseline (T1) and 6 months after chemotherapy (T3). Specific dietary supplements containing vitamin D as well as multivitamins were considered with regard to supplemental vitamin D intake. Most multivitamins available in the Netherlands contain vitamin D. The consistency of dietary supplement use was determined based on intake at baseline (T1) and 6 months after the end of chemotherapy (patient group) or 12 months after baseline (comparison group) (T3). Participants were categorized as consistent users, consistent nonusers, participants who started using supplements (‘starters’) and participants who stopped using supplements (‘stoppers’) during the study. In case of missing data on supplement use at one of the indicated time points, consistency of supplement use was classified as unknown. Information on clinical characteristics, including adjuvant or neoadjuvant chemotherapy and cancer stage, was collected from the medical records.
Statistical Analysis
Population characteristics were presented as mean ± standard deviation (SD) or numbers and percentages. Normality of the data was checked by visual inspection of the QQ-plots. In case of data not following a normal distribution, median and interquartile ranges (IQR) were presented. Changes in 25(OH)D3 levels over time were compared for patients and the comparison group using a linear mixed model analysis with three time points (T1–T3). Serum 25(OH)D3 levels were considered as dependent variable in these analyses. Time, group and their interaction (time*group) were included as fixed factors and subjects were defined as random factors in the model. A random intercept was included to take into account the individual variation in levels between participants and the correlation between observations in the same participant. The variance of components (VC) was used as covariance structure. The final model was adjusted for age at time of blood withdrawal (continuous) and season of each individual blood withdrawal (spring, summer, autumn, winter) as potential confounders, which were both included as fixed factors. Results of the mixed model analyses were presented as estimated marginal means and 95% confidence intervals (95% CI), which were compared in a post-hoc analysis with Sidak correction for multiple comparisons (Citation30). Explorative stratified analyses for timing of chemotherapy (neoadjuvant versus adjuvant) and menopausal status (pre-menopausal versus post-menopausal) were conducted. Moreover, a sensitivity analysis excluding the inconsistent users, i.e., those who reported changes in dietary supplement containing vitamin D or with incomplete data on supplement use, was performed. IBM SPSS Statistics version 23 was used for all analyses and the values of P < 0.05 were considered statistically significant.
Results
Data on circulating 25(OH)D3 levels at all time points were available for 95 breast cancer patients undergoing chemotherapy and 52 women without cancer (). Age of the participants was on average 52.0 (SD 8.8, range 25–69) and 53.4 (SD 8.9, range 31–71) years (women with and without breast cancer, respectively). Menopausal status was comparable for the two groups. Women with breast cancer tended to smoke more often (19%) at the time of assessment as compared to the women without cancer (8%). At baseline, the majority of the participants adhered to the guideline for physical activity (60–63% at T1). Six months after chemotherapy, a decline in adherence to this guideline was observed for the patients with breast cancer (48% versus 63% for women without cancer) ().
Table 1. Description of the study population.
The majority of the patients (n = 62, 65%) received chemotherapy in the adjuvant setting. A regimen with docetaxel, doxorubicin and cyclophosphamide (TAC) or with 5-fluorouracil, epirubicin, cyclophosphamide and docetaxel (FEC/DOC) was most commonly prescribed (37% and 33%, respectively). The median time between breast cancer diagnosis and start of chemotherapy was 25 days (IQR 19–37) for patients receiving neoadjuvant chemotherapy and 91 days (IQR 71–111) for patients receiving adjuvant chemotherapy. The participants of the current study were representative for the total study population of the COBRA study with regard to the studied variables (n = 181 patients and n = 180 women without cancer, data not shown).
