Abstract
Studies suggest consuming soy may protect women from breast cancer. In this study, lifetime exposure to 20%, 5% and 1% ISP in MTB-IGFIR mice (mammary-specific expression of IGF-IR) were evaluated to determine whether ISP could protect against mammary tumorigenesis. MTB-IGFIR mice fed ISP diets displayed increased mammary tumor incidence and reduced tumor latency compared to mice fed 20% casein. To evaluate whether a diet containing a less refined form of soy could protect against mammary tumor development MTB-IGFIR mice were fed Teklad 2018 (contains soybean meal). MTB-IGFIR mice fed the Teklad 2018 diet were completely protected against mammary tumor development. To determine whether dietary ISP was sufficient to induce mammary tumorigenesis, MTB-IGFIR mice were fed Teklad 2018ISP (soybean meal of Teklad 2018 was replaced with an equivalent amount of ISP). Only two of 10 MTB-IGFIR mice fed Teklad 2018ISP developed mammary tumors. This study demonstrates the complex interaction between soy and other dietary components in modifying mammary tumor development.
Introduction
The interest in soy based products for reducing breast cancer risk stems from epidemiologic studies showing that women from cultures consuming high levels of dietary soy have an approximately 3-fold reduced risk of developing breast cancer compared to women from cultures that typically consume small amounts of soy (Citation1–5). Soy products such as soybeans contain naturally occurring plant chemicals known as isoflavones. The main isoflavones in soy are genistein, daidzein and glycitein (Citation6) and these isoflavones are phytoestrogens and thus can interact with estrogen receptor-α (ERα) and ERβ (Citation7, Citation8). Most phytoestrogens, including soy isoflavones, have relatively weak estrogenic activity compared to endogenous estrogens (Citation9). Therefore, it is thought that dietary isoflavones compete with estrogen for ERs and reduce ER signaling. Since lifetime estrogen exposure is a breast cancer risk factor (Citation10), soy isoflavones presumably reduce breast cancer risk through suppressing ER signaling.
While most of the studies have focused on the isoflavone components of soy, soybeans also contain compounds such as protease inhibitors, phytosterols and saponins that may also influence breast cancer risk (Citation11–13). The levels of these components as well as the levels of isoflavones can be influenced by processing of soybeans (Citation14–16). Processing and refining of soybeans into a product known as isolated soy protein (ISP) or protein soy isolate removes most of the carbohydrates and fiber leaving a product that is approximately 90% protein. Asian cultures typically consume minimally processed soybeans while ISP is common in North America and this processing could impact the protective benefits of dietary soy against breast cancer development.
Since it is difficult to control for the contributions of other lifestyle factors to breast cancer risk in humans and human prevention trials would take decades to complete, several studies have utilized rodent models to investigate the impact of soy on mammary tumor development. Animal models using chemical carcinogens or oncogenic transgenes have showed mixed results (reviewed in (Citation17)) with some studies showing that soy decreases tumor latency or increases tumor incidence while other studies demonstrate that soy protects against mammary tumor development or had no effect.
Given our previous findings that extremely high levels of dietary ISP promoted mammary tumor development (Citation18) and the inconsistent published results regarding the protective effects of dietary soy on mammary tumor development, this study investigated whether dietary soy could protect against mammary tumor development using different concentrations and formulations of soy.
Materials and Methods
Ethics
Animals were housed and cared for following guidelines established by the Central Animal Facility at the University of Guelph and the guidelines established by the Canadian Council of Animal Care. This study was approved by the Animal Care Committee at the University of Guelph (AUP# 3123 and 3994).
Mice and Diets
MTB-IGFIR transgenic mice overexpress the type I insulin-like growth factor receptor (IGF-IR) in mammary epithelial cells in a doxycycline inducible manner and have been previously described (Citation19). Six experimental diets were created by Envigo (Madison, WI). For the 20% casein diet, all the dietary protein was derived from casein. Similar, for the 20% ISP diet, all the dietary protein was derived from isolated soy protein (ISP). The 5% ISP and 1% ISP diets contained 5% ISP or 1% ISP, respectively and the remaining protein was provided by casein. The casein and soy diets are based on the AIN-93G diet. Dietary components are listed in Supplementary Tables 1 and 2. The isolated soy protein used by Envigo typically contains approximately 1980 ppm genistein + daidzein aglycone units. Therefore the 20% ISP diet contains approximately 400 mg/kg of isoflavones while the 5% ISP and 1% ISP contain approximately 100 mg/kg and 20 mg/kg, respectively. Our study also included a standard rodent diet, Teklad 2018 (Envigo, Madison, WI). Isoflavone levels (daidzein and genistein) of Teklad 2018 were measured every quarter by Envigo and this diet was used from January 2013 until November 2017. Average isoflavone levels in Teklad 2018 during this time frame were 276 ± 11 mg/kg. The final diet was Teklad 2018ISP and in this diet the soybean meal of Teklad 2018 was replaced with an equivalent amount of ISP (5.3% ISP). Mice were exposed to one of the six diets throughout embryonic and postnatal development by feeding the specified diet to mating mice and maintaining the offspring on the appropriate diet following weaning. At either postnatal day 45 (PND45) or PND100 female MTB-IGFIR mice were switched to their designated diet + 100 mg of doxycycline per kilogram of food to induce expression of the IGF-IR transgene in mammary epithelial cells (Citation18, Citation19). Mice were maintained on the doxycycline supplemented diet until the end of the study.
