Abstract
Metalloendocrinology is a new interdisciplinary field, which was established due to the importance of connections between inorganic chemicals and hormonal mechanisms. The role of cadmium in hormone-related tumors is an excellent example of this connection, as cadmium mimics estrogen in the human body. Since endometrial cancer (EC) is hormone-related, it is well-suited for assessing the estrogenic effects of cadmium. Therefore, the present study aims to explore the role of dietary cadmium intake in the progression-free survival (PFS) and overall survival (OS) in women with EC. Dietary cadmium intake was estimated based on a large cohort of Swedish women (n = 416) with EC. Median dietary cadmium intake was then analyzed in relation to different tumor characteristics and clinical outcomes. Cox proportional hazard models were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs). Median daily dietary cadmium intake in the cohort was 13.1 μg (interquartile range 25%-75%=6.4). High dietary cadmium intake (μg/day) was associated with significantly decreased OS in the study cohort (HR = 0.956, 95% CI = 0.914-1.001, p = 0.05). Dietary cadmium intake was not associated with PFS (HR = 0.975, 95% CI = 0.924-1.028, p = 0.348). Therefore, our results indicate that high dietary cadmium intake could be associated with poor outcome in women with EC.
Introduction
Endometrial cancer (EC) is the most common gynaecological tumor in high-income countries, and incidence rates for the disease have increased over time (Citation1). In 2016, the incidence rate of EC was 27.7 per 100 000 women in Sweden (Citation2). The majority of EC cases are diagnosed at an early stage (Citation3), and more than 95% of EC develops in women over 40 years of age, but 4% of women with EC are under 40 years old (Citation4). Median age at EC diagnosis among Swedish women is 71 years old, according to the Ministry of Health and Social Affairs and the Swedish Cancer Society (Citation2). Various patient characteristics and histopathological tumor features have a significant influence on patient prognosis (Citation5).
There are several established risk factors for EC, including hypertension and diabetes, as well as conditions associated with excess estrogen exposure, such as early menarche, late menopause, and high body mass index (Citation5). Environmental factors that mimic the effects of estrogen could also be associated with a higher incidence of EC (Citation6).
Cadmium is a heavy metal and an environmental contaminant, which has been classified as a group 1 carcinogen (Citation7). Although environmental quality standards exist for cadmium, it has been found in a few Swedish lakes, watercourses, and coastal waters (Citation8). Dietary intake is the most significant source of cadmium exposure in people not exposed through their occupation or habitat in specifically polluted areas in Sweden (Citation9); therefore, both cadmium levels in food items and food consumption patterns play a role in total dietary cadmium intake (Citation10). Cereal products and vegetables are the most common sources, partly due to their high consumption but also because cadmium is mainly absorbed by crops from the soil (Citation9). Tobacco products are the second most common source of cadmium intake in the population, due to high concentrations in cigarettes and snuff (Citation7,Citation11).
Cadmium absorption in humans is low (3%-5%), but the biological half-life of cadmium in the liver and kidneys of humans’ ranges from 10 to 30 years (Citation7). Interestingly, low iron status increases cadmium absorption in the intestine, which leads to higher cadmium concentrations in women than men (Citation12,Citation13). The metal has many adverse effects, including on kidneys and bone (Citation14,Citation15). In addition, cadmium has a mutagenic effect and increases estrogen receptor α-mediated cell proliferation (Citation16–18). Thus, it has an estrogenic effect, resulting in hyperplasia, increased uterine weight, and endometrial thickness (Citation19). Recently, the new scientific field metalloendocrinology was introduced (Citation20). It shows the essential role of inorganic chemicals in the development of hormone-dependent conditions. The present study aims to explore the role of dietary cadmium intake in the disease progression and survival of women with EC.