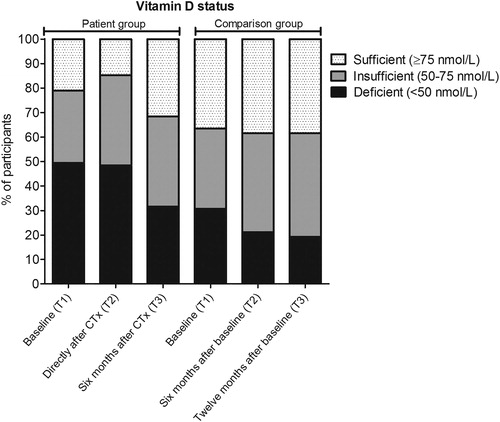
In this study, 79% of the women with breast cancer showed an insufficient vitamin D status (<75 nmol/L) before the start of chemotherapy and 50% of the women even showed a vitamin D deficiency (<50 nmol/L). In the comparison group, insufficient and deficient levels of 25(OH)D3 at start of the study were found in 63% and 31% of the women, respectively (). It should be noted, however, that these descriptive data did not take the season of blood sampling into account, which slightly differed between the groups. Therefore, we continued with linear mixed model analyses to confirm these findings while considering season of blood sampling as well as age at time of blood withdrawal.
Figure 1. Percentage of participants with a sufficient, insufficient or deficient vitamin D status. Vitamin D status in breast cancer patients (n = 95) and women without cancer (n = 52) according to the time of sample collection. Baseline samples were collected before start of chemotherapy (CTx) for patients with breast cancer.
The estimated marginal means (95% CI) retrieved from the linear mixed model analysis are presented in . A statistically significant interaction for time and participant group was found (P = 0.001). Post-hoc analyses demonstrated that before start of chemotherapy, 25(OH)D3 levels were lower in the patients with breast cancer (55.8, 95% CI 51.2–60.4 nmol/L) as compared to the comparison group (67.2, 95% CI 61.1–73.3 nmol/L) (P = 0.003). Also directly after chemotherapy, patients had lower 25(OH)D3 levels (50.7, 95% CI 46.1–55.2 nmol/L) as compared to women without cancer (68.7, 95% CI 62.6–74.9 nmol/L) (P < 0.001), whereas 6 months after chemotherapy these differences between the groups disappeared (66.7, 95% CI 62.1–71.2 and 69.8, 95% CI 63.6–76.0 nmol/L, respectively, P = 0.421) (). Directly after chemotherapy, 25(OH)D3 levels were on average 5.1 (95% CI –10.7–0.5) nmol/L lower as compared to pre-chemotherapy values (T2 versus T1, P = 0.082) in the patients. In this group, 25(OH)D3 levels increased again 6 months after chemotherapy (T3 versus T2 = 16.0, 95% CI 10.4–21.7 nmol/L, P < 0.001) and ended up higher as compared to pre-chemotherapy values (T3 versus T1 = 10.9, 95% CI 5.5–16.4 nmol/L, P < 0.001), whereas 25(OH)D3 levels of the women without cancer did not statistically significantly change over time (). Stratified analyses for patients receiving neoadjuvant versus adjuvant chemotherapy or for pre-menopausal versus post-menopausal participants showed similar findings ().
Figure 2. Changes in circulating 25(OH)D3 levels. Estimated marginal means (95% confidence intervals) of the circulating 25(OH)D3 levels throughout the course of chemotherapy (CTx) in patients with breast cancer and at comparable time points for women from the comparison group based on a linear mixed model analysis. The analyses were adjusted for age and season of each blood withdrawal. aStatistically significant interaction for time*group in the linear mixed model analysis. *Indicates a statistically significant difference (Sidak-adjusted P < 0.05) for the comparison between patients and women without cancer identified through a pairwise post-hoc analysis. **Indicates a statistically significant change over time in the patients with breast cancer (Sidak-adjusted P < 0.05). A] All patients with breast cancer (n = 95) and women without cancer (n = 52), B] Patients receiving adjuvant chemotherapy (n = 62) and women without cancer (n = 52), C] Patients receiving neoadjuvant chemotherapy (n = 33) and women without cancer (n = 52), D] Pre-menopausal patients (n = 53) and women without cancer (n = 29), E] Post-menopausal patients (n = 40) and women without cancer (n = 22).