Mammary Tumor Onset and Tissue Collection
Mammary tumor onset was determined by palpation. Tumor onset was determined as the number of days after the IGF-IR transgene was induced and tumor free curves were plotted using GraphPad Prism 8 (GraphPad Software, La Jolla, CA). Statistical differences in tumor-free curves were determined using a log-rank test of each comparison and Bonferroni’s method for multiple comparisons in GraphPad Prism 8. Mammary tumors were collected once they reached approximately 10% of the mouse’s body weight. Mammary tumors were divided with a portion being fixed in 10% formalin for paraffin sectioning or flash frozen for RNA and protein analysis. The lungs from each mouse were collected and all lobes were fixed in formalin and embedded in paraffin for sectioning.
For the normal mammary glands, mice were fed 20% casein, 20% ISP, 5% ISP, 1% ISP or Teklad 2018 (without doxycycline) throughout embryonic and postnatal development and mammary glands were collected at PND45, PND55, and PND100. One 4th mammary gland was wholemounted as described in (Citation19). The other 4th mammary gland was formalin fixed and paraffin embedded while the remaining mammary glands were flash frozen.
Duct Length, Duct Area and Terminal End Bud Number
Mammary wholemounts were performed as previously described (Citation19). Wholemounted mammary glands were captured using a Canon 6 D digital camera (Canon Canada, Mississauga, ON) and the images were imported into Aperio ImageScope (Leica Biosystems, Concord, ON). The distance measured from the edge of the lymph node (closest to the nipple) to the tips of the three longest ducts were averaged to provide a measure of duct length. To calculate duct area, mammary ducts from wholemount images were manually traced in Photoshop CS5 (Adobe, San Jose, CA) using a brush size of 10px and black color using a Wacom CTE-440 tablet (Wacom Technology Corporation, Portland, OR). Traced images then had the lymph node removed in Photoshop and were then imported into Image J (Citation20). Each image was measured in Image J using a threshold of 12 and the area occupied by ducts determined. The number of terminal end buds (TEBs) was determined through manual counting of the wholemount images.
Staining and Immunohistochemistry
Mammary tumors and mammary glands were stained with hematoxylin and eosin for histological examination. Mammary tumors were also stained with Gomori trichrome (Newcomer Supply, Middleton, WI). Immunohistochemistry was performed as previously described (Citation21) using antibodies against Krt5 (ab53121), Krt14 (ab7800), and p63 (ab124762) obtained from Abcam, Toronto, ON, Canada. The p63 antibody was used at a dilution of 1:750 while the antibodies for Krt5 and Krt14 were used at a dilution of 1:100. The presence of lung metastases was determined using hematoxylin and eosin stained lung tissue.
RNA Extraction, RNA Sequencing and Real-Time PCR
RNA was extracted from mammary tissue using the mirVana miRNA isolation kit (Life Technologies, Burlington, ON, Canada) following the manufacturer’s instructions. For the normal, postnatal day (PND) 55 mammary glands, RNA sequencing was performed at the Genome Quebec Innovation Center at McGill University (Montreal, QC) using the Illumina Hiseq 2500 v4 PE125 as previously described (Citation22). For the mammary tumors, RNA sequencing was performed by Novogene (Chula Vista, CA) using Illumina Hiseq 4000. Data analysis was performed using Genialis software (Genialis Inc, Houston, TX) using their general RNA sequencing pipeline. Briefly this pipeline uses BBDuk to remove adapters and trim reads, STAR to align the reads and featureCounts to provide gene-level counts. The RNA sequencing data has been uploaded to GEO under accession number GSE122316.
Statistics
For the tumor free curves, statistical differences were determined using a log-rank (Mantel-Cox) test and Bonferroni’s method for multiple comparisons and values were considered statistically significant at p < 0.05. For comparing means of multiple groups, a one-way ANOVA followed by multiple comparisons using a Tukey’s test was used and values were considered statistically significant at p < 0.05. Metastatic frequencies were evaluated using a Fisher’s exact test. All statistical tests were performed using GraphPad Prism 8 software (GraphPad Software, San Diego, CA).
