Abstract
Background
Despite professional recommendations malnutrition is not adequately addressed in cancer patients. Here, we explored whether nutritional status (NS) is associated with HRQoL in men with metastatic castrate-resistant prostate cancer (mCRPC). Methods: Men with mCRPC enrolled into this prospective observational study were allocated to one of the four NS categories based on clinical, laboratory, and patient self-reported criteria: well-nourished (WN), nutritional risk without criteria for cachexia/sarcopenia (NR), sarcopenia, and cachexia. The HRQoL was evaluated by the Functional Assessment of Cancer Therapy-Prostate (FACT-P) questionnaire. Association between NS and self-reported HRQoL was sought by the linear regression model, which was adjusted for known prognostic variables and body mass index. Results: Over the period of two years, 141 patients were enrolled. Their median age was 74.1 years (IQR 68.6–79.4 years) and majority of them were minimally symptomatic. Fifty-nine patients (41.8%) were WN, followed by 24 (17%), 42 (29.8%), and 16 (11.4%) patients with NR, sarcopenia, and cachexia, respectively. As compared to WN patients, all three other NS categories were significant negative predictors of HRQoL (P < 0.04). Conclusions: Abnormal NS is highly prevalent in men with mCRPC and is negatively associated with their HRQoL, which supports the recommendation for management of malnutrition in these patients.
Introduction
Prostate cancer is the most common neoplasm in men with a mortality rate of 10.1 per 100,000 European Union citizens in 2018 (Citation1). Prostate cancer patients, especially those with advanced disease may be at high risk for cancer-related malnutrition because both cancer and its treatment can threaten their nutritional status (NS) (Citation2). Moreover, they often have various comorbid conditions related to their age which can further increase risk for the development of malnutrition. The cornerstone of treatment of advanced prostate cancer is long-term androgen deprivation therapy (ADT), which has various adverse effects on body, including alterations in body composition with a gain in fat mass and loss of skeletal muscle mass (i.e. sarcopenia) (Citation3–6). In castrate-resistant disease, cancer grows and spreads despite castrate level of testosterone (i.e., metastatic castrate-resistant prostate cancer (mCRPC)) (Citation7). As increased body weight is linked to an increased risk for recurrence and mortality in men with prostate cancer, urologists and oncologists are usually primarily focused on high body mass index (BMI) and weight loss management (Citation8). However, there is a growing concern that nutritional disorders, mainly malnutrition, occur among overweight and obese cancer patients (Citation9).
Advanced prostate cancer usually has a prolonged disease course of several years, during which malnutrition can develop gradually and insidiously. Malnutrition can lead to serious consequences, including adverse impact on health and survival and added healthcare costs in patients with cancer (Citation10–12). Recently, the European Society for Clinical Nutrition and Metabolism (ESPEN) recommended three key steps to update nutritional care for cancer patients: i) the screening for nutritional risk, ii) expansion of nutrition-related assessments to include evaluation of food intake, body composition, inflammatory biomarkers, resting energy expenditure, and physical function, and iii) use of multimodal therapeutic interventions, including individually adapted strategy for increased nutritional intake, measures for lessening inflammation, and hyper-metabolic stress, as well as increasing physical activity (Citation13). However, patients with cancer who are at risk for malnutrition may not receive nutritional support for various reasons. Cancer-related malnutrition may not be recognized or its severity might be underestimated when recognized by health professionals (Citation10, Citation14, Citation15). Furthermore, patients and their relatives may underestimate the importance of malnutrition and decline nutritional intervention recommended by health professionals (Citation16). Further evidence that malnutrition has substantial impact on clinically relevant outcomes such as length of survival or its quality might help to improve the implementation of nutritional care in the care of patients with cancer, particularly in specific sub-populations of patients such as men with mCRPC.
In this prospective observational cohort study, we aimed to assess the association between professionally assessed NS and health-related quality of life (HRQoL) in patients with early mCRPC. We hypothesized that poor NS is independently associated with worse HRQoL in these patients.
