Abstract
Background: This pilot, double-blind, comparator-controlled trial evaluated the safety and tolerability of an oral targeted medical nutrition (TMN) supplement for the management of cachexia in patients with non-small-cell lung cancer (NSCLC).
Methods: Patients receiving first-line chemotherapy for NSCLC with weight loss or low BMI were randomized 1:1 to receive juice-based TMN (∼200 kcal; 10 g whey protein; ≥2.0 g eicosapentaenoic acid/docosahexaenoic acid in fish oil; and 10 μg 25-hydroxy-vitamin D3) or a milk-based isocaloric comparator twice daily for 12 weeks (ClinicalTrials.gov: NCT02515032). Primary endpoints included number/type of adverse events and changes in vital signs/laboratory parameters. Secondary endpoints included measures of clinical relevance. Survival was an exploratory endpoint.
Results: The TMN group (n = 26; mean 64.4 years) experienced fewer adverse events (64 vs. 87) than the comparator group (n = 29; mean 66.0 years), including fewer cases of neutropenia (0 vs. 4). Compliance was slightly lower in the TMN (58.5%) vs. comparator group (73.6%). There were no statistically significant between-group differences in efficacy endpoints. Fewer (4 vs. 10) patients who received TMN than comparator had died by 1-year post baseline.
Conclusions: TMN was well tolerated. Trends for improved clinical outcomes with TMN identified in this study warrant further investigation.
Introduction
Cachexia is a complex wasting syndrome, known to have a negative impact on clinical outcomes in patients with cancer and several other chronic diseases (Citation1). In patients with cancer, cachexia is defined as “a multifactorial syndrome characterized by an ongoing loss of skeletal muscle mass (with or without loss of fat mass) that cannot be fully reversed by conventional nutritional support, and leads to progressive functional impairment” (Citation2). The spectrum of cancer cachexia ranges from early-stage with minimal weight loss and/or muscle loss, to severe weight loss, muscle wasting, and severely impaired performance status (Citation2).
Both “pre-cachexia” (early-stage weight loss combined with systemic inflammation and anorexia) and overt cachexia are now known to be key determinants of outcomes, including survival, in patients with non-small-cell lung cancer (NSCLC) (Citation3,Citation4) – the most common type of lung cancer (Citation5). Importantly, cachexia has been shown to have a negative impact on response to chemotherapy (Citation3). Although the relationship between cachexia and chemotherapy is complicated by chemotherapy-induced weight loss (Citation6), patients with weight loss before chemotherapy are less likely to complete three treatment cycles and are more likely to suffer from chemotherapy-associated toxicities than those with a stable weight (Citation7). Cachectic patients with NSCLC also report reduced health-related quality of life (HRQoL) and suffer an increased rate of decline in physical function compared with those without cachexia (Citation4,Citation8). Ultimately, cachexia, including that which develops during chemotherapy, may have an impact on survival (Citation3). Because of its negative impact on prognosis, cancer cachexia should be treated comprehensively (Citation9).
Early multimodal therapy, including nutrition that has been specially formulated to treat the characteristic metabolic alterations of cachexia, is thought to be the best approach to treatment of patients with cancer (Citation10,Citation11). Nutritional supplements, including anti-inflammatory omega-3 polyunsaturated fatty acids (n-3 PUFAs), protein, and/or specific amino acids have been shown to have beneficial effects in cachectic patients with NSCLC. In randomized trials, supplementation with n-3 PUFAs has been shown to improve clinical response to chemotherapy (Citation12), as well as chemotherapy tolerability measures, including neutrophil and/or lymphocyte numbers (Citation13). Consumption of n-3 PUFA-containing nutritional supplements is also reportedly associated with improvements in HRQoL, exercise capacity (Citation14,Citation15), body weight, and muscle mass (Citation15) – the latter two being evident even during chemotherapy (Citation16,Citation17).
Protein supplementation is acknowledged to be a crucial determinant for muscle maintenance in cancer, not least because intake of high quality protein is known to be required for optimal skeletal muscle protein synthesis (Citation18). Nutritional supplements containing leucine and n-3 PUFAs have been associated with improvements in the rate of muscle protein synthesis in patients with cancer (Citation19), and supplementation with a cysteine-rich protein has been found to lead to substantial weight gain in patients with NSCLC (Citation20). Deficiency of vitamin D is often observed in patients with cancer (Citation21) and patients receiving chemotherapy may be at increased risk of severe deficiency (Citation22,Citation23). Given that vitamin D deficiency has been associated with poor prognosis for patients with cancer (Citation24), supplementation with vitamin D could be beneficial in selected patients.
This pilot, randomized, double-blind, comparator-controlled trial is the first to evaluate the safety and tolerability of a targeted medical nutrition (TMN) ready to drink supplement containing a combination of n-3 PUFAs, 25-hydroxy-vitamin D3 and high-quality whey protein, compared with an isocaloric comparator matched for energy content, in pre-cachectic and cachectic patients with NSCLC.