Methods
Study Population
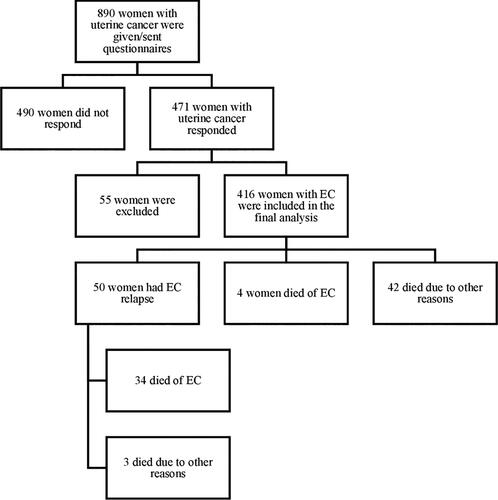
The study population included 890 women diagnosed with uterine cancer and admitted to the Karolinska University Hospital Solna within the period 2007-2012. All women underwent hysterectomy and bilateral salpingo-oophorectomy, and tumor tissue samples were collected during the operation. Pathologists examined the samples, performed immunohistochemical analyses and summarized the results into a pathologic-anatomic diagnosis, which was entered into patients’ medical records. If needed, patients received adjuvant treatment at the Karolinska University Hospital. After cancer treatment, and if the disease had not spread, gynecologists outside of the hospital conducted follow-up visits, which included collection of patient history, vaginal examination, and transvaginal ultrasound. All patients with suspected relapse were referred back to the Karolinska University Hospital for more detailed examinations. All women in the study population were given a written description of the study and letter of consent. Upon agreement to participate in the study and completion of the letter of consent, women were given or sent two printed questionnaires, which were completed at home, with full anonymity guaranteed. All described methods were carried out in accordance with relevant guidelines and regulations.
Questionnaires and Clinical Data
Questionnaire one covered lifestyle and dietary habits (Appendix S1) and Questionnaire two covered selected socioeconomic and reproductive factors (Appendix S2), with two reminders sent at an interval of 2 mo. Questionnaire one covered eight different subjects: physical activity and exercise, sun habits, eating habits, dietary habits in the last year, dietary supplements and medicine, alcohol, tobacco, and outdoor activities; there were 1-14 items in each subject. The subject “physical activity and exercise” has been validated and contains five different options for each item. The time spent doing each specific activity a day was multiplied by its energy usage in metabolic equivalents (METs) and summarized in MET-hours per day (Citation21). We used the item “watching TV/reading” to assess leisure time physical activity, with sitting ≥5 h per day described as inactivity as per Friberg et al. (Citation22). In the case of missing data on this item, the gap was considered as zero. The subject “dietary habits in the last year” was a food frequency questionnaire previously designed by Terry et al. at the Institute of Environmental Medicine at Karolinska Institutet (Citation23). This questionnaire has been validated and is based on internationally accepted questions. The subject “tobacco” included cigarette and snuff use and was divided into never, former, or current tobacco use. The standard portion of snuff was considered to be 1 g of smokeless tobacco.
Questionnaire two was also developed at Karolinska Institutet (personal communication, M. Mints) and consisted of four different subjects: physical parameters (height and weight), women’s health questions (age at menarche and menopause, etc.), general health questions (diabetes and hypertensive heart disease), and other questions (education, family history, etc.). Both questionnaires included the patient’s personal registration number.
The hospital’s medical records programme, Take Care, is used in Stockholm County and Karolinska University Hospital to handle electronic medical records with regular updates regarding patients’ diagnosis, treatments, follow-up visits, relapses, survival, and other medical conditions. Patients’ personal registration number was used by Take Care to collect information on different clinical variables. The latest review of survival data was performed in February 2019.
Analytical Cohort
The study sample was formed based on responses to Questionnaire 1, which was returned by 471 women (response rate = 53%). We then excluded 24 patients treated outside Stockholm County, as we had no access to their medical records; 23 patients diagnosed with cancer of the uterus that was not EC; three patients who received primary treatment before 2007 and were admitted to Karolinska University Hospital due to EC relapse after study initiation; four patients who completed the questionnaires improperly; and one patient diagnosed with ovarian cancer (Citation24). Therefore, 416 women were included in the final analysis.
Assessment of Dietary Cadmium Exposure
Median daily dietary cadmium intake was calculated for each participating patient based on the food frequency questionnaire. The cadmium content of different food items was taken from the reports of the National Food Agency (NFA; Uppsala, Sweden) (Citation25), which have been monitoring cadmium levels in different food items since the 1970s. The mean cadmium level for each food item in NFA reports is based on analyses ranging from a few to several hundred. Only the most recent reports were used in order not to comprise the quality of the data (Citation9,Citation26–34).