Patients and Methods
Study Design
We conducted a prospective observational cohort study to seek the association between NS and HRQoL in patients with early mCRPC (i.e., before they received any potentially effective systemic therapy for mCRPC such as novel antiandrogens [abiraterone acetate or enzalutamide] and/or chemotherapy). All consecutive patients with early mCRPC who were referred from urology or radiation oncology services to medical oncologists at the Institute of Oncology Ljubljana between July 2016 and July 2018 were prospectively assessed for the eligibility for participation in this trial. We excluded patients with the following limitations/conditions: i) cognitive decline disabling to fill the self-reported questionnaires, ii) Eastern Cooperative Oncology Group (ECOG) performance status (PS) ≥3, iii) nutritional counseling in the last six months, iv) inserted heart device precluding bioelectrical impedance analysis (BIA), and v) unwillingness to participate in the study. All participating patients provided a written informed consent. This study, coded as MZ 0120-272/2016-3, received the approval of the institutional review board and National Ethics Review Board (on May 31st, 2016) and is in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
Assessment of the NS
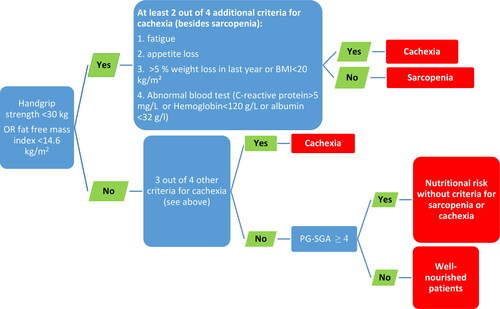
The BMI was calculated according to the established formula (Citation17). Categories of the NS were determined on the basis of the following assessments and criteria: i) handgrip strength assessed by the handgrip dynamometry, ii) patient generated-subjective global assessment (PG-SGA) questionnaire, and iii) Evans’ criteria for cachexia (>5% weight loss in the last year or BMI <20 kg/m2, handgrip strength <30 kg, fatigue, appetite loss, abnormal blood test: C-reactive protein (CRP) >5 mg/L, or hemoglobin (Hb) <120 g/L, or albumin <32 g/L) (Citation18, Citation19). The Evans’ criteria were used for the assessment as they better predict survival as compared to Fearon’s cancer cachexia criteria (Citation20). Four categories of NS were defined: well-nourished (WN), nutritional risk without criteria for cachexia/sarcopenia (NR), sarcopenia, and cachexia. The allocation process was followed according to the algorithm shown in .
Sarcopenia was determined on the basis of functional performance assessed by the handgrip dynamometry (cutoff 30 kg) or fat-free mass index (FFMI cutoff 14.6 kg/m2) (Citation19,Citation21) (). Every patient was encouraged to squeeze the handle of the Jamar hand-held hydraulic dynamometer as hard as possible with his dominant arm. As previously described, two tests were performed (Citation22). Then, we assessed body composition by the BIA device and calculated FFMI (fat-free mass/height squared). Performing the test, we followed the procedure described previously; wiping the dorsal part of a foot and hand then posing electrodes. For standardization, a patient had to have an empty bladder, since water could profoundly disturb the measure of BIA (Citation23, Citation24). If a patient was not sarcopenic (i.e., normal handgrip and FFMI) nor cachectic, but still had an abnormal result from the PG-SGA (≥4 points) he was allocated to the NR. The PG-SGA is a scored nutritional assessment tool, developed specifically for cancer patients and could differentiate well-nourished patients from patients at nutritional risk (Citation25). This tool includes BMI, weight changes, symptoms related to food intake, functional capacity and its difference, some comorbidities and physical examination of muscle/fat deficit, and the presence of edema (Citation22, Citation26). As previously described, patients with a score of ≥4 are considered to be at higher nutritional risk (Citation26). Patients with normal handgrip muscle strength, without Evans` criteria for cachexia and PG-SGA score ≤3 were allocated into the WN. Finally, on the basis of calculated BMI, the distribution of overweight patients across four NS categories was assessed.
Assessment of the HRQoL
The HRQoL measurements Licensor FACIT Organization provided us with the grant permission for the application of the validated HRQoL questionnaire (Slovenian version) Functional Assessment of Cancer Treatment (FACT-P). It consists of generic cancer HRQoL and 12-item prostate cancer subscale; generic cancer subscales evaluate physical, social/family, emotional, and functional aspects of wellbeing; prostate-specific subscale considers issues such as sexuality, bowel and bladder function as well as pain (Citation27). The score ranges from 0 to 156 points; the higher scores reflect better HRQoL (Citation28).
Statistical Analysis
With the power of 0.8 and an estimated difference in the FACT-P score of five points between consecutive groups of NS and predicted standard deviation (SD) of 20 points, a sample size of at least 100 patients was required. Data are presented as medians and interquartile ranges (IQR) or frequencies where appropriate. Linear assumptions such as linearity, homoscedasticity, independence, and normality of residuals were assessed. In the case of missing data in the FACT-P questionnaire, we followed the instructions provided on the FACIT Organization website.