Results
Determine Whether Different Concentrations of Dietary ISP Protected against Mammary Tumor Development
Our original study demonstrated that MTB-IGFIR transgenic mice fed a diet containing 20% ISP developed mammary tumors more frequently and more rapidly than MTB-IGFIR transgenic mice fed a control, casein diet (Citation18). Since 20% ISP is an extremely high amount of dietary soy, this paper also evaluated two lower concentrations of dietary soy; 5% ISP and 1% ISP to determine whether lower concentrations of dietary soy could protect against mammary tumor development.
MTB-IGFIR transgenic mice were fed matched diets containing 20% casein, 20% ISP, 5% ISP, or 1% ISP throughout their lifetime. Mammary tumor development was induced at postnatal day 45 (PND45) or PND100 by switching the MTB-IGFIR females to the appropriate diet containing 100 mg/kg of doxycycline (doxycycline induces IGF-IR transgene expression in mammary epithelial cells of MTB-IGFIR mice (Citation19)). shows the tumor-free curves for mice fed the different diets. Mice fed the 20% casein diet had significantly prolonged tumor latency compared to (i) mice fed the 20% ISP and 5% ISP diets when the IGF-IR transgene was induced at PND45 () and (ii) mice fed the 5% ISP and 1% ISP diets when the IGF-IR transgene was induced at PND100 (). Tumor-free curves for mice fed the 20%, 5% and 1% ISP diets were not significantly different suggesting that the level of ISP in the diet did not influence tumor development. Tumor incidence () was also significantly higher in mice fed the 20% ISP, 5% ISP or 1% ISP diets compared to mice fed the 20% casein diet when the IGF-IR transgene was induced at PND100. Therefore, the presence of ISP in the diet shortened mammary tumor latency and increased mammary tumor incidence.
Figure 1. Tumor-free curves of MTB-IGFIR transgenic mice with lifetime exposure 20% Casein (▪), 20% ISP (◆), 5% ISP (▼) or 1% ISP (▲) when overexpression of the IGF-IR transgene was initiated at PND45 (A) or PND100 (B). Mice fed the 20% casein diet had significantly different (p < 0.05) tumor free curves from the 20% ISP and 5% ISP diets when the IGF-IR transgene was induced at PND45 (A) and the 5% ISP and 1% ISP diets when the IGF-IR transgene was induced at PND100 (B). There were no significant differences in tumor onset when comparing the different ISP diets.
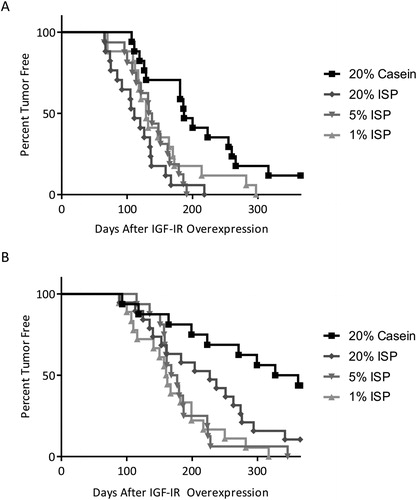
Table 1. Tumor characteristics of MTB-IGFIR mice fed the various diets.
Histologically, tumors from MTB-IGFIR transgenic mice fed the 20% casein, 1% ISP, 5% ISP or 20% ISP diets typically presented as solid sheet of tumor cells () with little intervening collagen () and variable amounts of necrosis. However, there were tumors that presented with nests of tumor cells separated by collagen (Supplementary Figure 1C,D) that were found in both casein-fed and ISP-fed mice. Most of the tumors also presented with regions of squamous differentiation (Supplementary Figure 1E) which were characterized by altered cell morphology and cells staining positive for Krt5 (Supplementary Figure 1F), Krt14 (Supplementary Figure 1G) or p63 (Supplementary Figure 1H). Although the percentage of tumors with squamous differentiation were frequently higher in the ISP diets compared to the casein diet these values were not statistically significant.
To determine whether the casein or ISP diets influenced mammary tumor metastasis, the percentage of mice with lung metastases was determined. When the IGF-IR transgene was induced at PND45, mice fed the 20% casein diet had the highest percentage of lung metastases, but this difference was not statistically significant (Supplementary Table 3). When the IGF-IR transgene was induced at PND100, mice fed the 5% ISP diet displayed the highest percentage of mice containing lung metastases and was significantly different from the 20% ISP mice but not the 20% casein or 1% ISP mice (Supplementary Table 3). Therefore, there was no consistent association between a particular diet and metastatic frequency.