Cadmium levels for each food item were averaged. There is no variation in the cadmium levels found in particular food items in Sweden, nor is there any artificial cadmium contamination of soil in the country. Moreover, there are only a few companies in Sweden that produce food items (Citation10). Therefore, we consider our dietary cadmium intakes to be nationally representative. For a few food items (white bread loaf, fiber-enriched bread, granary wholemeal bread, i.e., whole grain bread, oranges and other citrus fruits, and raisins), Danish data on cadmium levels were used (Citation35).
Average daily dietary cadmium intake (μg cadmium per day) was estimated for each participating patient by multiplying the frequency of consumption of different foods by the average daily consumption calculated from the mean values of age-specific (<66, ≥66 years) portion sizes of scale-weighted foods recorded for four 1-week periods 3-4 mo, apart, by 213 women randomly selected from the cohort (Citation10). The age-specific portion sizes were provided by the Nutritional Epidemiology Unit of the Karolinska Institute. Cadmium intake from the air is less than 1% of total cadmium exposure, and average cadmium intake from water (community-provided tap and private wells) is 0.2%; thus, both of these exposures were ignored in our calculations (Citation10).
Cadmium intake from tobacco was estimated as follows: mean cadmium level in each cigarette/snuff × mean number of cigarettes/snuff patches used × mean number of years of tobacco use (Citation11,Citation36). Cadmium intake from tobacco was analyzed independently from dietary intake due to a high possibility of statistical errors (Citation36).
Statistical Analyses
Descriptive statistics were used to describe our analytical cohort. Cox proportional hazard models were used for the statistical analyses. All women were considered at risk of relapse or death from the time of EC diagnosis. End of follow-up was identified at death or 1 February 2019, whichever occurred first.
We estimated crude and adjusted HRs by tertiles of daily dietary cadmium intake, based on the distribution among the cohort members, using the lowest tertile as the reference group. We also evaluated predefined, specified, individual characteristics as potential effect modifiers: educational level (<high school; high school or equal; >high school), tobacco use (never; current and/or former), body mass index (<25; ≥25), and leisure time physical activity (MET score, continuous). Effect modifications were examined by including the terms into the model, and they were all tested by the Wald test. Statistical analyses were performed with IBM SPSS Statistics for Macintosh, Version 25.0 (IBM Corp., Armonk, NY, USA) in August 2019.
Results
In this retrospective cohort study of women with EC from the Stockholm region, we estimated average daily dietary cadmium intake among 416 women with EC ().
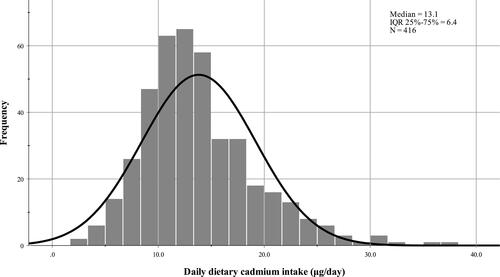
Median daily dietary cadmium intake in the cohort was 13.1 μg/day (interquartile range [IQR] 25%-75%=6.4) (). At the same time, median weekly cadmium intake was 1.3 μg/kg of body weight. The dominant source of dietary cadmium intake was cereal products and vegetables (75.3%).
Baseline characteristics of the full cohort by tertiles of median daily dietary cadmium intake (μg/day) are summarized in . Median age and body mass index at the moment of diagnosis was 67.0 years and 26.0 kg/m2, respectively. The most common tumor type was early-stage diploid endometroid carcinoma.
Table 1. Baseline characteristics of the full cohort by tertiles of median daily dietary cadmium (Cd) intake (μg/day).
Median daily cadmium intake from tobacco was similar between current and former smokers/snuff users ().
Table 2. Daily cadmium (Cd) intake from tobacco (mg/day).
In the entire cohort, median follow-up was 8.5 years. Using the Cox proportional hazard model, we observed that the daily dietary cadmium intake (μg/day) was significantly associated with decreased OS (HR = 0.956, 95% CI = 0.914-1.001, p = 0.05) (). Physical activity decreased probability of death (p < 0.0001).
Figure 3. Progression free survival (A and C) and overall survival (B and D) in the whole cohort (A and B) and in each tertile of median daily dietary cadmium (Cd) intake (μg/day).