In the first part of the analysis, an association between BMI and HRQoL was sought. Linear regression model was adjusted for possible confounders: i) duration of previous ADT, ii) Charlson index of comorbidity, iii) pain according to the visual analogue scale (VAS), iv) age, v) laboratory measures (Hb and prostate-specific antigen [PSA]), and vi) the presence of visceral metastases. Selected cofounders are patient- and disease-related and can affect outcome of patients with advanced prostate cancer. In the second part of the analysis, an association between NS categories and HRQoL was sought. Confounders described above and BMI were included in the multiple linear regression model. The proportion of explained variation (R2) was adjusted for the number of covariates. P values of <0.05 were deemed statistically significant, and 95% confidence interval (CI) and percentage of explained variability for significant results were provided when appropriate. No adjustments for multiple comparisons were made.
Results
Patients’ Characteristics
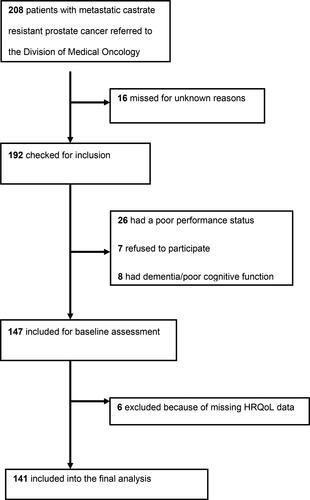
Over the period of two years 208 men with mCRPC were assessed for the participation in the study. Overall, 45 patients were ineligible to participate in the study. No patient received nutritional counseling before the presentation to a medical oncologist. One hundred and forty-one patients were included into the final analysis (). The median age of patients was 74.1 years (IQR 68.6–79.4 years) and 18 (12.8%) patients had visceral metastases. Similarly, other characteristics of patients were also in line with the presentation of early mCRPC ().
Table 1. Patients’ characteristics.
Assessment of NS and HRQoL
Median BMI was 26.9 kg/m2 (IQR 24.5–30.0 kg/m2). The median handgrip strength and FFMI were 31.75 kg (IQR 22.68–38.56 kg) and 18.5 kg/m2 (IQR 17.2–20.1 kg/m2), respectively (). Fifty-nine (41.8%) patients were WN, followed by 24 (17%), 42 (29.8%), and 16 (11.4%) patients with NR, sarcopenia, and cachexia, respectively. Among 42 patients with sarcopenia, 41 (98%) had handgrip strength <30 kg and only one patient had FFMI <14.6 kg/m2. Obese patients (i.e., BMI >30 kg/m2) were identified across all NS categories: 18 (30.5%) among WN, followed by 4 (16.7%), 8 (19%), and 2 (12.5%) with NR, sarcopenia, and cachexia, respectively. The proportion of men with BMI ≥25 kg/m2 was 77.3% in groups with WN and NR and 79.3% in groups with sarcopenia and cachexia (). The median P-FACT score was 103.5 (IQR 86.8 − 124.2).
Table 2. Distribution of BMI across NS categories.
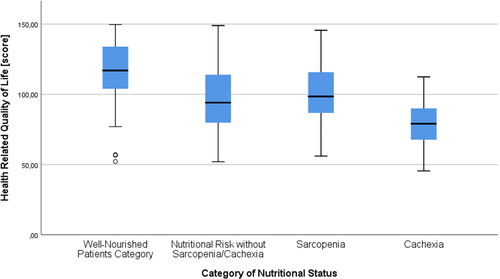
In patients who were WN or had NR, sarcopenia, and cachexia median FACT-P scores were 117 (IQR 103.72 − 134), 94 (IQR 76.92 − 115), 98.47 (IQR 86.47 − 116.02), and 79.1 (IQR 67.34 − 90.25), respectively (). Ten (7%) patients filled in the FACT-P and PG-SGA questionnaires with the help of relatives or investigators.
Association between BMI and HRQoL
In the simple linear regression model, there was a significant positive correlation between BMI and HRQoL score (β = 1.46, 95% CI [0.51–2.42], P = 0.002, R2 = 6.8%). After adjustments – which did not include NS categories – the correlation between BMI and HRQoL remained significant (β = 0.99, 95% CI [0.14–1.84], P = 0.01). Beside BMI significant positive and negative predictors of HRQoL in the multiple regression model were Hb and pain, respectively (R2 = 32.4%) ().