RNA was extracted from mammary tumors of from mice fed 20% casein, 20% ISP or 1% ISP diets (tumors from 5% ISP fed mice were not sequenced). RNA sequencing was performed to determine whether mammary tumors that developed in mice fed ISP or casein diets differed in their gene expression. Hierarchical clustering (Supplementary Figure 2) revealed that that tumors induced by the different diets failed to form discrete clusters. When mammary tumors from mice fed the 20% casein diet were compared to tumors from 20% ISP fed mice, only 10 genes were differentially expressed (log2 fold change ≥ 1, FDR < 0.01; Supplementary Figure 3). Similarly, only 40 genes were differentially expressed in mammary tumors from mice fed 20% casein compared to mammary tumors from mice fed 1% ISP (log2 fold change ≥ 1, FDR < 0.01; ). Only two genes, Pde6a and Nyx, were shared between the comparisons and both genes were elevated in the tumors from casein fed mice compared to ISP fed mice.
Examine Whether a Diet Containing a Less Refined Soy Product Protects against Mammary Tumor Development
Soy can be consumed in a variety of forms, some of which are more highly processed than others. ISP, as used in the 20% ISP, 5% ISP and 1% ISP diets, is one of the most highly processed forms of soy where most of the carbohydrates and fibers are removed leaving a product that is approximately 90% protein. In contrast, less processed forms of soy such as soybean meal contain compounds including phytosterols and saponins that are not typically found in ISP (Citation14). To investigate whether a diet containing a less refined form of soy (∼5% soybean meal) could inhibit mammary tumor development, MTB-IGFIR mice were exposed to Teklad 2018 throughout embryonic and postnatal development. Surprisingly none of the MTB-IGFIR mice fed Teklad 2018 developed mammary tumors (mice were followed for 1 year after IGF-IR transgene induction; ).
Table 2. Tumor characteristics of MTB-IGFIR mice fed Teklad 2018 or Teklad 2018ISP.
Determine Whether Dietary ISP is Sufficient to Induce Mammary Tumor Development
The observation that diets containing even small amounts of ISP enhanced mammary tumor development compared to diets lacking ISP suggest that ISP may promote mammary tumorigenesis. To determine whether ISP is sufficient to induce mammary tumor development, one additional diet was tested, Teklad 2018ISP. Teklad 2018ISP was essentially identical to Teklad 2018 except the soybean meal was removed and replaced with an equivalent amount of ISP. If the presence of ISP was sufficient to promote mammary tumor development, then Teklad 2018ISP fed MTB-IGFIR mice should develop mammary tumors. Ten MTB-IGFIR mice with lifetime exposure to Teklad 2018ISP and IGF-IR transgene induction at PND45 were monitored for 12 mo, and two of the mice developed mammary tumors between 9 and 12 mo, of age ().
Determine Whether Diet Influences Mammary Tumor Susceptibility through Changes in Ductal Development
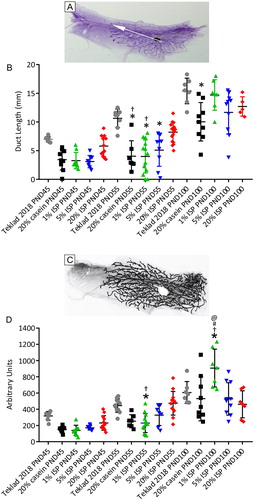
Given that mice fed Teklad 2018 failed to develop mammary tumors while mice fed the ISP diets frequently developed mammary tumors, an earlier timepoint, namely ductal development, was evaluated to determine whether the ISP diets induced changes in ductal development that would predict mammary tumor risk. Mice were fed Teklad 2018, 20% casein, 20% ISP, 5% ISP or 1% ISP diets (without doxycycline) throughout embryonic and postnatal development and mammary wholemount analysis was performed at PND45, PND55 and PND100. Supplementary Figure 4 shows representative PND55 wholemounts of mice fed the different diets. Ductal length was determined by measuring the three longest ducts in each wholemount () and then averaging the length of these three ducts. Quantification of ductal length is presented in . Mice fed the Teklad 2018 diet showed an increase in ductal length from PND45 to PND100 as expected. Mice fed the 20% casein, 1% ISP or 5% ISP diets had significantly shorter ducts at PND 55 compared to mice fed Teklad 2018 while mice fed the 20% ISP diet had ductal development most similar to mice fed Teklad 2018. Mice fed the 20% casein or 1% ISP diet also had significantly shorter ducts than mice fed the 20% ISP diet at PND55. By PND 100 there was no significant difference in ductal length between any of the mice fed ISP diets or Teklad 2018 while mice fed the 20% casein diet still had ducts that were significantly shorter than mice fed Teklad 2018. A similar pattern emerged when evaluating duct area however only mice fed the 1% ISP diet had significantly less ductal area than mice fed either the Teklad 2018 diet or 20% ISP diet at PND55 (). As a final measure of ductal development, the number of terminal end buds were counted. The number of TEBs in each mammary gland was highly variable and there were no signification differences in TEB number between mice fed the various diets.