Methods
Study Design
This 12-week, randomized, double-blind, parallel-group, comparator-controlled, multicenter trial was designed primarily to assess the safety and tolerability of TMN (Nutrifriend Cachexia; Smartfish, Oslo, Norway) in patients with NSCLC (ClinicalTrials.gov identifier: NCT02515032). The study involved 16 sites across four countries (Croatia, Italy, Slovakia, and Sweden). It was conducted in accordance with good clinical practice and the principles of the Declaration of Helsinki. The study protocol and its amendments were reviewed and approved by an Independent Ethics Committee or Institutional Review Board at each study site. All patients provided written informed consent.
Patients were randomized in a 1:1 ratio to receive TMN or an isocaloric comparator drink. Randomization was implemented by Trial Form Support AB (Lund, Sweden); patients were assigned a three-digit number that allocated them to a treatment group. Patients at each site were assigned to these numbers sequentially. Access to randomization details was restricted (ensuring investigator, patient, and study sponsor blinding) until study end.
The primary objective of the trial was to assess the safety and tolerability of TMN during chemotherapy. Secondary objectives were the evaluation of the efficacy of TMN in improving measures of clinical relevance, including changes in body weight, muscle function, and lipid profiles, and compliance with TMN as an add-on nutritional supplement. Analyses of survival and chemotherapy-related outcomes were also evaluated.
Participants
Patients eligible for inclusion in the study were those initiating first-line standard chemotherapy as treatment for NSCLC; patients were required to initiate their first cycle of platinum-based chemotherapy at the baseline visit. Participants received chemotherapy every 3 weeks thereafter during the 12-week study. All standard of care medications for NSCLC were allowed, and patients continued in the study if chemotherapy was terminated or altered. All participants must have been classified as having weight loss grade 0–3 according to the classification by Martin et al. (based on weight loss/low body mass index [BMI]) (Citation25), and to have experienced less than 11% involuntary weight loss in the 12 months before randomization. Patients must also have had a functional performance status of 2 or less as measured by the Eastern Cooperative Oncology Group scale (Citation26). Patients with a second invasive malignancy, brain metastases, or relapse of NSCLC within the 2 years before randomization were excluded. Full inclusion and exclusion criteria are listed in Supplemental Table 1.
Nutritional Intervention
Patients were randomized at baseline to receive either the juice-based TMN drink (∼200 kcal; 10 g whey protein concentrate; 11 g fat including ≥2.0 g docosahexaenoic acid + eicosapentaenoic acid [∼1,200 mg DHA + ∼800 mg EPA] in fish oil; 20 g carbohydrate and 10 μg 25-hydroxy-vitamin D3 per 200 ml) or a milk-based isocaloric comparator drink (∼200 kcal; 6 g milk protein; 11 g fat including sunflower oil in place of DHA- and EPA-containing fish oil; 20 g carbohydrate and no 25-hydroxy-vitamin D3). Concentrations of components of TMN were chosen based on published literature on n-3 PUFA-containing supplements (Citation14,Citation27–29) and on n-3 PUFA-containing medical nutrition products from Smartfish (Citation30). The isocaloric comparator drink was formulated by Smartfish for the purpose of this study. Patients were instructed to drink two 200 ml packages of study product daily for 12 weeks. The study products were presented in white boxes (identical size and shape) and labeled “A” and “B”, ensuring that the contents of the two test products were not identifiable. Labels in the local language containing storage recommendations were also added, according to local country regulations. Patients were asked not to consume any other products containing n-3 PUFAs or supplements with more than 150% of the recommended dietary allowance of vitamin D during the study. No exercise regimen was specified in order to limit exercise as a potential confounding factor. However, self-reported exercise was recorded throughout the study.
Outcome Measures
Primary outcomes of interest were safety and tolerability endpoints; these comprised number and type of adverse events (AEs) that occurred during the study, as well as changes in vital signs (resting systolic and diastolic blood pressure, heart rate), physical examination results, laboratory safety parameters (hematology, clinical chemistry), and concomitant medications from baseline to week 12. Several clinical measures of efficacy were included as secondary endpoints, including changes in body weight, body composition, fasting plasma levels of triglyceride and cholesterol, fasting plasma levels of inflammatory biomarkers, hand grip strength, and daily walking distance (measured by pedometer) from baseline to week 12. Appetite was evaluated using the Council on Nutrition Appetite Questionnaire (Citation31) and a study-specific palatability questionnaire. Compliance with study product was recorded using a study-specific drink consumption diary. Changes in fasting plasma levels of omega-3, omega-6, and vitamin D3 from baseline to week 12 were also assessed. Overall survival and chemotherapy-related outcomes, including tumor size, response to chemotherapy, and chemotherapy tolerability, were examined as exploratory endpoints. Full details of all study endpoints and how they were measured are provided in Supplemental Table 2.
Patients were assessed for all clinical and laboratory measures specified in the protocol at screening (days −30 to −1), baseline (day 1), and every 3 weeks thereafter (weeks 3–12). Follow-up assessment of treatment outcome in terms of survival was completed at 12 months post-baseline; information was obtained via the patient’s general practitioner or through hospital records for patients who had not withdrawn consent during the 12-week treatment period.