Table 3. A multiple linear regression model for HRQoL without NS categories.
Association between NS Categories and HRQoL
For all three abnormal NS categories there was a significant negative association with HRQoL when compared with WN category; β = −18.3, 95% CI [−28.9 to −7.6] for NR vs. WN; β = −17.5 95% CI [−26.4 to −8.6] for sarcopenia vs. WN; and β = −36.5, 95% CI [−48.9 to − 24.2] for cachexia vs. WN; P < 0.001; R2 = 23.2% (). After adjustments, which also included BMI, significant negative predictors of HRQoL were abnormal NS categories and pain (R2 = 36.3%) (). As FACT-P questionnaire includes four questions related to the pain an exploratory analysis with the omission of questions related to the pain in the FACT-P questionnaire and its re-scoring was conducted to test the robustness of results of our final model. In the exploratory analysis NS and pain remained significant negative predictors of the HRQoL ().
Table 4. Multivariable regression model for HRQoL with NS categories.
Discussion
Both the length of survival and quality of life are important for patients with advanced cancer. Here, we show that abnormal NS is highly prevalent and inversely associated with HRQoL in men with early mCRPC before they receive a potentially effective therapy for their advanced and incurable disease. The NS determined by the multidisciplinary professional assessment is better predictor of HRQoL than BMI in these patients.
In our study, we identified overweight and obese patients within all four NS categories; 79.3% of patients with sarcopenia and cachexia were overweight or obese (). Similarly, advanced muscle wasting was present in 34% of patients with advanced renal cell carcinoma who had BMI greater than 25 kg/m2 and sarcopenic obesity in 27% of patients with mCRPC (Citation29, Citation30). According to our definition of sarcopenia, which also included assessment of muscle strength almost 30% of all patients had sarcopenia and 19% of these were obese (). Our definition of sarcopenia is in line with the current broad European consensus which beside the low quantity and quality of muscle mass highlights the importance of low muscle strength for the definition of sarcopenia (Citation31). Sarcopenic obesity is independently associated with higher mortality and a higher rate of complications during systemic and surgical cancer treatment across multiple cancer sites and treatment plans (Citation32, Citation33). However, currently there is a lack of consensus on the definition of sarcopenic obesity and clear diagnostic criteria for sarcopenic obesity with wide clinical applicability and clear cutoff values (Citation34).
It has been hypothesized that patients with sarcopenia are generally unfit and unable to tolerate stress when they receive antineoplastic therapy (Citation32). While prognostic effect of the BMI is controversial, sarcopenia is clearly associated with poor tolerability of chemotherapy with docetaxel and detrimental effect on the outcome of men with mCRPC (Citation35, Citation36). Modern anticancer targeted agents can also cause skeletal muscle wasting and low muscle mass is a risk factor for toxicity of these small-molecule targeted agents (Citation29, Citation37) Furthermore, sarcopenia and cachexia are associated with increased clearance of novel immunotherapeutic agents such as checkpoint inhibitors (e.g., pembrolizumab) and poorer survival of cancer patients treated with immunotherapy (Citation38, Citation39). As NS has an impact on tolerability of anticancer treatment and new anticancer drugs start to open new avenues of treatment of men with mCRPC nutritional support should be an essential part of management of these patients in everyday clinical practice (Citation40). Also, nutritional assessment and nutritional intervention could be an integral part of large randomized clinical trials which evaluate new anticancer therapies to study the mutual interplay between malnutrition and safety and efficacy of new anticancer drugs in advanced prostate cancer. Of note, although anabolic steroid analogs of testosterone such as nandrolone and oxandrolone may have some nutritional benefits in cancer patients they should not be used in patients with prostate cancer as they are agonists of the androgen receptor and my promote cancer growth (Citation13).