![Figure 2. Changes in circulating 25(OH)D3 levels. Estimated marginal means (95% confidence intervals) of the circulating 25(OH)D3 levels throughout the course of chemotherapy (CTx) in patients with breast cancer and at comparable time points for women from the comparison group based on a linear mixed model analysis. The analyses were adjusted for age and season of each blood withdrawal. aStatistically significant interaction for time*group in the linear mixed model analysis. *Indicates a statistically significant difference (Sidak-adjusted P < 0.05) for the comparison between patients and women without cancer identified through a pairwise post-hoc analysis. **Indicates a statistically significant change over time in the patients with breast cancer (Sidak-adjusted P < 0.05). A] All patients with breast cancer (n = 95) and women without cancer (n = 52), B] Patients receiving adjuvant chemotherapy (n = 62) and women without cancer (n = 52), C] Patients receiving neoadjuvant chemotherapy (n = 33) and women without cancer (n = 52), D] Pre-menopausal patients (n = 53) and women without cancer (n = 29), E] Post-menopausal patients (n = 40) and women without cancer (n = 22).](/cms/asset/3ae1c0fd-4b4c-4833-a470-5ee1a15d7e48/hnuc_a_1559938_f0002_b.jpg)
Table 2. Changes in 25OHD levels over time.
Before start of chemotherapy (T1), 32% (n = 30) of the patients and 31% (n = 16) of the women without cancer reported use of dietary supplements containing vitamin D in the past month. Six months after chemotherapy (T3), this percentage increased to 47% (n = 45) of the patients and 29% (n = 15) of the women without cancer. Consistent vitamin D supplement use was reported in 23% (n = 22) of the patients and 23% (n = 12) of the women without cancer (). Throughout the study, 21% (n = 20) of the patients reported that they started taking dietary supplements with vitamin D, whereas 6% (n = 6) patients stopped using supplements with vitamin D. For the group of women without cancer, 6% (n = 3) started and 6% (n = 3) stopped use of dietary supplements with vitamin D. Given the dynamics of supplement use in this study population, we explored to what extent changes in supplement use may have accounted for the observed changes in 25(OH)D3 levels. A sensitivity analyses excluding participants who reported changes in dietary supplement use (n = 26 breast cancer patients and n = 6 women without cancer) or with unavailable data on supplement use (n = 13 breast cancer patients and n = 6 women without cancer) showed similar findings (). Thus, also for this population of consistent users and nonusers of dietary supplements containing vitamin D, 25(OH)D3 levels declined directly after chemotherapy and increased again 6 months after chemotherapy in the breast cancer patients (n = 56), but remained stable in the women without cancer (n = 40) ().
Figure 3. Use of dietary supplements containing vitamin D. Dietary supplements with vitamin D as well as multivitamins containing vitamin D were considered. Use of these supplements was reported by the participants at start of the study (T1) and 6 months after the end of chemotherapy for patients (i.e. 12 months after start of the study for the comparison group) (T3). Consistency is reflecting use or nonuse of these supplements at the indicated time points, whereas inconsistency refers to changes in dietary supplement use (i.e., starting of stopping use of dietary supplements containing vitamin D). A] Patients with breast cancer (n = 95), B] Women without cancer from the comparison group (n = 52).
![Figure 3. Use of dietary supplements containing vitamin D. Dietary supplements with vitamin D as well as multivitamins containing vitamin D were considered. Use of these supplements was reported by the participants at start of the study (T1) and 6 months after the end of chemotherapy for patients (i.e. 12 months after start of the study for the comparison group) (T3). Consistency is reflecting use or nonuse of these supplements at the indicated time points, whereas inconsistency refers to changes in dietary supplement use (i.e., starting of stopping use of dietary supplements containing vitamin D). A] Patients with breast cancer (n = 95), B] Women without cancer from the comparison group (n = 52).](/cms/asset/26dfe728-b251-4bff-a32f-3b155c9e5b84/hnuc_a_1559938_f0003_b.jpg)
Discussion
The aim of this study was to assess changes in circulating 25(OH)D3 levels throughout the course of chemotherapy in patients with stage I–III breast cancer as compared to a group of women without cancer. We have shown that suboptimal levels of 25(OH)D3 are common among breast cancer patients as well as women without cancer. Importantly, 25(OH)D3 levels before start of chemotherapy were lower in patients with breast cancer as compared to women without cancer and further declined during chemotherapy. The proportion of patients with a sufficient vitamin D status (15%) became alarmingly small directly after chemotherapy. Six months after the end of chemotherapy, 25(OH)D3 levels in breast cancer patients returned to levels observed in the comparison group independent of the season of blood sampling or changes in dietary supplement use. Circulating levels of 25(OH)D3 remained stable throughout the study in women without cancer.