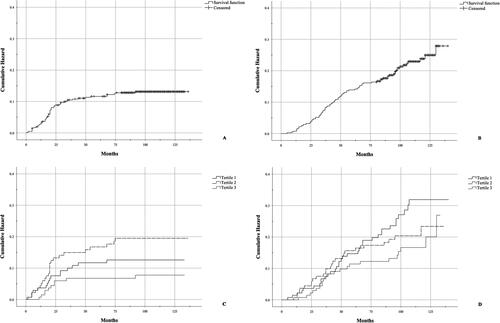
Daily dietary cadmium intake was not associated with PFS (HR = 0.975, 95% CI = 0.924-1.028, p = 0.348). However, the frequency of PFS events differed by tertile of median daily dietary cadmium intake: 12.9%, 18.1%, and 7.9%. The middle tertile had a significantly higher number of the PFS events when compared to tertiles 1 and 3 combined (HR = 1.804, 95% CI = 1.056-3.079, p = 0.031). PFS was decreased among women with type 2 EC (p < 0.0001) and more advanced-stage disease (FIGO stage III and IV) (p < 0.0001). Combining former and current smokers was not significantly associated with both PFS and OS (HR = 0.020, 95% CI = 0.571-1.823, p = 0.945; HR = 0.218, 95% CI = 0.503-1.286, p = 0.363).
When combined into a Cox proportional hazard model, we observed no significant input from the potential effect modifiers (educational level, tobacco use, body mass index, and leisure time physical activity) by tertiles of daily dietary cadmium intake ().
Table 3. HR of EC by different baseline characteristics among the PFS events and OS events according to daily dietary cadmium exposure.
Discussion
In the current study, we found that high median daily dietary cadmium intake significantly decreases OS in women with EC. However, daily dietary cadmium intake is not associated with PFS. To the best of our knowledge, our study is the first to report on the effects of cadmium on EC prognosis and outcome. The median daily dietary cadmium intake in our cohort of women with EC was 13.1 μg/day. Julin et al. reported similar values (14 μg/day) among women in the same region of Sweden (Citation10). Meanwhile, the median weekly cadmium intake was 1.3 μg/kg of body weight, which is consistent with results obtained by the Swedish National Food Agency in 2015 (Citation9).
The Panel on Contaminants in the Food Chain stated that a tolerable weekly cadmium intake is 2.5 μg/kg of body weight (Citation37). This suggests that the patients in our cohort benefited from a high level of consumer protection. Moreover, due to Swedish regulations, Swedish women overall do not belong to a vulnerable group regarding cadmium exposure (Citation38). Therefore, the results of our current study could be applicable to countries with similar strict regulations. However, the role of cadmium in the prognosis and outcome of hormone-related tumors like EC in polluted areas with high levels of cadmium exposure has yet to be described, and additional experimental and epidemiological studies in the respective areas are needed.
The dominant source of dietary cadmium intake was from cereal products and vegetables, which is in agreement with statements from the European Food Safety Authority (Citation37). High cadmium intake is generally related to a healthier diet, since the main source of cadmium is vegetables and cereals. Nevertheless, we found that high median daily dietary cadmium intake increased the risk of death from EC. And, it would be interesting to explore further the association between dietary cadmium intake and OS in women with EC, especially in a cadmium-polluted area.
We also observed that women with type 2 EC and advanced-stage disease had a worse prognosis. In contrast, vigorous physical activity was associated with a better outcome. This is consistent with previous studies that have reported the benefits of regular physical activity in patients with cancer (Citation39,Citation40).
Although our study is the first to investigate the effects of cadmium on PFS and OS in women with EC, previous studies have examined the relationship between cadmium intake and the risk of developing EC.
For example, a meta-analysis of eight studies, which included a study by Akesson et al., examined the association between dietary cadmium intake and overall cancer risk and showed no significant correlation (Citation41). At the same time, subgroup analyses including study design, geographic location, and type of cancer showed a positive association between dietary cadmium intake and cancer risk in studies from high-income countries, especially with hormone-related cancers such as EC. Another prospective study examined the connection between dietary cadmium intake and epithelial ovarian cancer risk over almost 19 years and found no significant correlation (Citation42). One possible reason for this result may be that estrogen is not an aetiologic factor in ovarian cancer, but it may play a significant role in EC (Citation43–45).
Our study has several strengths, such as the large number of women in the study sample, the use of individual clinical and pathological profiles, and the use of a self-created, detailed food-cadmium database that used the most up-to-date sources. The number of EC cases (n = 416) is considered to be high due to the specific study design. All women included in the study had a well-documented family history of EC and completed the detailed food frequency questionnaire. Another strength of the present study was the long-term follow-up (median follow-up was 102.0 mo,).