We showed that poor NS is highly prevalent and unfavorably associated with HRQoL before any potentially effective systemic treatment is administered in patients with mCRPC. Furthermore, the association between NS determined at enrollment and HRQoL assessed six months after enrollment was also significant in our cohort of patients (Citation41). This suggests that introduction and short-term treatment with a potentially effective anticancer therapy (mostly novel antiandrogens) does not mitigate the unfavorable association between NS and HRQoL. The association between NS and HRQoL has previously been studied in other settings of prostate cancer but not in mCRPC (Citation4). The cohort of men with mCRPC enrolled in our study was homogeneous and NS was assessed prospectively by well-established clinical, laboratory, and patient self-reported criteria. There is no doubt that cancer-related malnutrition is prognostic for survival in cancer patients and that nutritional intervention improves nutritional outcomes, however, currently there is a lack of robust evidence from randomized controlled trials (RCTs) that nutritional intervention improves clinically relevant endpoints such as HRQoL, tolerability of treatment and overall survival (Citation42). Results of our study are in line with the current ESPEN recommendations of nutritional support in patients with cancer, however a need for well-designed RCTs to investigate the impact of nutritional intervention on clinically relevant endpoints in cancer remains (Citation13, Citation43). Importantly, men with advanced prostate cancer should have access to the nutritional evaluation and support early in the course of their disease to decrease the risk of complications related to malnutrition in later stages of their disease (i.e., mCRPC), when they usually receive more toxic treatments. Unfortunately, access to the nutrition services which would meet long-term needs in prostate cancer patients may be limited worldwide (Citation44).
Malnutrition and muscle loss in cancer patients is multifactorial and may occur not only due to the inadequate food intake and catabolic metabolic derangements but also due to the decreased physical activity. Therefore, nutritional care should always be accompanied by the exercise training when feasible (Citation13). This is particularly important for men with advanced prostate cancer who often suffer not only from ADT-related sarcopenia but also osteoporosis and consequently bone fractures. Different types of exercise, which can also be adjusted to the deteriorating elderly patients may increase muscle mass and mineral bone density (Citation45). Relatively brief exposure to exercise significantly enhances muscle mass, strength, physical function, and balance in hypogonadal men with prostate cancer as compared to the regular care (Citation46). Also, exercise is an important adjunct to the antiresorptive therapy in men with prostate cancer who receive ADT (Citation47). Men with advanced prostate cancer should have access to special supportive care programs, which would offer comprehensive health lifestyle services, including nutritional support and exercise programs.
Our study has several limitations. First, our results might be different if an alternative validated questionnaire for the assessment of HRQoL were used. Also, the FACT-P questionnaire was explicitly developed for comparison of the HRQoL between interventional and control arm within randomized clinical trials and not for prospective cohort studies such as ours. As only 7% of patients filled in the FACT-P and PG-SGA questionnaires with the assistance of relatives or the investigator it is very unlikely that this introduced substantial bias into our analysis. Secondly, due to the absence of a broad and unequivocal multifactorial definition of NS in nutritional science society, one might define the categories of the NS differently, which could lead to different conclusions. However, criteria for the allocation of men into the NS categories were defined a priori in our study which reduces a risk for potential allocation bias. Thirdly, approximately one quarter of all patients with mCRPC were not eligible for nutritional assessment in this study and therefore less selective exclusion criteria should be considered in similar studies in the future. Finally, our findings may not be applicable to patients with other types of cancer.
In conclusion, according to the results of our study almost 60% of patients with mCRPC have abnormal NS, which is negatively associated with their HRQoL. Professional nutritional assessment and support should be widely available for men with advanced prostate cancer within specific supportive care programs.
Author Contribution Statement
All authors have made a significant contribution to a journal article and share responsibility and accountability for the results. All authors have read and agreed to the published version of the manuscript.
Supplemental Material
Download PDF (71.3 KB)Acknowledgments
Authors wish to thank data manager Minka Macanovic as well as dieticians Denis Mlakar Mastnak and Eva Justin for help with conducting the study.
Disclosure Statement
The authors declare no conflict of interest.