Vitamin D deficiencies are commonly reported in patients with breast cancer (Citation10,Citation14–21). Also in our study, half of the breast cancer patients (50%) had a vitamin D deficiency at start of the study. Various, but not all, studies agreed that higher vitamin D levels were associated with better breast cancer outcomes and survival (Citation7–11). Most of these studies assessed serum 25OHD levels up to one year prior to or shortly after breast cancer diagnosis. It should be noted that various factors, including changes in diet and lifestyle after cancer diagnosis and cancer treatments may impact circulating 25OHD levels. This raises the clinically relevant question whether 25OHD levels are subjective to changes after cancer diagnosis.
Only few studies have examined 25OHD levels throughout the course of chemotherapy in breast cancer patients. A small study among nine women with breast cancer suggested that serum 25OHD levels remained stable during chemotherapy with FEC (Citation25). Others, however, have reported an increased prevalence of vitamin D deficiencies after neoadjuvant chemotherapy among 77 women with locally advanced breast cancer (44% before treatment versus 73% around the last cycle) (Citation17). Similarly, 73 patients with breast cancer from Iran showed declined levels of serum 25(OH)D3 (minus 13%) 8 months after the end of adjuvant chemotherapy as compared to pre-chemotherapy values (Citation23). Charehbili et al. have studied the effects of neoadjuvant chemotherapy, with or without the bisphosphonate zoledronic acid, on 25(OH)D3 levels in 73 stage II–III breast cancer patients with HER2-negative tumors participating in the NEOZOTAC trial (Citation22). For patients receiving neoadjuvant TAC without zoledronic acid and vitamin D/calcium (n = 34), the decline in 25(OH)D3 levels was –16 nmol/L (SD 25.2) (Citation22). It should be noted, however, that these analyses were not adjusted for season of blood sampling. In our study, an adjusted decrease of 5 nmol/L (95% CI –10.7–0.5) was found 1–3 weeks after the last cycle of chemotherapy compared to pre-chemotherapy values (P = 0.082). Hence, our findings are consistent with the results of the NEOZOTAC trial (Citation22), which was conducted among a comparable group of breast cancer patients in the Netherlands. However, the magnitude of the decrease in 25(OH)D3 levels directly after chemotherapy was modest in our study compared to the NEOZOTAC trial. Potential explanations for this discrepancy may be the timing of blood sampling which was conducted before the last cycle of chemotherapy in the NEOZOTAC trial and 1–3 weeks after chemotherapy in our study. Possibly, levels of 25(OH)D3 already started to recover shortly after chemotherapy. Also baseline 25(OH)D3 levels, which were higher for the NEOZOTAC participants as compared to our population (deficiency in 38% and 50% of the patients, respectively), or different chemotherapeutic settings (neoadjuvant TAC and various neoadjuvant or adjuvant regimens, respectively) may explain these findings.