Statistical Analysis
It was determined a priori that 25 evaluable patients in each of the treatment groups would be sufficient to meet the primary objective of this pilot study, based on previous experience with similar studies. Safety analyses were performed for all patients who consumed any amount of study product – this was the safety analysis set. All safety endpoints were summarized using descriptive statistics. Efficacy analyses were first performed on data from all patients who underwent a post-baseline assessment – the full analysis set (FAS). Secondly, planned analyses were performed on data from a group of patients with high (>70%) compliance, who had completed week 12 of the study – the per protocol set (PPS). This threshold for compliance was selected based on a previous study of the same TMN in patients with chronic obstructive pulmonary disease (COPD), which reported 79% compliance (Citation30).
For continuous secondary efficacy endpoints, differences between the two treatment groups from baseline to week 12 were assessed using analysis of covariance (ANCOVA) with baseline value as covariate. Planned analyses were also performed on blood pressure, and post hoc analyses were performed on neutrophil parameters and heart rate using the same method, owing to the known effects of n-3 PUFAs on these parameters (Citation32–34). Between-group differences in exploratory endpoints were assessed by Fisher’s exact test or ANCOVA, as appropriate. No adjustments were made for multiple comparisons due to the exploratory nature of the analysis. All tests were two-sided and statistical significance was set at P ≤ 0.05. Data were analyzed using Statistical Analysis System (v9.3) software. Data are presented as mean change from baseline to week 12, alongside baseline-adjusted P values, unless otherwise specified. Estimated effect sizes are presented as mean (95% confidence interval) for statistically significant differences.
Results
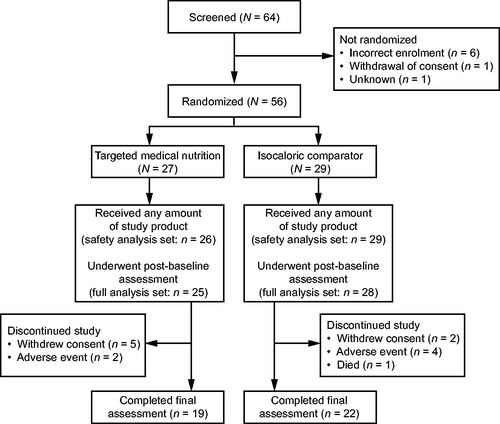
Between 19 October 2015 and 16 March 2017, 64 patients were screened, and 55 were randomized to receive either TMN (n = 26) or an isocaloric comparator (n = 29) (safety analysis set) (). Of these, 53 patients were included in the FAS (TMN, n = 25; comparator, n = 28), and 27 were included in the PPS (TMN, n = 11; comparator, n = 16). Baseline demographics and characteristics including age, sex, BMI, weight loss, and stage of NSCLC were similar between the two treatment groups (). There was a slight difference between groups in the levels of Martin et al. weight loss grade (Citation25), with patients in the comparator group having a more severe grade of weight loss/lower BMI than those in the TMN group. However, the two groups were well balanced when considering cachexia as defined by Fearon et al. (Citation2). Two-thirds of patients received cisplatin- and one-third received carboplatin-based therapies, and the distribution did not differ between treatment groups.
Table 1. Baseline patient demographics and characteristics.
Safety
A smaller number of AEs were reported in the TMN group than in the isocaloric comparator group (64 vs. 87 events, respectively), and AEs occurred in fewer patients in the TMN group than the comparator group (18 vs. 26 patients, respectively). Four patients in the comparator group experienced neutropenia compared with no patients in the TMN group. Two AEs in the TMN group were considered to be related to study product compared with three AEs in the comparator group (). Three patients in the TMN group and nine patients in the comparator group experienced serious AEs (), none of which was considered study product-related. One patient enrolled in the isocaloric comparator group died during the 12-week treatment period owing to NSCLC, but this was not deemed related to study product by the investigator.
Table 2. Related adverse events and serious adverse events experienced by patients in the TMN and isocaloric comparator groups.
Changes from baseline to week 12 in vital signs were slightly different between the two groups (). Heart rate decreased in the TMN group, while it increased in the comparator group. This difference did not reach statistical significance in the FAS (−2.9 vs. +5.8 bpm; P = 0.07) but was significant in the PPS (−11.5 vs. +6.7; P = 0.04; effect size, −9.7 [−18.8; −0.7]). Increases in blood pressure were not significantly different between the groups but were numerically smaller in the TMN group than in the comparator group ().
Table 3. Change from baseline to week 12 in vital signs and laboratory safety parameters in the TMN and isocaloric comparator groups.
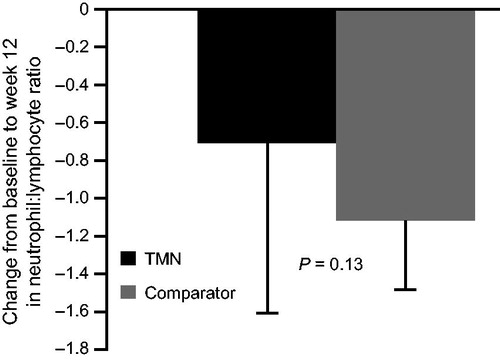
Changes in laboratory safety parameters were generally similar between the groups (). The neutrophil:lymphocyte ratio decreased to a lesser extent in the TMN group than in the comparator group; this difference was not statistically significant when evaluating the FAS or the PPS ().