Additional information
Funding
References
- Malvezzi M, Carioli G, Bertuccio P, Boffetta P, Levi F, La Vecchia C, Negri E. European cancer mortality predictions for the year 2018 with focus on colorectal cancer. Ann Oncol. 2018;29(4):1016–1022. doi:https://doi.org/10.1093/annonc/mdy033
- Cushen SJ, Power DG, McDermot R, O'Sullivan K, Maceneaney P, Daly L, Ryan AM. Body composition as a predictor of chemotherapy toxicity in patients with metastatic prostate cancer treated with docetaxel (abstr). Proc Nutr Soc. 2015;74(OCE2):E165. doi:https://doi.org/10.1017/S0029665115001834
- van Londen GJ, Levy ME, Perera S, Nelson JB, Greenspan SL. Body composition changes during androgen deprivation therapy for prostate cancer: a 2-year prospective study. Crit Rev Oncol Hematol. 2008;68(2):172–177. doi:https://doi.org/10.1016/j.critrevonc.2008.06.006
- Østergren PB, Kistorp C, Bennedbaek FN, Faber J, Sønksen J, Fode M. The use of exercise interventions to overcome adverse effects of androgen deprivation therapy. Nat Rev Urol. 2016;13(6):353–364. doi:https://doi.org/10.1038/nrurol.2016.67
- Smith MR, Finkelstein JS, McGovern FJ, Zietman AL, Fallon MA, Schoenfeld DA, Kantoff PW. Changes in body composition during androgen deprivation therapy for prostate cancer. J Clin Endocrinol Metab. 2002;87(2):599–603. doi:https://doi.org/10.1210/jcem.87.2.8299
- Alibhai SMH, Breunis H, Timilshina N, Naglie G, Tannock I, Krahn M, Warde P, Fleshner NE, Canning SD, Tomlinson G, et al. Long-term impact of androgen-deprivation therapy on physical function and quality of life. Cancer. 2015;121(14):2350–2357. doi:https://doi.org/10.1002/cncr.29355
- Parker C, Gillessen S, Heidenreich A, Horwich A, Committee EG. Cancer of the prostate: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26:v69–v77. doi:https://doi.org/10.1093/annonc/mdv222
- Cao Y, Ma J. Body mass index, prostate cancer-specific mortality, and biochemical recurrence: a systematic review and meta-analysis. Cancer Prev Res. 2011;4(4):486–501. doi:https://doi.org/10.1158/1940-6207.CAPR-10-0229
- Martin L, Birdsell L, MacDonald N, Reiman T, Clandinin MT, McCargar LJ, Murphy R, Ghosh S, Sawyer MB, Baracos VE, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. JCO. 2013;31(12):1539–1547. doi:https://doi.org/10.1200/JCO.2012.45.2722
- Planas M, Álvarez-Hernández J, León-Sanz M, Celaya-Pérez S, Araujo K, García de Lorenzo A. Prevalence of hospital malnutrition in cancer patients: a sub-analysis of the PREDyCES® study. Support Care Cancer. 2016;24(1):429–435. doi:https://doi.org/10.1007/s00520-015-2813-7
- Pressoir M, Desné S, Berchery D, Rossignol G, Poiree B, Meslier M, Traversier S, Vittot M, Simon M, Gekiere JP, et al. Prevalence, risk factors and clinical implications of malnutrition in French Comprehensive Cancer Centres. Br J Cancer. 2010;102(6):966–971. doi:https://doi.org/10.1038/sj.bjc.6605578
- Gellrich N-C, Handschel J, Holtmann H, Krüskemper G. Oral cancer malnutrition impacts weight and quality of life. Nutrients. 2015;7(4):2145–2160. doi:https://doi.org/10.3390/nu7042145
- Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, Bozzetti F, Fearon K, Hütterer E, Isenring E, Kaasa S, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr. 2017;36(1):11–48. doi:https://doi.org/10.1016/j.clnu.2016.07.015
- Hébuterne X, Lemarié E, Michallet M, de Montreuil CB, Schneider SM, Goldwasser F. Prevalence of malnutrition and current use of nutrition support in patients with cancer. JPEN J Parenter Enteral Nutr. 2014;38(2):196–204. doi:https://doi.org/10.1177/0148607113502674
- Attar A, Malka D, Sabaté JM, Bonnetain F, Lecomte T, Aparicio T, Locher C, Laharie D, Ezenfis J, Taieb J, et al. Malnutrition is high and underestimated during chemotherapy in gastrointestinal cancer: an AGEO prospective cross-sectional multicenter study. Nutr Cancer. 2012;64(4):535–542. doi:https://doi.org/10.1080/01635581.2012.670743