Based on our data, we also had the opportunity to study changes in 25(OH)D3 levels over a prolonged time period and to compare trajectories in 25(OH)D3 levels with those of women without cancer. Interestingly, 6 months after the end of chemotherapy, serum 25(OH)D3 levels returned to values observed in women without cancer. As a consequence, also the prevalence of vitamin D deficiencies among the breast cancer patients decreased from 50% before start of chemotherapy to 32% 6 months after the end of chemotherapy. These results are in agreement with the findings of Kim et al. who demonstrated that serum 25OHD levels in 93 Korean women with breast cancer receiving adjuvant chemotherapy decreased during the first 6 months after diagnosis, but recovered 12 months after diagnosis (Citation24). We have now added further evidence suggesting that serum 25(OH)D3 levels even returned to 25(OH)D3 levels observed in women without cancer. Various mechanisms may explain the recovery of 25(OH)D3 levels 6 months after the end of chemotherapy. Our first thought was that patients, who may become more health conscious during and after cancer treatment (Citation31), could have changed their use of dietary supplements containing vitamin D. Dietary supplements containing vitamin D substantially contribute to 25(OH)D3 levels (Citation32,Citation33). At the start of our study, 32% of the patients (and 31% of the women without cancer) reported use of dietary supplements containing vitamin D in the past month. At the end of the study, the percentage of patients who reported use of dietary supplements containing vitamin D in the previous month increased to 47%, which is in line with previous studies showing that dietary supplement use is common among cancer survivors in general, and breast cancer survivors in particular (Citation34–36). However, our sensitivity analysis for which the participants who changed their use of dietary supplements containing vitamin D were excluded, showed similar results and provided compelling evidence that changes in dietary supplement use were not primarily responsible for the observed increases in serum 25(OH)D3 levels after chemotherapy.
Alternative explanations for the observed recovery of 25(OH)D3 levels may refer to other behavioral changes or biological aspects. Potential diet and lifestyle behaviors that the patients adopted after cancer diagnosis (Citation37,Citation38), such as changed dietary habits, increased physical activity, more outdoor activities and sunlight exposure, may have contributed to increased 25(OH)D3 levels. In the current study, data on sun exposure were not available. Our data on adherence to the national recommendations for physical activity did not provide evidence that women with breast cancer increased their physical activity after the end of chemotherapy, although changes in type of activities or outdoor behaviors cannot be excluded. From a tumor biology point of view, removal of the tumor by surgical resection and chemotherapy may have also resulted in recovery of circulating 25(OH)D3 levels. Previous studies suggested that regulation of the vitamin D pathway may be altered in breast cancer cells (Citation39,Citation40). For example, more stable CYP24 mRNA profiles, responsible for clearing of the active metabolite 1,25(OH)2D, were found in human breast cancer cells as compared to normal human mammary epithelial cells (Citation41). Also (epigenetic) deregulation and altered expression levels of the vitamin D receptor (VDR) gene during breast carcinogenesis have been described (Citation39,Citation40,Citation42,Citation43). Moreover, exogenous 25OHD exposure resulted in local accumulation of 25OHD and the active metabolite 1,25(OH)2D in tumor tissue of a breast cancer mouse model (Citation44). Altogether, these findings may point toward altered metabolism of vitamin D during carcinogenesis and potentially increased demands of breast cancer cells. This hypothesis remains, however, speculative and needs to be confirmed, since the exact role of uptake, storage and metabolism of vitamin D in breast cancer tissue is not fully understood (Citation39) and cannot be addressed in our study. Also alternative mechanisms or personal or clinical factors determining vitamin D levels in patients receiving chemotherapy, need to be explored in future studies.
Potential limitations of our study include the relatively small sample size and our heterogeneous population in terms of tumor characteristics, timing of treatment, and cytotoxic regimens. This can, however, also be considered a strength, since the population of breast cancer patients in the Netherlands is characterized by heterogeneity as well. It should be recognized that patients and women from the comparison group slightly differed with regard to some characteristics (e.g., smoking status, BMI and physical activity). These differences may have potentially impacted comparisons between the groups, but are not likely to affect analyses dedicated to changes in 25(OH)D3 levels within the respective groups. Strengths of our study are the measurements of 25(OH)D3 levels at multiple time points after diagnosis and the assessment of dietary supplement use throughout the course of cancer therapy. Moreover, the consideration of a comparison group of women without cancer can be considered a major strength of the current study.