Figure 2. Change from baseline to week 12 in the TMN group and the isocaloric comparator group in neutrophil:lymphocyte ratio. Data are mean ± SEM for the full analysis set; P value was estimated by analysis of covariance adjusted for baseline value. Mean change from baseline to week 12 was calculated as: week 12 value minus baseline value for each patient, divided by the n number. TMN group, n = 18; comparator group, n = 19. SEM, standard error of the mean; TMN, targeted medical nutrition.
No patient had an abnormal result that was not related to NSCLC during the physical examination (routine assessment of cardiovascular system, respiratory system, abdomen, skin, and nervous system) at week 12. Use of concomitant medications was similar between the groups and did not change over the course of the study.
Secondary Efficacy Outcomes
Patients in the TMN and comparator groups gained body weight to a similar extent over 12 weeks (FAS: +0.8 kg vs. +0.6 kg; NS; PPS: +1.7 kg vs. +0.5 kg; NS) (), and thus the increase in BMI was also similar (FAS: +0.4 kg/m2 vs. +0.2 kg/m2; NS; PPS: +0.7 kg/m2 vs. +0.2 kg/m2; NS). There were no between-group differences in changes in waist and calf circumference, or in appendicular lean body mass (DXA FAS: +173.5 g vs. +87.9 g, NS) or fat mass (DXA FAS: +437.6 g vs. +1,188.8 g, NS), as assessed by dual-energy X-ray absorptiometry and computerized tomography (CT) scan. Changes in muscle area and visceral adipose tissue, as assessed by CT scan, did not differ significantly between the groups.
Table 4. Change from baseline to week 12 in secondary efficacy endpoints in the TMN and isocaloric comparator groups.
A difference in change in triglyceride levels was observed between the TMN and comparator groups (); this was not statistically significant in the FAS analysis (+0.09 mmol/L vs. +0.31 mmol/L; NS), but was significant when considering the PPS (−0.28 mmol/L vs. +0.43 mmol/L; P < 0.01; effect size, 0.63 [0.47; 0.84]). Changes in total cholesterol, and high-density lipoprotein (HDL) and low-density lipoprotein (LDL) cholesterol levels were similar between the two groups (). The ratio of HDL to LDL also remained similar between the two groups, as did fasting blood glucose and serum insulin levels.
Changes in dominant and non-dominant hand grip strength favored the TMN group numerically, but were not statistically significant in analyses of the FAS or the PPS (). Likewise, there were numerical improvements in daily walking distance for the TMN vs. the comparator group (), which did not reach statistical significance. Self-reported exercise was not reported routinely, about two thirds of the patients reported some exercise, mainly walking. The distribution of patients exercising was similar between the groups. No formal statistical analysis has been done on these data.
Changes in inflammatory biomarkers were similar between the groups when considering the FAS and PPS; concentrations of interleukin (IL)-6, IL-8, and C-reactive protein (CRP) decreased in both groups, whereas concentrations of tumor necrosis factor and IL-15 increased (all NS for TMN vs. comparator). Inflammation-based modified Glasgow Prognostic Scores (mGPS) were similar in the two groups and did not seem to be a predictor of mortality in this setting.
Changes in scores on the Council on Nutrition Appetite Questionnaire and palatability questionnaire were minor and did not differ between the groups.
Compliance
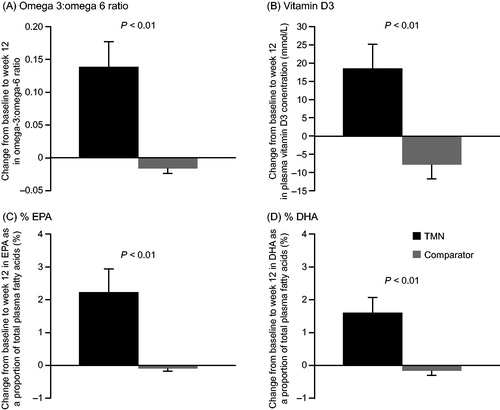
Compliance to study products was reasonably good in both groups but tended to be lower in the TMN group than the comparator group (58.5% vs. 73.6%; P = 0.06). In the FAS, significant increases were seen by week 12 in the TMN group vs. the comparator group in omega-3:omega-6 (O3:O6) ratio (+0.1403 vs. −0.0162; P < 0.01; effect size, 0.15 [0.1; 0.2]), as well as EPA (+2.2% vs. −0.1%; P < 0.01; effect size, 2.3 [1.1; 3.5]) and DHA (+1.6% vs. −0.1%; P < 0.01; effect size, 1.6 [0.8; 2.4]) as a proportion of the total fatty acids, and vitamin D3 levels (+18.6 nmol/L vs. −7.9 nmol/L; P < 0.01; effect size, 25.3 [13.0; 37.7]) (). These differences were also significant in the PPS (O3:O6 ratio: P < 0.01; %EPA: P < 0.01; %DHA: P < 0.01; vitamin D3: P < 0.01).
Figure 3. Change from baseline to week 12 in the TMN group and the isocaloric comparator group in (a) plasma omega-3 to omega-6 ratio, (b) plasma vitamin D3 level, (c) EPA as a proportion of total plasma fatty acids, and (d) DHA as a proportion of total plasma fatty acids. Data are mean ± SEM for the full analysis set; P values were estimated by analysis of covariance adjusted for baseline value. Mean change from baseline to week 12 was calculated as: week 12 value minus baseline value for each patient, divided by the n number. TMN group, n = 16–17; comparator group, n = 22. DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; SEM, standard error of the mean; TMN, targeted medical nutrition.