- Gyan E, Raynard B, Durand J-P, Lacau Saint Guily J, Gouy S, Movschin ML, Khemissa F, Flori N, Oziel-Taieb S, Bannier Braticevic C, et al. Malnutrition in patients with cancer: comparison of perceptions by patients, relatives, and physicians-results of the NutriCancer2012 Study. JPEN J Parenter Enteral Nutr. 2017;42:260. doi:https://doi.org/10.1177/0148607116688881
- Barao K, Forones NM. Body mass index: different nutritional status according to WHO, OPAS and Lipschitz classifications in gastrointestinal cancer patients. Arq Gastroenterol. 2012;49(2):169–71. doi:https://doi.org/10.1590/S0004-28032012000200013
- Evans WJ, Morley JE, Argilés J, Bales C, Baracos V, Guttridge D, Jatoi A, Kalantar-Zadeh K, Lochs H, Mantovani G, et al. Cachexia: a new definition. Clin Nutr. 2008;27(6):793–799. doi:https://doi.org/10.1016/j.clnu.2008.06.013
- Cederholm T, Barazzoni R, Austin P, Ballmer P, Biolo G, Bischoff SC, Compher C, Correia I, Higashiguchi T, Holst M, et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin Nutr. 2017;36(1):49–64. doi:https://doi.org/10.1016/j.clnu.2016.09.004
- Vanhoutte G, van de Wiel M, Wouters K, Sels M, Bartolomeeussen L, De Keersmaecker S, Verschueren C, De Vroey V, De Wilde A, Smits E, et al. Cachexia in cancer: what is in the definition? BMJ Open Gastroenterol. 2016;3(1):e000097. doi:https://doi.org/10.1136/bmjgast-2016-000097
- Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12(5):489–495. doi:https://doi.org/10.1016/S1470-2045(10)70218-7
- Reijnierse EM, de Jong N, Trappenburg MC, Blauw GJ, Butler-Browne G, Gapeyeva H, Hogrel J-Y, McPhee JS, Narici MV, Sipilä S, et al. Assessment of maximal handgrip strength: how many attempts are needed? J Cachexia Sarcopenia Muscle. 2017;8(3):466–474. doi:https://doi.org/10.1002/jcsm.12181
- Grundmann O, Yoon SL, Williams JJ. The value of bioelectrical impedance analysis and phase angle in the evaluation of malnutrition and quality of life in cancer patients – a comprehensive review. Eur J Clin Nutr. 2015;69(12):1290–1297. doi:https://doi.org/10.1038/ejcn.2015.126
- Barbosa-Silva MC, Barros AJ, Wang J, Heymsfield SB, Pierson RN. Bioelectrical impedance analysis: population reference values for phase angle by age and sex. Am J Clin Nutr. 2005;82(1):49–52. doi:https://doi.org/10.1093/ajcn/82.1.49
- Bauer J, Capra S. Comparison of a malnutrition screening tool with subjective global assessment in hospitalised patients with cancer – sensitivity and specificity. Asia Pac J Clin Nutr. 2003;12:257–260.
- Citak E, Tulek Z, Uzel O. Nutritional status in patients with head and neck cancer undergoing radiotherapy: a longitudinal study. Support Care Cancer. 2019;27(1):239–247. doi:https://doi.org/10.1007/s00520-018-4319-6
- Cella D, Nichol MB, Eton D, Nelson JB, Mulani P. Estimating clinically meaningful changes for the Functional Assessment of Cancer Therapy-Prostate: results from a clinical trial of patients with metastatic hormone-refractory prostate cancer . Value Health. 2009;12(1):124–129. doi:https://doi.org/10.1111/j.1524-4733.2008.00409.x
- Cella D, Petrylak DP, Fishman M, Teigland C, Young J, Mulani P. Role of quality of life in men with metastatic hormone-refractory prostate cancer: how does atrasentan influence quality of life? Eur Urol. 2006;49(5):781–789. doi:https://doi.org/10.1016/j.eururo.2005.12.058
- Antoun S, Birdsell L, Sawyer MB, Venner P, Escudier B, Baracos VE. Association of skeletal muscle wasting with treatment with sorafenib in patients with advanced renal cell carcinoma: results from a placebo-controlled study. JCO. 2010;28(6):1054–1060. doi:https://doi.org/10.1200/JCO.2009.24.9730
- Cushen SJ, Power DG, Murphy KP, McDermott R, Griffin BT, Lim M, Daly L, MacEneaney P, O' Sullivan K, Prado CM, et al. Impact of body composition parameters on clinical outcomes in patients with metastatic castrate-resistant prostate cancer treated with docetaxel. Clin Nutr ESPEN. 2016;13:e39–e45. doi:https://doi.org/10.1016/j.clnesp.2016.04.001
- Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31. doi:https://doi.org/10.1093/ageing/afy169