The retrospective design of the study may lead to misclassification. Moreover, the individual daily dietary cadmium intake was estimated by formula. Therefore, errors due to self-reported dietary intake cannot be excluded. Furthermore, our study used no biomarkers from blood or urine to validation the assessment of cadmium intake during the long study period. Today, it is recommended to check levels of the metal in blood and urine (Citation46,Citation47), and Julin et al. (Citation48) validated the relationship between dietary cadmium intake and cadmium concentrations in urine and blood.
In summary, the present study found that high median daily dietary cadmium intake could be associated with poor in women with EC.
Author Contributions
ZR, EÖ and MM conceived and designed the study. ZR, EÖ and MM did the data curation. ZR, IG and EÖ conducted the data analysis and drafted the initial manuscript. MM helped with results interpretation and gave critical comments for the manuscript. MM did the project administration and secured funding for data collection. All authors contributed to the final version of the manuscript.
Ethics Approval and Consent to Participate
All patients were given a written description of the study and completed an informed consent. Full anonymity of questionnaire information was guaranteed, as per the consent letter. To minimize integrity violations, we unidentified the patients after data collection. The ethical review board in Stockholm (Regionala etikprövningsnämnden i Stockholm) approved this project (dr nr 2006/649 and dr nr 2010/1536-31/2).
Acknowledgments
We like to acknowledge the Institute of Environmental Medicine at KI for the questionnaire, the Swedish company EDB for scanning the raw data and generating Microsoft Excel tables, and Elisabeth Berg for helpful advice on statistics. Also, we thank senior chemist Barbro Kollander from the National Food Agency for provided reports on cadmium concentrations in different food items. Moreover, we are grateful to Agneta Åkesson, Bettina Julin, and Alicja Wolk for raw data from their study and helpful advice.
Availability of Data and Materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Disclosure Statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
References
- Lortet-Tieulent J, Ferlay J, Bray F, Jemal A. International patterns and trends in endometrial cancer incidence, 1978-2013. J Natl Cancer Inst. 2018;110(4):354–61. doi:https://doi.org/10.1093/jnci/djx214
- Bergman O, Fredholm L, Hont G, Johansson E, Ljungman P, Munck-Wikland E, Nahi H, Zedenius J. Cancer i siffror 2018. Utgiven av Socialstyrelsen och Cancerfonden (2018). Available online: https://www.socialstyrelsen.se/globalassets/sharepoint-dokument/artikelkatalog/statistik/2018-6-10.pdf
- National Cancer Institute. Endometrial cancer treatment Physician Data Query (PDQ). 2019. Available online: http://www.cancer.gov/cancertopics/pdq/treatment/endometrial/healthprofessional (accessed on 15 October 2019).
- Lee NK, Cheung MK, Shin JY, Husain A, Teng NN, et al. Prognostic factors for uterine cancer in reproductive-aged women. Obstet Gynecol. 2007;109:655–62. doi:https://doi.org/10.1097/01.aog.0000255980.88205.15
- Colombo N, Creutzberg C, Amant F, Bosse T, González-Martín A, Ledermann J, Marth C, Nout R, Querleu D, Mirza MR, ESMO-ESGO-ESTRO Endometrial Consensus Conference Working Group, et al. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: diagnosis, treatment and follow-up. Ann Oncol. 2016;27(1):16–41. doi:https://doi.org/10.1093/annonc/mdv484
- Safe S. Cadmium's disguise dupes the estrogen receptor. Nat Med. 2003;9(8):1000–1. doi:https://doi.org/10.1038/nm0803-1000
- Scientific Opinion of the Panel on Contaminants in the Food Chain on a request from the European Commission on cadmium in food EFSA. The EFSA Journal. 2009;980:1–139.
- Kemikalieinspektionen. Fördjupad utvärdering av Giftfri miljö 2019. 2019. Available online: https://www.kemi.se/download/18.60cca3b41708a8aecdbc324b/1587049628882/rapport-2-19-fordjupad-utvardering-av-giftfri-miljo-2019.pdf
- Livsmedelsverket. Swedish Market Basket Survey 2015 – per capita-based analysis of nutrients and toxic compounds in market baskets and assessment of benefit or risk. Rapport 26. 2017. Available online: https://www.livsmedelsverket.se/globalassets/publikationsdatabas/rapporter/2017/swedish-market-basket-survey-2015-livsmedelsverkets-rapportserie-nr-26-20172.pdf
- Julin B. Dietary cadmium exposure and the risk of hormone-related cancers. Stockholm, Sweden: Karolinska Institutet; 2012.