Exploratory Outcomes
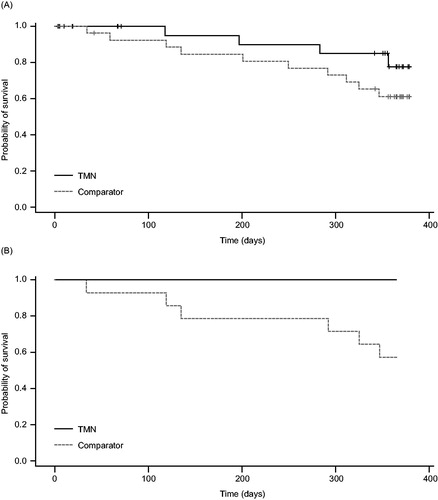
Survival over 12 months from baseline was numerically higher in the TMN group than the comparator group. shows the Kaplan–Meier survival curve; a total of four patients in the TMN group and 10 patients in the comparator group had died by the end of the 12-month follow-up period. This difference was not statistically significant. Post hoc analysis of survival in the subset of patients with pre-cachexia revealed a significant between-group difference; no patients died in the TMN group, compared with six in the comparator group (0 vs. 6 patients; P = 0.02; ).
Figure 4. Kaplan–Meier curves showing survival from baseline to 1 year (a) overall and (b) in the subset of patients with pre-cachexia, in the TMN group and the isocaloric comparator group. a) TMN group, n = 25; comparator group, n = 28; P = 0.18. b) TMN group, n = 14; comparator group, n = 14; P = 0.02. TMN, targeted medical nutrition.
Patients in both groups responded similarly to chemotherapy as measured by several exploratory endpoints. The same number of patients in the TMN and comparator groups had either a complete or a partial response to chemotherapy by the end of the study (8 vs. 8 patients; NS). The mean change in tumor size was also similar in both groups (−23.2 mm vs. −19.8 mm; NS). Exploratory endpoints concerning chemotherapy tolerability were also similar between the groups. A similar number of patients in both groups developed dose-limiting toxicity during the study (20 vs. 23 patients; NS). The dose and/or dose frequency was lowered, and the type of chemotherapy was changed in numerically fewer patients in the TMN vs. the comparator group (4 vs. 7 patients; NS). Likewise, numerically fewer patients in the TMN vs. the comparator group required a delay between chemotherapy cycles (5 vs. 8 patients; NS). None of these conclusions was different when considering the populations in the PPS.
Discussion
In this pilot study, we have demonstrated for the first time the safety and tolerability of a targeted nutritional supplement containing n-3 PUFAs, vitamin D3, and whey protein in pre-cachectic and cachectic patients with NSCLC receiving chemotherapy. TMN was well tolerated with a favorable safety profile compared with a comparator that was matched for energy content. Signs of potential clinical benefits warrant exploration in further trials.
Nutritional support is recommended for malnourished patients receiving anticancer treatment (Citation35,Citation36), and it is important to demonstrate the safety and tolerability of nutritional interventions in this vulnerable population. In this study, the AE profiles of both study products were similar. However, several safety parameters favored the TMN intervention. Blood pressure increased to a lesser extent, and heart rate decreased in patients who received TMN vs. those who received the isocaloric comparator, suggesting that TMN may have a protective effect on these vital signs. These findings are in line with previous studies that have reported antihypertensive and heart rate-lowering effects of high-dose n-3 PUFAs – especially in patients with high blood pressure and heart rate at baseline (Citation30,Citation32,Citation33).
Signs of a modest protective effect of TMN on immune function – including a numerically smaller reduction in neutrophil:lymphocyte ratio and neutrophil count, and a lower incidence of neutropenia than that observed with the comparator drink – could warrant further exploration in larger trials. Although mGPS did not seem to be a predictor of mortality in this setting, mGPS or other similar measures may be useful in future studies. Chemotherapy commonly induces neutropenia and impairment of neutrophil function (Citation37,Citation38) – effects that may lead to a need for either a dose delay or dose reduction. It has been suggested that n-3 PUFAs could have a role in maintaining immune function during chemotherapy. In one randomized controlled trial, 2 g of fish oil daily reduced the risk of neutropenia in patients with cancer who were receiving chemotherapy (Citation39).
Several other recent clinical trials focusing on n-3 PUFA supplementation have shown improved responses to chemotherapy, for example in terms of tumor response rate, number of treatment cycles received, and 1-year survival (Citation12,Citation40). It is, however, noteworthy that one study in mice suggested that n-3 PUFAs could be associated with resistance to chemotherapy (Citation41). Nevertheless, there is no evidence that fish oil supplementation is associated with worse outcomes in humans; it is important to point out that the current TMN, rich in n-3 PUFAs, did not lead to any impairments in response to chemotherapy in this clinical trial, in terms of the proportion of patients with a complete or partial response and mean reduction in tumor size. Together with previous studies (Citation12,Citation40), these findings illustrate the potential limitations of extrapolating animal-derived data to the clinic. Moreover, the tolerability results in this study could point towards improved chemotherapy tolerability with this TMN, as measured by numerically fewer patients experiencing AEs, developing dose-limiting toxicity, and requiring dose reductions in the TMN vs. the comparator group. These findings could have important implications for the nutritional management of patients receiving chemotherapy if replicated in larger trials.