In conclusion, our results confirmed that vitamin D deficiencies are common among Dutch patients with breast cancer. After the end of chemotherapy, circulating 25(OH)D3 levels recovered and within 6 months even returned to levels observed in women without cancer. However, suboptimal levels of vitamin D were still observed in the majority of the women participating in this study, which may have important clinical implications. This finding underpins the relevance of awareness and potentially careful monitoring of vitamin D levels in this vulnerable population of women approaching menopausal age with an increased risk of declined bone health. In addition, our findings may provide interesting leads for further studies dedicated to the hypothesis that low serum 25(OH)D3 levels are, at least partly, resulting from breast tumor biology.
Acknowledgments
We gratefully acknowledge all COBRA participants. We would like to thank staff of the following hospitals: Ziekenhuis Gelderse Vallei, Maxima Medisch Centrum, Reinier de Graaf Ziekenhuis, Onze Lieve Vrouwen Gasthuis, Amphia Ziekenhuis, Canisius Wilhelmina Ziekenhuis, Radboud Universitair Medisch Centrum, Alexander Monro Ziekenhuis, St. Antonius Ziekenhuis, St. Anna Ziekenhuis and Flevoziekenhuis. We thank Hendriek Boshuizen for her statistical advice and Yfke de Vries, Anja de Kruijf, Celine Kelfkens, Lisette Kamps, and Monique Derks for their assistance during recruitment of the participants and collection of data.
Disclosure Statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Cranney A, Horsley T, O'Donnell S, Weiler H, Puil L, et al.: Effectiveness and safety of vitamin D in relation to bone health. Evid Rep Technol Assess (Full Rep), Report no. 158, 1–235, 2007.
- Chung M, Balk EM, Brendel M, Ip S, Lau J, et al.: Vitamin D and calcium: a systematic review of health outcomes. Evid Rep Technol Assess (Full Rep), Report no. 183, 1–420, 2009.
- Cranney A, Weiler HA, O'Donnell S, and Puil L: Summary of evidence-based review on vitamin D efficacy and safety in relation to bone health. Am J Clin Nutr 88, 513S–519s, 2008.
- Martin-Herranz A and Salinas-Hernandez P: Vitamin D supplementation review and recommendations for women diagnosed with breast or ovary cancer in the context of bone health and cancer prognosis/risk. Crit Rev Oncol Hematol 96,91–99, 2015.
- Abdel-Razeq H and Awidi A: Bone health in breast cancer survivors. J Cancer Res Ther 7,256–263, 2011.
- Chen Z, Maricic M, Bassford TL, Pettinger M, Ritenbaugh C, et al.: Fracture risk among breast cancer survivors: results from the Women's Health Initiative Observational Study. Arch Intern Med 165,552–558, 2005.
- Vrieling A, Seibold P, Johnson TS, Heinz J, Obi N, et al.: Circulating 25-hydroxyvitamin D and postmenopausal breast cancer survival: influence of tumor characteristics and lifestyle factors? Int J Cancer 134,2972–2983, 2014.
- Kim Y and Je Y: Vitamin D intake, blood 25(OH)D levels, and breast cancer risk or mortality: a meta-analysis. Br J Cancer 110,2772–2784, 2014.
- Rose AA, Elser C, Ennis M, and Goodwin PJ: Blood levels of vitamin D and early stage breast cancer prognosis: a systematic review and meta-analysis. Breast Cancer Res Treat 141, 331–339, 2013.
- Yao S, Kwan ML, Ergas IJ, Roh JM, Cheng TD, et al.: Association of serum level of vitamin D at diagnosis with breast cancer survival: a case-cohort analysis in the pathways study. JAMA Oncol 3,351–357, 2017.
- Vaughan-Shaw PG, O'Sullivan F, Farrington SM, Theodoratou E, Campbell H, et al.: The impact of vitamin D pathway genetic variation and circulating 25-hydroxyvitamin D on cancer outcome: systematic review and meta-analysis. Br J Cancer 116,1092–1110, 2017.