- Song M-A, Marian C, Brasky TM, Reisinger S, Djordjevic M, Shields PG. Chemical and toxicological characteristics of conventional and low-TSNA moist snuff tobacco products. Toxicol Lett. 2016;245:68–77. doi:https://doi.org/10.1016/j.toxlet.2016.01.012
- Bárány E, Bergdahl IA, Bratteby L-E, Lundh T, Samuelson G, Skerfving S, Oskarsson A. Iron status influences trace element levels in human blood and serum. Environ Res. 2005;98(2):215–23. doi:https://doi.org/10.1016/j.envres.2004.09.010
- Akesson A, Berglund M, Schütz A, Bjellerup P, Bremme K, Vahter M. Cadmium exposure in pregnancy and lactation in relation to iron status. Am J Public Health. 2002;92(2):284–7. doi:https://doi.org/10.2105/ajph.92.2.284
- Jarup L, Akesson A. Current status of cadmium as an environmental health problem. Toxicol Appl Pharmacol. 2009;238:201–8. doi:https://doi.org/10.1016/j.taap.2009.04.020
- Engström A, Michaëlsson K, Suwazono Y, Wolk A, Vahter M, Åkesson A. Long-term cadmium exposure and the association with bone mineral density and fractures in a population-based study among women. J Bone Miner Res. 2011;26(3):486–95. doi:https://doi.org/10.1002/jbmr.224
- Jin YH, Clark AB, Slebos RJC, Al-Refai H, Taylor JA, Kunkel TA, Resnick MA, Gordenin DA. Cadmium is a mutagen that acts by inhibiting mismatch repair. Nat Genet. 2003;34(3):326–9. doi:https://doi.org/10.1038/ng1172
- Slebos RJC, Li M, Evjen AN, Coffa J, Shyr Y, Yarbrough WG. Mutagenic effect of cadmium on tetranucleotide repeats in human cells. Mutat Res. 2006;602(1–2):92–9. doi:https://doi.org/10.1016/j.mrfmmm.2006.08.003
- Brama M, Gnessi L, Basciani S, Cerulli N, Politi L, Spera G, Mariani S, Cherubini S, Scotto d'Abusco A, Scandurra R, et al. Cadmium induces mitogenic signaling in breast cancer cell by an ERalpha-dependent mechanism. Mol Cell Endocrinol. 2007;264(1–2):102–8. doi:https://doi.org/10.1016/j.mce.2006.10.013
- Johnson MD, Kenney N, Stoica A, Hilakivi-Clarke L, Singh B, Chepko G, Clarke R, Sholler PF, Lirio AA, Foss C, et al. Cadmium mimics the in vivo effects of estrogen in the uterus and mammary gland. Nat Med. 2003;9(8):1081–4. doi:https://doi.org/10.1038/nm902
- Stevenson MJ, Uyeda KS, Harder NHO, Heffern MC. Metal-dependent hormone function: the emerging interdisciplinary field of metalloendocrinology. Metallomics. 2019;11(1):85–110. doi:https://doi.org/10.1039/C8MT00221E
- Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, O'Brien WL, Bassett DR, Schmitz KH, Emplaincourt PO, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9 Suppl):S498–S504. doi:https://doi.org/10.1097/00005768-200009001-00009
- Friberg E, Mantzoros CS, Wolk A. Physical activity and risk of endometrial cancer: a population-based prospective cohort study. Cancer Epidemiol Biomarkers Prev. 2006;15(11):2136–40. doi:https://doi.org/10.1158/1055-9965.EPI-06-0465
- Terry P, Vainio H, Wolk A, Weiderpass E. Dietary factors in relation to endometrial cancer: a nationwide case-control study in Sweden. Nutr Cancer. 2002;42(1):25–32. doi:https://doi.org/10.1207/s15327914nc421_4
- Kurman RJ, C ML, Herrington CS, Young RH. WHO classification of tumours of female reproductive organs. Lyon: IARC; 2014.