Survival data also support a potential beneficial effect of TMN in patients with NSCLC receiving chemotherapy, with a greater number of patients in the TMN group than the comparator group surviving to 1-year post-baseline. It is encouraging that the probability of survival was greater for pre-cachectic patients in the TMN group than the comparator group, although these findings should be interpreted with caution because this trial was not designed to detect differences in survival. These data, however, support the notion that early nutritional intervention, before substantial weight loss has already occurred (Citation25), may be particularly important for these patients. Overall, together with the findings relating to immune function, these results suggest that TMN has a favorable safety profile in patients with NSCLC who are receiving chemotherapy and support further studies investigating the importance of early nutritional intervention in patients with NSCLC.
In this study, we report signs of positive effects of TMN on several measures of clinical relevance in patients with cancer that could be explored further. Malnutrition and weight loss are common contributors to poor prognosis in patients with cancer and are often worsened during chemotherapy (Citation6). Importantly, in this study, TMN was associated with body weight gain over a treatment period of 12 weeks. Interestingly, patients who received TMN gained weight to a similar degree to those who received the isocaloric comparator, despite the fact that the TMN group had lower study product intake based on compliance data. Therefore, it seems likely that weight gain could be increased further with greater supplement consumption. While patients gained weight, there was no detectable change in body composition in either group. Although an increase in lean muscle mass would have been preferable in these patients, it is noteworthy that lean body mass was not lost in either of the groups and any weight gain is itself likely to be beneficial for long-term prognosis (Citation3,Citation7). Furthermore, we cannot exclude the possibility that TMN had positive effects on muscle quality that could influence survival.
The impact of TMN on metabolic markers – triglyceride levels in particular – was generally favorable. This study was not powered to detect a between-group difference in these measures; however, these results are of interest for the evaluation of TMN. The directions of change observed were positive and in line with previous studies of high-dose n-3 PUFAs (Citation42), as well as a previous study of the same TMN in patients with COPD (Citation30). Importantly, the HDL:LDL ratio did not change; thus, the overall change in lipid profile was considered beneficial.
Exercise capacity as measured by daily walking distance improved numerically in patients in the TMN group relative to those in the comparator group. Numerical changes in grip strength also suggested a slight potential improvement in muscle function for patients in the TMN vs. the comparator group. In a previous controlled pilot trial of TMN in cachectic patients with COPD, TMN was associated with clinically relevant improvements in exercise tolerance, but not exercise capacity or muscle function, in the absence of a prespecified exercise regimen (Citation30). These pilot trials have not included prespecified exercise regimens in order to avoid the potential confounding effects, but it may be of interest in the future to determine whether any effects of nutritional supplementation could be potentiated by an exercise regimen.
Compliance with nutritional supplementation in chemotherapy-treated patients with cancer is challenging, owing to treatment-induced changes in taste and appetite (Citation43). Fish oil-containing supplements in particular may be poorly tolerated; in a recent randomized controlled trial, compliance to an EPA-containing nutritional supplement in patients with cancer was reported to be 48% over 6 weeks (Citation44). In comparison, patients in the present study had 58% compliance with TMN over a longer duration (12 weeks) and there were no differences in palatability vs. the comparator. Therefore, the reason for the lower than expected compliance with TMN in this study remains to be understood. One explanation could be that the TMN product has a thicker consistency than the comparator and thus could be more filling. However, a different patient group previously had high (∼80%) compliance with the same TMN (Citation30), suggesting that there may be one or more factors, such as changes in taste, affecting compliance in this particular population of patients with cancer treated with chemotherapy (Citation30). Further studies could help to clarify this point.
This study has several strengths; the patient withdrawal rate was low, and patients were well characterized. The main limitation of the study was its small sample size: the study was not powered to detect between-group differences in the secondary and exploratory outcomes studied, several of which might have been expected to improve with TMN vs. comparator as previously reported (Citation30). Rather, the study has been useful for identifying parameters that warrant further investigation in larger trials. The use of an isocaloric comparator in this study allowed evaluation of the combination of ingredients in the TMN, although the protein content of the two drinks was slightly different. Future trials should use a comparator with matched protein content and include measurement of food consumption using a quantitative tool.
In conclusion, this pilot, randomized, double-blind, controlled trial demonstrated that TMN containing high-dose n-3 PUFAs, vitamin D, and high-quality protein has a favorable safety profile in pre-cachectic and cachectic patients with NSCLC receiving chemotherapy. Further trials are warranted to investigate potential effects on clinical outcomes.
Authors' Contributions
All authors contributed to study design, interpretation of data and drafting of the manuscript. F. Lonnqvist and M. Bech contributed to data acquisition and analysis. All authors approved the final version of the article for publication.
Supplemental Material
Download MS Word (53.3 KB)Acknowledgments
The authors thank Katie Pillidge, PhD, of PharmaGenesis London, London, UK for medical writing support in the preparation of this manuscript and Mikaela Alenäs at Trial Form Support for statistical analyses, funded by Smartfish AB, Stockholm, Sweden.