Figure 2. Mammary duct length and area. A representative wholemount showing how the length of the mammary ducts was measured (A; white arrow). Quantification of ductal length is presented in (B). Mice fed the 20% casein, 1% ISP or 5% ISP diets had significantly shorter ducts at PND 55 compared to the Teklad 2018 (*) while mice fed the 20% ISP diet had ductal development most similar to mice fed the Teklad 2018 diet. Mice fed the 20% casein or 1% ISP diet also had significantly shorter ducts than mice fed the 20% ISP diet (Ɨ) at PND55. Panel C show a representative wholemount tracing and quantification of mammary ductal area is plotted in panel D. Mice fed the 1% ISP diet had significantly reduced mammary ductal area compared to mice fed Teklad 2018 (*) or mice fed 20% ISP (Ɨ) at PND55 but significantly higher ductal area than all the other diets at PND100. * significantly different (p < 0.05) than Teklad 2018, Ɨ significantly different than 20% ISP, # significantly different than 5% ISP and @ significantly different than 1% ISP.
Next, RNA sequencing was performed on PND55 mammary glands from mice fed Teklad 2018, 20% casein, 20% ISP, and 1% ISP diets (mammary glands from 5% ISP fed mice were not sequenced). Hierarchical clustering did not reveal any consistent patterns across the samples however the mammary glands from four of the five mice fed Teklad 2018 did cluster relatively closely together (Supplementary Figure 5). Given that mammary glands from mice fed 1% ISP or 20% ISP diets almost always developed mammary tumors and mice fed Teklad 2018 never developed mammary tumors, gene expression profiles of PND55 mammary glands from mice fed these diets were compared. A false discover rate (FDR) < 0.01 and a log2 fold change ≥ 1 was used to identify significant, differentially expressed genes. As shown in , 140 genes were shared in the mammary glands from mice fed the 1% or 20% ISP diets compared to mice fed the Teklad 2018 diet. Enrichr (Citation23, Citation24) was then used to analyze these 140 differentially expressed genes and the top three gene ontology molecular functions were all related to actin binding (). As the altered expression in actin binding genes was somewhat surprising, the Mouse Gene Atlas component of Enrichr was also used to evaluate these 140 differentially expressed genes and this analysis confirmed that these genes were frequently expressed in non-lactating mammary glands ().
Figure 3. RNA sequencing data from normal mammary glands. Venn diagrams of the genes significantly, differentially expressed in the PND55 mammary glands from mice 20% ISP or 1% ISP diets compared to mice fed the Teklad 2018 diet (A) or the 20% casein diet (B). The tables show the top three gene ontology molecular functions and mouse tissues (Mouse Gene Atlas) associated with these differentially expressed genes shared by the ISP diets compared to the Teklad 2018 diet (A) and 20% casein diet (B).
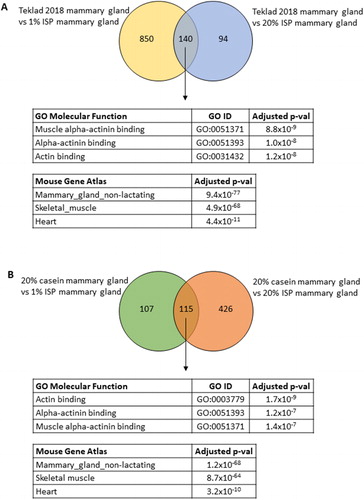
Since the enrichment of genes associated with actin binding in pubertal mammary glands in ISP fed mice compared to Teklad 2018 fed mice was unanticipated, PND55 mammary glands of 20% casein fed and ISP fed mice were also compared. As shown in , 115 genes were shared by the two ISP diets compared to the casein diet. Pathway analysis of these 115 shared genes revealed alterations in actin binding and these genes were frequently expressed in non-lactating mammary glands ()
Discussion
A previously published manuscript from our lab demonstrated that diets containing 20% ISP promoted mammary tumor development in MTB-IGFIR transgenic mice compared to a casein diet (Citation18). This finding was surprising since most epidemiologic studies suggest that women from cultures consuming high levels of dietary soy have an approximately 3-fold reduced risk of developing breast cancer compared to women from cultures that consume small amounts of dietary soy (Citation1–5). Since 20% ISP is an extremely high level of ISP, this study evaluated two additional concentrations of ISP, 1% ISP and 5% ISP, to determine whether more moderate levels of ISP could inhibit mammary tumorigenesis in MTB-IGFIR mice. Similar to the 20% ISP diet, diets containing 1% ISP or 5% ISP promoted mammary tumor development in MTB-IGFIR mice compared to the 20% casein diet.