- Brouwer-Brolsma EM, Bischoff-Ferrari HA, Bouillon R, Feskens EJ, Gallagher CJ, et al.: Vitamin D: Do we get enough? A discussion between vitamin D experts in order to make a step towards the harmonisation of dietary reference intakes for vitamin D across Europe. Osteoporos Int 24,1567–1577, 2013.
- Holick MF: The vitamin D deficiency pandemic: approaches for diagnosis, treatment and prevention. Rev Endocr Metab Disord 18, 153–165, 2017.
- Aguirre M, Manzano N, Salas Y, Angel M, Díaz-Couselo FA, et al.: Vitamin D deficiency in patients admitted to the general ward with breast, lung, and colorectal cancer in Buenos Aires, Argentina. Arc Osteoporos 11, 1–4, 2016.
- Crew KD, Shane E, Cremers S, McMahon DJ, Irani D, et al.: High prevalence of vitamin D deficiency despite supplementation in premenopausal women with breast cancer undergoing adjuvant chemotherapy. Jco 27, 2151–2156, 2009.
- Hauser K, Walsh D, Shrotriya S, and Karafa M: Low 25-hydroxyvitamin D levels in people with a solid tumor cancer diagnosis: the tip of the iceberg? Support Care Cancer 22, 1931–1939, 2014.
- Jacot W, Pouderoux S, Thezenas S, Chapelle A, Bleuse JP, et al.: Increased prevalence of vitamin D insufficiency in patients with breast cancer after neoadjuvant chemotherapy. Breast Cancer Res Treat 134,709–717, 2012.
- Hatse S, Lambrechts D, Verstuyf A, Smeets A, Brouwers B, et al.: Vitamin D status at breast cancer diagnosis: correlation with tumor characteristics, disease outcome, and genetic determinants of vitamin D insufficiency. Carcinogenesis 33,1319–1326, 2012.
- Janbabai G, Shekarriz R, Hassanzadeh H, Aarabi M, and Borhani SS: A survey on the relationship between serum 25-hydroxy vitamin D level and tumor characteristics in patients with breast cancer. Int J Hematol-Oncol Stem Cell Res 10, 30–36, 2016.
- Yao S, Sucheston LE, Millen AE, Johnson CS, Trump DL, et al.: Pretreatment serum concentrations of 25-hydroxyvitamin D and breast cancer prognostic characteristics: a case-control and a case-series study. PLoS One 6,e17251, 2011.
- Imtiaz S, Siddiqui N, Raza SA, Loya A, and Muhammad A: Vitamin D deficiency in newly diagnosed breast cancer patients. Indian J Endocr Metab 16, 409–413, 2012.
- Charehbili A, Hamdy NA, Smit VT, Kessels L, van Bochove A, et al.: Vitamin D (25-0H D3) status and pathological response to neoadjuvant chemotherapy in stage II/III breast cancer: data from the NEOZOTAC trial (BOOG 10-01). Breast 25, 69–74, 2016.
- Safaei-Nodehi R, Esmaili J, Sharifian R, Movaseghi S, and Parkhideh S: Does adjuvant chemotherapy change bone mineral density and related serum biomarkers in women with breast cancer? Caspian J Intern Med 8, 91–98, 2017.
- Kim HJ, Koh BS, Yu JH, Lee JW, Son BH, et al.: Changes in serum hydroxyvitamin D levels of breast cancer patients during tamoxifen treatment or chemotherapy in premenopausal breast cancer patients. Eur J Cancer 50,1403–1411, 2014.
- Kailajarvi ME, Salminen EK, Paija OM, Virtanent AM, Leino AE, et al.: Serum bone markers in breast cancer patients during 5-fluorouracil, epirubicin and cyclophosphamide (FEC) therapy. Anticancer Res 24, 1271–1274, 2004.
- de Vries YC, van den Berg MM, de Vries JH, Boesveldt S, de Kruif JT, et al.: Differences in dietary intake during chemotherapy in breast cancer patients compared to women without cancer. Support Care Cancer 28, 2581–2591, 2017.