Disclosure Statement
A. Laviano and M. Muscaritoli have received personal fees from Smartfish. P.C. Calder has received a research grant and personal fees from Smartfish. A.M.W.J. Schols received fees to her institution from Smartfish and Nutricia. F. Lonnqvist is a medical adviser to and has received personal fees from Smartfish. M. Bech is an employee of Smartfish.
Additional information
Funding
References
- Evans WJ, Morley JE, Argiles J, Bales C, Baracos V, et al.: Cachexia: a new definition. Clin Nutr 27, 793–799, 2008.
- Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, et al.: Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 12, 489–495, 2011.
- Kimura M, Naito T, Kenmotsu H, Taira T, Wakuda K, et al.: Prognostic impact of cancer cachexia in patients with advanced non-small cell lung cancer. Support Care Cancer 23, 1699–1708, 2015.
- LeBlanc TW, Nipp RD, Rushing CN, Samsa GP, Locke SC, et al.: Correlation between the international consensus definition of the Cancer Anorexia-Cachexia Syndrome (CACS) and patient-centered outcomes in advanced non-small cell lung cancer. J Pain Symptom Manage 49, 680–689, 2015.
- Molina JR, Yang P, Cassivi SD, Schild SE, and Adjei AA: Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc 83, 584–594, 2008.
- Kiss N: Nutrition support and dietary interventions for patients with lung cancer: current insights. Lung Cancer (Auckl) 7, 1–9, 2016.
- Ross PJ, Ashley S, Norton A, Priest K, Waters JS, et al.: Do patients with weight loss have a worse outcome when undergoing chemotherapy for lung cancers? Br J Cancer 90, 1905–1911, 2004.
- Bye A, Sjoblom B, Wentzel-Larsen T, Gronberg BH, Baracos VE, et al.: Muscle mass and association to quality of life in non-small cell lung cancer patients. J Cachexia Sarcopenia Muscle 8, 759–767, 2017.
- Muscaritoli M, Molfino A, Lucia S, and Rossi Fanelli F: Cachexia: a preventable comorbidity of cancer. A T.A.R.G.E.T. approach. Crit Rev Oncol Hematol 94, 251–259, 2015.
- Laviano A, Di Lazzaro Giraldi G, and Koverech A: Does nutrition support have a role in managing cancer cachexia? Curr Opin Support Palliat Care 10, 288–292, 2016.
- Muscaritoli M, Molfino A, Gioia G, Laviano A, and Rossi Fanelli F: The “parallel pathway”: a novel nutritional and metabolic approach to cancer patients. Intern Emerg Med 6, 105–112, 2011.
- Murphy RA, Mourtzakis M, Chu QS, Baracos VE, Reiman T, et al.: Supplementation with fish oil increases first-line chemotherapy efficacy in patients with advanced nonsmall cell lung cancer. Cancer 117, 3774–3780, 2011.
- Sanchez-Lara K, Turcott JG, Juarez-Hernandez E, Nunez-Valencia C, Villanueva G, et al.: Effects of an oral nutritional supplement containing eicosapentaenoic acid on nutritional and clinical outcomes in patients with advanced non-small cell lung cancer: randomised trial. Clin Nutr 33, 1017–1023, 2014.
- van der Meij BS, Langius JA, Spreeuwenberg MD, Slootmaker SM, Paul MA, et al.: Oral nutritional supplements containing n-3 polyunsaturated fatty acids affect quality of life and functional status in lung cancer patients during multimodality treatment: an RCT. Eur J Clin Nutr 66, 399–404, 2012.
- van der Meij BS, van Bokhorst-de van der Schueren MA, Langius JA, Brouwer IA, and van Leeuwen PA: n-3 PUFAs in cancer, surgery, and critical care: a systematic review on clinical effects, incorporation, and washout of oral or enteral compared with parenteral supplementation. Am J Clin Nutr 94, 1248–1265, 2011.
- Murphy RA, Mourtzakis M, Chu QS, Baracos VE, Reiman T, et al.: Nutritional intervention with fish oil provides a benefit over standard of care for weight and skeletal muscle mass in patients with nonsmall cell lung cancer receiving chemotherapy. Cancer 117, 1775–1782, 2011.
- de van der Schueren MAE, Laviano A, Blanchard H, Jourdan M, Arends J, et al.: Systematic review and meta-analysis of the evidence for oral nutritional intervention on nutritional and clinical outcomes during chemo(radio)therapy: current evidence and guidance for design of future trials. Ann Oncol 29, 1141–1153, 2018.
- Devries MC, and Phillips SM: Supplemental protein in support of muscle mass and health: advantage whey. J Food Sci 80, A8–A15, 2015.
- Deutz NE, Safar A, Schutzler S, Memelink R, Ferrando A, et al.: Muscle protein synthesis in cancer patients can be stimulated with a specially formulated medical food. Clin Nutr 30, 759–768, 2011.
- Tozer RG, Tai P, Falconer W, Ducruet T, Karabadjian A, et al.: Cysteine-rich protein reverses weight loss in lung cancer patients receiving chemotherapy or radiotherapy. Antioxid Redox Signal 10, 395–402, 2008.