In an attempt to understand the mechanisms through which ISP promoted mammary tumor development, RNA sequencing was performed on mammary tumors from mice fed 20% casein, 1% ISP, and 20% ISP diets. Mammary tumors from casein-fed or ISP-fed mice were genetically similar indicating that ISP did not produce mammary tumors with a distinct phenotype compared mice fed a casein diet. Since the mammary tumors were highly similar, gene expression of normal mammary glands from casein-fed and ISP-fed mice were also evaluated to determine whether ISP altered mammary developmental characteristics that might render the mammary glands more susceptible to tumor development. RNA sequencing revealed that mice fed ISP diets increased gene expression associated with actin/actinin binding compared to mice fed casein diets or a standard rodent chow, Teklad 2018. One possibility is that the higher expression of actin binding proteins increases the stiffness of the mammary gland or ducts and mammary stiffness has been associated with increased breast cancer risk (Citation25–27). However, additional studies would be required to determine whether dietary ISP alters the stiffness of the mammary environment.
In addition to the casein and ISP diets, mammary tumor development in MTB-IGFIR mice was evaluated following lifetime exposure to a standard rodent diet, Teklad 2018. None of the 10 MTB-IGFIR mice fed the Teklad 2018 diet developed mammary tumors. There are several differences between the casein/ISP diets (which are based on AIN-93G) and the Teklad 2018 diet including the type of dietary soy. Teklad 2018 contains soybean meal which is a less refined form of soy than ISP. As the soy refinement process can remove compounds such as protease inhibitors, phytosterols and saponins and some of these components have been implicated in regulating breast cancer risk (Citation11–14, Citation16), it was possible that the less refined soy found in Teklad 2018 could protect against mammary tumorigenesis. To evaluate this possibility a special Teklad 2018 diet was created where the soybean meal was removed from Teklad 2018 and replaced with an equivalent amount of ISP (5.3% ISP). Two of the 10 MTB-IGFIR mice fed the 5.3% ISP-substituted Teklad 2018 diet (Teklad 2018ISP) developed mammary tumors. Thus, replacing the soybean meal of Tekalad 2018 with an equivalent amount of ISP induced a small, non-significant increase in tumor incidence but this 20% tumor incidence was significantly lower than the 100% mammary tumor incidence observed in MTB-IGFIR mice fed the 5% ISP diet. Therefore, it appears that dietary soy interacts with other dietary components to influence mammary tumor development. Differences between the AIN-93G-based diets (casein and ISP diets) and the Teklad 2018-based diets include the levels of vitamin A, vitamin K, several B vitamins (B1, B2, B3, B5, B6, B7, and B9) and the complexity of the carbohydrates. Vitamins B2 (riboflavin), B6 (pyridoxine), and B9 (folate) have been implicated in reducing breast cancer risk (Citation28–30) while higher blood glucose levels associated with simple carbohydrates like those found in the casein and ISP diets have been associated with increased breast cancer risk in humans (Citation31–34) and rodents (Citation35–37).
While these differences in dietary ISP, vitamins, and carbohydrates could directly affect mammary epithelial cells they could also impact the composition of intestinal and/or colonic bacteria. Soy isoflavones are metabolized by intestinal bacteria and thus the amount and type of soy isoflavones entering the circulation are dependent the abundance of different bacterial species (Citation38). Composition of the intestinal microbiota can be influenced by the amount and type of dietary carbohydrate, fiber, fats, protein, vitamins and minerals (Citation39–41). Therefore, the differences in the composition of the AIN-93G-based and Teklad 2018-based diets may influence the metabolism of the dietary soy and, in turn, affect the composition and amount of isoflavones absorbed by the mice.
In summary, this study has shown that ISP can promote mammary tumor development in mice fed AIN-93G-based diets. However, the ability of ISP to act as a tumor promoter appears to be influenced by other dietary factors such as the complexity of dietary carbohydrates and the levels of vitamins. This complex interplay between soy isoflavones and other dietary components requires careful consideration when designing future studies. With respect to dietary guidelines for cancer prevention, maintaining a healthy diet that provides the recommended levels of all micronutrients along with complex carbohydrates, will likely be more beneficial than supplementing the diet with individual components including soy products.
Competing Interests
The authors declare that they have no competing interests.