- Swedish National Food Agency (Livsmedelsverket). Link: https://www.livsmedelsverket.se (accessed on 25 December 2019).
- Ingrid Nordlander, Bitte Aspenström-Fagerlund, Anders Glynn, Anna Törnkvist, Tatiana Cantillana, Karin Neil Persson, Frida Broman, Livsmedelsverket och Kinfe Girma, Jordbruksverket. Kontroll av restsubstanser i levande djur och animaliska livsmedel - Resultat 2014. Rapport 12. 2015. Available online: https://www.livsmedelsverket.se/globalassets/publikationsdatabas/rapporter/2015/kontroll-av-restsubstanser-i-levande-djur-och-animaliska-livsmedel---resultat-2014.pdf
- Kajsa Gustavsson, Ingrid Nordlander, Bitte Aspenström-Fagerlund, Anders Glynn, Ingrid Nilsson, Anna Törnkvist, Lina Thebo, Karin Neil Persson, Livsmedelsverket, Eva Persson, Läkemedelsverket och Kinfe Girma, Jordbruksverket. Kontroll av restsubstanser i levande djur och animaliska livsmedel - Resultat 2011. Rapport 9. 2012. Available online: https://www.livsmedelsverket.se/globalassets/publikationsdatabas/rapporter/2012/2012_livsmedelsverket_9_restsubstansrapport_2011.pdf
- Lars Jorhem, Christina Åstrand, Birgitta Sundström, Joakim Engman, Barbro Kollande. Fisk och skaldjur, metaller i livsmedel - fyra decenniers analyser. Rapport 25. 2014. Available online: https://www.livsmedelsverket.se/globalassets/publikationsdatabas/rapporter/2014/fisk-och-skaldjur-metaller-i-livsmedel---fyra-decenniers-analyser-rapport-25-2014
- Lars Jorhem, Christina Åstrand, Birgitta Sundström, Joakim Engman, Barbro Kollander. Spannmål, nötter och fröer, metaller i livsmedel - fyra decenniers analyser. Rapport 1. 2015. Available online: https://www.livsmedelsverket.se/globalassets/publikationsdatabas/rapporter/2015/metaller-i-livsmedel---fyra-decenniers-analyser-av-spannmal-notter-och-froerrapport-1-2015.pdf
- Lars Jorhem, Christina Åstrand, Birgitta Sundström, Joakim Engman, Barbro Kollander. Frukt, bär, grönsaker och svamp, metaller i livsmedel - fyra decenniers analyser. Rapport 10. 2016. Available online: https://www.livsmedelsverket.se/globalassets/publikationsdatabas/rapporter/2016/metaller-i-livsmedel---fyra-decenniers-analyser-av-frukt-bar-gronsaker-och-svamp_rapport_10_2016.pdf
- Lars Jorhem, Christina Åstrand, Birgitta Sundström, Joakim Engman, Barbro Kollander. Kött, chark, mejeri och drycker, metaller i livsmedel - fyra decenniers analyser. Rapport 28. 2017. Available online: https://www.livsmedelsverket.se/globalassets/publikationsdatabas/rapporter/2017/metaller-i-kott-chark-mejerivaror-och-drycker-fyra-decenniers-analyser-rapportserie-nr-28-2017.pdf
- Monika Pearson, Joakim Engman, Bodil Rundberg, Anna von Malmborg, Sören Wretling, Veronica Öhrvik. Grönsaker och rotfrukter - analys av näringsämnen. Rapport 10. 2013. Available online: https://www.livsmedelsverket.se/globalassets/publikationsdatabas/rapporter/2013/2013_livsmedelsverket_10_gronsaker_och_rotfrukter_analys_av_naringsamnen.pdf
- Veronica Öhrvik, Joakim Engman, Rasmus Grönholm, Anders Staffas, Hanna Sara Sandler, Anna von Malmborg. Grönsaker och rotfrukter - analys av näringsämnen. Rapport 3. 2016. Available online: https://www.livsmedelsverket.se/globalassets/publikationsdatabas/rapporter/2016/gronsaker-svamp-och-frukt---analys-av-naringsamnen---rapport-3_2016.pdf
- Veronica Öhrvik, Joakim Engman, Rasmus Grönholm, Anders Staffas, Hanna Sara Strandler, Anna von Malmborg. Drycker - analys av näringsämnen. Rapport 20. 2015. Available online: https://www.livsmedelsverket.se/globalassets/publikationsdatabas/rapporter/2015/drycker---analys-av-naringsamnen---rapport-20_2015.pdf