- Strohle A, Zanker K, and Hahn A: Nutrition in oncology: the case of micronutrients (review). Oncol Rep 24, 815–828, 2010.
- Crew KD, Shane E, Cremers S, McMahon DJ, Irani D, et al.: High prevalence of vitamin D deficiency despite supplementation in premenopausal women with breast cancer undergoing adjuvant chemotherapy. J Clin Oncol 27, 2151–2156, 2009.
- Fakih MG, Trump DL, Johnson CS, Tian L, Muindi J, et al.: Chemotherapy is linked to severe vitamin D deficiency in patients with colorectal cancer. Int J Colorectal Dis 24, 219–224, 2009.
- Drake MT, Maurer MJ, Link BK, Habermann TM, Ansell SM, et al.: Vitamin D insufficiency and prognosis in non-Hodgkin's lymphoma. J Clin Oncol 28, 4191–4198, 2010.
- Martin L, Senesse P, Gioulbasanis I, Antoun S, Bozzetti F, et al.: Diagnostic criteria for the classification of cancer-associated weight loss. J Clin Oncol 33, 90–99, 2015.
- Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, et al.: Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5, 649–655, 1982.
- Colomer R, Moreno-Nogueira JM, Garcia-Luna PP, Garcia-Peris P, Garcia-de-Lorenzo A, et al.: N-3 fatty acids, cancer and cachexia: a systematic review of the literature. Br J Nutr 97, 823–831, 2007.
- Weed HG, Ferguson ML, Gaff RL, Hustead DS, Nelson JL, et al.: Lean body mass gain in patients with head and neck squamous cell cancer treated perioperatively with a protein- and energy-dense nutritional supplement containing eicosapentaenoic acid. Head Neck 33, 1027–1033, 2011.
- Wigmore SJ, Ross JA, Falconer JS, Plester CE, Tisdale MJ, et al.: The effect of polyunsaturated fatty acids on the progress of cachexia in patients with pancreatic cancer. Nutrition 12, S27–S30, 1996.
- Calder PC, Laviano A, Lonnqvist F, Muscaritoli M, Ohlander M, et al.: Targeted medical nutrition for cachexia in chronic obstructive pulmonary disease: a randomized, controlled trial. J Cachexia Sarcopenia Muscle 9, 28–40, 2017.
- Wilson MM, Thomas DR, Rubenstein LZ, Chibnall JT, Anderson S, et al.: Appetite assessment: simple appetite questionnaire predicts weight loss in community-dwelling adults and nursing home residents. Am J Clin Nutr 82, 1074–1081, 2005.
- Geleijnse JM, Giltay EJ, Grobbee DE, Donders AR, and Kok FJ: Blood pressure response to fish oil supplementation: metaregression analysis of randomized trials. J Hypertens 20, 1493–1499, 2002.
- Mozaffarian D, Geelen A, Brouwer IA, Geleijnse JM, Zock PL, et al.: Effect of fish oil on heart rate in humans: a meta-analysis of randomized controlled trials. Circulation 112, 1945–1952, 2005.
- Calder PC: n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr 83, 1505S–1519S, 2006.
- Thompson KL, Elliott L, Fuchs-Tarlovsky V, Levin RM, Voss AC, et al.: Oncology evidence-based nutrition practice guideline for adults. J Acad Nutr Diet 117, 297–310.e47, 2017.
- August DA, and Huhmann MB, American Society for P, Enteral Nutrition Board of D: A.S.P.E.N. clinical guidelines: nutrition support therapy during adult anticancer treatment and in hematopoietic cell transplantation. JPEN J Parenter Enteral Nutr 33, 472–500, 2009.
- Zitvogel L, Apetoh L, Ghiringhelli F, and Kroemer G: Immunological aspects of cancer chemotherapy. Nat Rev Immunol 8, 59–73, 2008.
- Kim SK, and Demetri GD: Chemotherapy and neutropenia. Hematol Oncol Clin North Am 10, 377–395, 1996.
- Bonatto SJ, Oliveira HH, Nunes EA, Pequito D, Iagher F, et al.: Fish oil supplementation improves neutrophil function during cancer chemotherapy. Lipids 47, 383–389, 2012.
- Morland SL, Martins KJB, and Mazurak VC: n-3 polyunsaturated fatty acid supplementation during cancer chemotherapy. J Nutr Intermed Metabol 5, 107–116, 2016.
- Daenen LG, Cirkel GA, Houthuijzen JM, Gerrits J, Oosterom I, et al.: Increased plasma levels of chemoresistance-inducing fatty acid 16:4(n-3) after consumption of fish and fish oil. JAMA Oncol 1, 350–358, 2015.
- Balk EM, Lichtenstein AH, Chung M, Kupelnick B, Chew P, et al.: Effects of omega-3 fatty acids on serum markers of cardiovascular disease risk: a systematic review. Atherosclerosis 189, 19–30, 2006.
- Ravasco P: Aspects of taste and compliance in patients with cancer. Eur J Oncol Nurs 9, S84–S91, 2005.
- Solheim TS, Laird BJA, Balstad TR, Stene GB, Bye A, et al.: A randomized phase II feasibility trial of a multimodal intervention for the management of cachexia in lung and pancreatic cancer. J Cachexia Sarcopenia Muscle 8, 778–788, 2017.