Supplemental Material
Download Zip (38.1 KB)Availability of Data and Materials
The datasets generated and analyzed during the current study are available at the Gene Expression Omnibus under accession number GSE122316
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Wu AH, Lee E, Vigen C. Soy isoflavones and breast cancer. Am Soc Clin Oncol Educ Book. 2013;33:102–106. doi:10.1200/EdBook_AM.2013.33.102
- Qin LQ, Xu JY, Wang PY, Hoshi K. Soyfood intake in the prevention of breast cancer risk in women: a meta-analysis of observational epidemiological studies. J Nutr Sci Vitaminol. 2006;52(6):428–436. doi:10.3177/jnsv.52.428
- Trock BJ, Hilakivi-Clarke L, Clarke R. Meta-analysis of soy intake and breast cancer risk. J Natl Cancer Inst. 2006;98(7):459–471. doi:10.1093/jnci/djj102
- Enderlin CA, Coleman EA, Stewart CB, Hakkak R. Hakkak R: Dietary soy intake and breast cancer risk. Oncol Nurs Forum. 2009;36(5):531–539. doi:10.1188/09.ONF.531-539
- Dong JY, Qin LQ. Soy isoflavones consumption and risk of breast cancer incidence or recurrence: a meta-analysis of prospective studies. Breast Cancer Res Treat. 2011;125(2):315–323. doi:10.1007/s10549-010-1270-8
- Messina M, Wu AH. Perspectives on the soy-breast cancer relation. Am J Clin Nutr. 2009;89(5):1673S–1679S. doi:10.3945/ajcn.2009.26736V
- Murkies AL, Wilcox G, Davis SR. Clinical review 92: phytoestrogens. J Clin Endocrinol Metab. 1998;83(2):297–303. doi:10.1210/jcem.83.2.4577
- Price KR, Fenwick GR. Naturally occurring oestrogens in foods-a review. Food Addit Contam. 1985;2(2):73–106. doi:10.1080/02652038509373531
- Vitale DC, Piazza C, Melilli B, Drago F, Salomone S. Isoflavones: estrogenic activity, biological effect and bioavailability. Eur J Drug Metab Pharmacokinet. 2013;38(1):15–25. doi:10.1007/s13318-012-0112-y
- Lippman ME, Krueger KA, Eckert S, Sashegyi A, Walls EL, Jamal S, Cauley JA, Cummings SR. Indicators of lifetime estrogen exposure: effect on breast cancer incidence and interaction with raloxifene therapy in the multiple outcomes of raloxifene evaluation study participants. JCO. 2001;19(12):3111–3116. doi:10.1200/JCO.2001.19.12.3111
- Rowlands JC, Berhow MA, Badger TM. Estrogenic and antiproliferative properties of soy sapogenols in human breast cancer cells in vitro. Food Chem Toxicol. 2002;40(12):1767–1774. doi:10.1016/s0278-6915(02)00181-3
- Hsieh CC, Hernandez-Ledesma B, Jeong HJ, Park JH, de Lumen BO. Complementary roles in cancer prevention: protease inhibitor makes the cancer preventive peptide lunasin bioavailable. PLoS One. 2010;5(1):e8890. doi:10.1371/journal.pone.0008890
- Chatterjee C, Gleddie S, Xiao CW. Soybean bioactive peptides and their functional properties. Nutrients. 2018;10(9):1211. doi:10.3390/nu1009
- Barnes S. The biochemistry, chemistry and physiology of the isoflavones in soybeans and their food products. Lymphat Res Biol. 2010;8(1):89–98. doi:10.1089/lrb.2009.0030
- Setchell KD, Cole SJ. Variations in isoflavone levels in soy foods and soy protein isolates and issues related to isoflavone databases and food labeling. J Agric Food Chem. 2003;51(14):4146–4155. doi:10.1021/jf026199b
- Fang N, Yu S, Badger TM. Comprehensive phytochemical profile of soy protein isolate. J Agric Food Chem. 2004;52(12):4012–4020. doi:10.1021/jf049842y
- Moorehead RA. Rodent models assessing mammary tumor prevention by Soy or Soy Isoflavones. Genes (Basel). 2019;10:566. doi:10.3390/genes10080
- Watson KL, Stalker L, Jones RA, Moorehead RA. High levels of dietary soy decrease mammary tumor latency and increase incidence in MTB-IGFIR transgenic mice. BMC Cancer. 2015;15:37. doi:10.1186/s12885-015-1037-z
- Jones RA, Campbell CI, Gunther EJ, Chodosh LA, Petrik JJ, Khokha R, Moorehead RA. Transgenic overexpression of IGF-IR disrupts mammary ductal morphogenesis and induces tumor formation. Oncogene. 2007;26(11):1636–1644. doi:10.1038/sj.onc.1209955
- Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671–675. doi:10.1038/nmeth.2089
- Watson KL, Moorehead RA. Loss of Akt1 or Akt2 delays mammary tumor onset and suppresses tumor growth rate in MTB-IGFIR transgenic mice. BMC Cancer. 2013;13:375. doi:10.1186/1471-2407-13-375