- van den Ouweland JM, Beijers AM, and van Daal H: Overestimation of 25-hydroxyvitamin D3 by increased ionisation efficiency of 3-epi-25-hydroxyvitamin D3 in LC-MS/MS methods not separating both metabolites as determined by an LC-MS/MS method for separate quantification of 25-hydroxyvitamin D3, 3-epi-25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 in human serum. J Chromatogr B Analyt Technol Biomed Life Sci 967, 195–202, 2014.
- de Hollander EL, Zwart L, de Vries SI, and Wendel-Vos W: The SQUASH was a more valid tool than the OBiN for categorizing adults according to the Dutch physical activity and the combined guideline. J Clin Epidemiol 65, 73–81, 2012.
- Wendel-Vos GC, Schuit AJ, Saris WH, and Kromhout D: Reproducibility and relative validity of the short questionnaire to assess health-enhancing physical activity. J Clin Epidemiol 56, 1163–1169, 2003.
- Šidák Z: Rectangular confidence regions for the means of multivariate normal distributions. J Am Statis Assoc 62, 626–633, 1967.
- Demark-Wahnefried W, Peterson B, McBride C, Lipkus I, and Clipp E: Current health behaviors and readiness to pursue life-style changes among men and women diagnosed with early stage prostate and breast carcinomas. Cancer 88, 674–684, 2000.
- Heaney RP, Davies KM, Chen TC, Holick MF, and Barger-Lux MJ: Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr 77, 204–210, 2003.
- Rapuri PB, Gallagher JC, and Haynatzki G: Effect of vitamins D2 and D3 supplement use on serum 25OHD concentration in elderly women in summer and winter. Calcif Tissue Int 74, 150–156, 2004.
- Velicer CM and Ulrich CM: Vitamin and mineral supplement use among US adults after cancer diagnosis: a systematic review. J Clin Oncol 26,665–673, 2008.
- Pouchieu C, Fassier P, Druesne-Pecollo N, Zelek L, Bachmann P, et al.: Dietary supplement use among cancer survivors of the NutriNet-Santé cohort study. Br J Nutr 113, 1319–1329, 2015.
- Velentzis LS, Keshtgar MR, Woodside JV, Leathem AJ, Titcomb A, et al.: Significant changes in dietary intake and supplement use after breast cancer diagnosis in a UK multicentre study. Breast Cancer Res Treat 128, 473–482, 2011.
- Alfano CM, Day JM, Katz ML, Herndon JE, 2nd, Bittoni MA, et al.: Exercise and dietary change after diagnosis and cancer-related symptoms in long-term survivors of breast cancer: CALGB 79804. Psychooncology 18, 128–133, 2009.
- Steinhilper L, Geyer S, and Sperlich S: Health behavior change among breast cancer patients. Int J Public Health 58, 603–613, 2013.
- Welsh J: Function of the vitamin D endocrine system in mammary gland and breast cancer. Mol Cell Endocrinol 453, 88–95, 2017.
- Welsh J: Vitamin D and breast cancer: Past and present. J Ster Biochem Mol Biol 177, 15–20, 2018.
- Matilainen JM, Malinen M, Turunen MM, Carlberg C, and Väisänen S: The number of vitamin D receptor binding sites defines the different vitamin D responsiveness of the CYP24 gene in malignant and normal mammary cells. J Biol Chem 285, 24174–24183, 2010.
- Kemmis CM and Welsh J: Mammary epithelial cell transformation is associated with deregulation of the vitamin D pathway. J Cell Biochem 105,980–988, 2008.
- Marik R, Fackler M, Gabrielson E, Zeiger MA, Sukumar S, et al.: DNA methylation-related vitamin D receptor insensitivity in breast cancer. Cancer Biol Ther 10, 44–53, 2010.
- Rossdeutscher L, Li J, Luco A-L, Fadhil I, Ochietti B, et al.: Chemoprevention activity of 25-hydroxyvitamin D in the MMTV-PyMT mouse model of breast cancer. Cancer Prevent Res 8, 120–128, 2015.