- Larsen EHA, N L, Moller A, Petersen A, Mortensen GK, Petersen J. Monitoring the content and intake of trace elements from food in Denmark. Food Addit Contam. 2002;19(1):33–46. doi:https://doi.org/10.1080/02652030110087447
- He P, Lu Y, Liang Y, Chen B, Wu M, Li S, He G, Jin T. Exposure assessment of dietary cadmium: findings from Shanghainese over 40 years, China. BMC Public Health. 2013;13(1):590. doi:https://doi.org/10.1186/1471-2458-13-590
- European Food Safety Authority; Cadmium dietary exposure in the European population. EFSA Journal 2012;10(1):2551. [37 pp.] doi:https://doi.org/10.2903/j.efsa.2012.2551. Available online: www.efsa.europa.eu/efsajournal
- Kemikalieinspektionen. Kadmiumhalten måste minska - för folkhälsans skull. Rapport 1/11. 2011. Available online: https://www.kemi.se/download/18.6df1d3df171c243fb23a990c/1591454113135/rapport-1-11.pdf
- Stout NL, Baima J, Swisher AK, Winters-Stone KM, Welsh J. A systematic review of exercise systematic reviews in the cancer literature (2005-2017). Pm R. 2017;9(9S2):S347–s384. doi:https://doi.org/10.1016/j.pmrj.2017.07.074
- Kohler LN, Garcia DO, Harris RB, Oren E, Roe DJ, Jacobs ET. Adherence to diet and physical activity cancer prevention guidelines and cancer outcomes: A systematic review. Cancer Epidemiol Biomarkers Prev. 2016;25(7):1018–28. doi:https://doi.org/10.1158/1055-9965.Epi-16-0121
- Akesson A, Julin B, Wolk A. Long-term dietary cadmium intake and postmenopausal endometrial cancer incidence: a population-based prospective cohort study. Cancer Res. 2008;68(15):6435–41. doi:https://doi.org/10.1158/0008-5472.CAN-08-0329
- Julin B, Wolk A, Akesson A. Akesson A: Dietary cadmium exposure and risk of epithelial ovarian cancer in a prospective cohort of Swedish women. Br J Cancer. 2011;105(3):441–4. doi:https://doi.org/10.1038/bjc.2011.238
- Risch HA. Hormonal etiology of epithelial ovarian cancer, with a hypothesis concerning the role of androgens and progesterone. J Natl Cancer Inst. 1998;90(23):1774–86. doi:https://doi.org/10.1093/jnci/90.23.1774
- Lukanova A, Lundin E, Akhmedkhanov A, Micheli A, Rinaldi S, Zeleniuch-Jacquotte A, Lenner P, Muti P, Biessy C, Krogh V, et al. Circulating levels of sex steroid hormones and risk of ovarian cancer. Int J Cancer. 2003;104(5):636–42. doi:https://doi.org/10.1002/ijc.10990
- Lukanova A, Lundin E, Micheli A, Arslan A, Ferrari P, Rinaldi S, Krogh V, Lenner P, Shore RE, Biessy C, et al. Circulating levels of sex steroid hormones and risk of endometrial cancer in postmenopausal women. Int J Cancer. 2004;108(3):425–32. doi:https://doi.org/10.1002/ijc.11529
- Olsson I-M, Bensryd I, Lundh T, Ottosson H, Skerfving S, Oskarsson A. Cadmium in blood and urine-impact of sex, age, dietary intake, iron status, and former smoking-association of renal effects. Environ Health Perspect. 2002;110(12):1185–90. doi:https://doi.org/10.1289/ehp.021101185
- Larsson SC, Orsini N, Wolk A. Urinary cadmium concentration and risk of breast cancer: a systematic review and dose-response meta-analysis. Am J Epidemiol. 2015;182(5):375–80. doi:https://doi.org/10.1093/aje/kwv085
- Julin B, Vahter M, Amzal B, Wolk A, Berglund M, Åkesson A. Relation between dietary cadmium intake and biomarkers of cadmium exposure in premenopausal women accounting for body iron stores. Environ Health. 2011;10:105. doi:https://doi.org/10.1186/1476-069X-10-105