- Jones R, Watson K, Bruce A, Nersesian S, Kitz J, Moorehead R. Re-expression of miR-200c suppresses proliferation, colony formation and in vivo tumor growth of murine claudin-low mammary tumor cells. Oncotarget. 2017;8(14):23727–23749. doi:10.18632/oncotarget.15829
- Chen EY, Tan CM, Kou Y, Duan Q, Wang Z, Meirelles GV, Clark NR, Ma'ayan A. Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinform. 2013;14:128. doi:10.1186/1471-2105-14-128
- Kuleshov MV, Jones MR, Rouillard AD, Fernandez NF, Duan Q, Wang Z, Koplev S, Jenkins SL, Jagodnik KM, Lachmann A, et al. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016;44(W1):W90–W97. doi:10.1093/nar/gkw377
- Schedin P, Keely PJ. Mammary gland ECM remodeling, stiffness, and mechanosignaling in normal development and tumor progression. Cold Spring Harb Perspect Biol. 2011;3(1):a003228. doi:10.1101/cshperspect.a003228
- Lv Y, Chen C, Zhao B, Zhang X. Regulation of matrix stiffness on the epithelial-mesenchymal transition of breast cancer cells under hypoxia environment. Naturwissenschaften. 2017;104(5–6):38. doi:10.1007/s00114-017-1461-9
- Boyd NF, Li Q, Melnichouk O, Huszti E, Martin LJ, Gunasekara A, Mawdsley G, Yaffe MJ, Minkin S. Evidence that breast tissue stiffness is associated with risk of breast cancer. PLoS One. 2014;9(7):e100937. doi:10.1371/journal.pone.0100937
- Mokbel K, Mokbel K. Chemoprevention of breast cancer with vitamins and micronutrients: a concise review. In Vivo. 2019;33(4):983–997. doi:10.21873/invivo.11568
- Yu L, Tan Y, Zhu L. Dietary vitamin B2 intake and breast cancer risk: a systematic review and meta-analysis. Arch Gynecol Obstet. 2017;295(3):721–729. doi:10.1007/s00404-016-4278-4
- Chen P, Li C, Li X, Li J, Chu R, Wang H. Higher dietary folate intake reduces the breast cancer risk: a systematic review and meta-analysis. Br J Cancer. 2014;110(9):2327–2338. doi:10.1038/bjc.2014.155
- Mullie P, Koechlin A, Boniol M, Autier P, Boyle P. Relation between breast cancer and high glycemic index or glycemic load: a meta-analysis of prospective cohort studies. Crit Rev Food Sci Nutr. 2016;56(1):152–159. doi:10.1080/10408398.2012.718723
- Choi Y, Giovannucci E, Lee JE. Glycaemic index and glycaemic load in relation to risk of diabetes-related cancers: a meta-analysis. Br J Nutr. 2012;108(11):1934–1947. doi:10.1017/S0007114512003984
- Dong JY, Zhang L, Zhang YH, Qin LQ. Dietary glycaemic index and glycaemic load in relation to the risk of type 2 diabetes: a meta-analysis of prospective cohort studies. Br J Nutr. 2011;106(11):1649–1654. doi:10.1017/S000711451100540X
- de Beer JC, Liebenberg L. Does cancer risk increase with HbA1c, independent of diabetes? Br J Cancer. 2014;110(9):2361–2368. doi:10.1038/bjc.2014.150
- Thompson HJ, Neuhouser ML, Lampe JW, McGinley JN, Neil ES, Schwartz Y, McTiernan A. Effect of low or high glycemic load diets on experimentally induced mammary carcinogenesis in rats. Mol Nutr Food Res. 2016;60(6):1416–1426. doi:10.1002/mnfr.201500864
- Bojkova B, Kajo K, Garajova M, Kubatka P, Pec M, Kiskova T, Orendas P, Kassayova M, Korpova M, Miklosova M, et al. Rosiglitazone shows partial oncostatic effect in rat mammary carcinogenesis. Neoplasma. 2013;60(1):46–55.:doi:10.4149/neo_2013_007
- Wellberg EA, Johnson S, Finlay-Schultz J, Lewis AS, Terrell KL, Sartorius CA, Abel ED, Muller WJ, Anderson SM. The glucose transporter GLUT1 is required for ErbB2-induced mammary tumorigenesis. Breast Cancer Res. 2016;18(1):131. doi:10.1186/s13058-016-0795-0
- Peiroten A, Bravo D, Landete JM. Bacterial metabolism as responsible of beneficial effects of phytoestrogens on human health. Crit Rev Food Sci Nutr. 2020;60(11):1922–1937. doi:10.1080/10408398.2019.1622505
- Sonnenburg ED, Sonnenburg JL. Starving our microbial self: the deleterious consequences of a diet deficient in microbiota-accessible carbohydrates. Cell Metab. 2014;20(5):779–786. doi:10.1016/j.cmet.2014.07.003
- Eswaran S, Muir J, Chey WD. Fiber and functional gastrointestinal disorders. Am J Gastroenterol. 2013;108(5):718–727. doi:10.1038/ajg.2013.63
- Forgie AJ, Fouhse JM, Willing BP. Diet-microbe-host interactions that affect gut mucosal integrity and infection resistance. Front Immunol. 2019;10:1802. doi:10.3389/fimmu.2019.01802