- Baracos VE, Arribas L. Sarcopenic obesity: hidden muscle wasting and its impact for survival and complications of cancer therapy. Ann Oncol. 2018;29(suppl_2):ii1–ii9. doi:https://doi.org/10.1093/annonc/mdx810
- Laviano A, Rossi Fanelli F. Nutritional status is a predictor of outcome in cancer patients, irrespective of stage. Intern Emerg Med. 2017;12(1):135–136. doi:https://doi.org/10.1007/s11739-016-1539-y
- Donini LM, Busetto L, Bauer JM, Bischoff S, Boirie Y, Cederholm T, Cruz-Jentoft AJ, Dicker D, Frühbeck G, Giustina A, et al. Critical appraisal of definitions and diagnostic criteria for sarcopenic obesity based on a systematic review. Clin Nutr. 2020;39(8):2368–2388. doi:https://doi.org/10.1016/j.clnu.2019.11.024
- Armstrong AJ, Halabi S, de Wit R, Tannock IF, Eisenberger M. The relationship of body mass index and serum testosterone with disease outcomes in men with castration-resistant metastatic prostate cancer. Prostate Cancer Prostatic Dis. 2009;12(1):88–93. doi:https://doi.org/10.1038/pcan.2008.36
- Ohtaka A, Aoki H, Nagata M, Kanayama M, Shimizu F, Ide H, Tsujimura A, Horie S. Sarcopenia is a poor prognostic factor of castration-resistant prostate cancer treated with docetaxel therapy. Prostate Int. 2019;7(1):9–14. doi:https://doi.org/10.1016/j.prnil.2018.04.002
- Massicotte M-H, Borget I, Broutin S, Baracos VE, Leboulleux S, Baudin E, Paci A, Deroussent A, Schlumberger M, Antoun S, et al. Body composition variation and impact of low skeletal muscle mass in patients with advanced medullary thyroid carcinoma treated with vandetanib: results from a placebo-controlled study. J Clin Endocrinol Metab. 2013;98(6):2401–2408. doi:https://doi.org/10.1210/jc.2013-1115
- Turner DC, Kondic AG, Anderson KM, Robinson AG, Garon EB, Riess JW, Jain L, Mayawala K, Kang J, Ebbinghaus SW, et al. Pembrolizumab exposure–response assessments challenged by association of cancer cachexia and catabolic clearance. Clin Cancer Res. 2018;24(23):5841–5849. doi:https://doi.org/10.1158/1078-0432.CCR-18-0415
- Roch B, Coffy A, Jean-Baptiste S, Palaysi E, Daures J-P, Pujol J-L, Bommart S. Cachexia – sarcopenia as a determinant of disease control rate and survival in non-small lung cancer patients receiving immune-checkpoint inhibitors. Lung Cancer. 2020;143:19–26. doi:https://doi.org/10.1016/j.lungcan.2020.03.003
- Yap TA, Zivi A, Omlin A, de Bono JS. The changing therapeutic landscape of castration-resistant prostate cancer. Nat Rev Clin Oncol. 2011;8(10):597–610. doi:https://doi.org/10.1038/nrclinonc.2011.117
- Cavka L, Pohar Perme M, Zakotnik B, Rotovnik Kozjek N, Seruga B. 676P prognostic role of nutritional status (NS) for health-related quality of life (HRQoL) in men with advanced prostate cancer (abstr). Abstract Book of the ESMO Virtual Congress 2020. Ann Oncol. 2020;31:S540. doi:https://doi.org/10.1016/j.annonc.2020.08.935
- de van der Schueren MAE, Laviano A, Blanchard H, Jourdan M, Arends J, Baracos VE. Systematic review and meta-analysis of the evidence for oral nutritional intervention on nutritional and clinical outcomes during chemo(radio)therapy: current evidence and guidance for design of future trials. Ann Oncol. 2018;29(5):1141–1153. doi:https://doi.org/10.1093/annonc/mdy114
- Arends J, Baracos V, Bertz H, Bozzetti F, Calder PC, Deutz NEP, Erickson N, Laviano A, Lisanti MP, Lobo DN, et al. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin Nutr. 2017;36(5):1187–1196. doi:https://doi.org/10.1016/j.clnu.2017.06.017
- McLaughlin K, Hedden L, Pollock P, Higano C, Murphy RA. Assessing the nutritional needs of men with prostate cancer. Nutr J. 2019;18(1):81. doi:https://doi.org/10.1186/s12937-019-0506-7
- Benedetti MG, Furlini G, Zati A, Letizia Mauro G. The effectiveness of physical exercise on bone density in osteoporotic patients. Biomed Res Int. 2018;2018:1–10. doi:https://doi.org/10.1155/2018/4840531
- Galvão DA, Taaffe DR, Spry N, Joseph D, Newton RU. Combined resistance and aerobic exercise program reverses muscle loss in men undergoing androgen suppression therapy for prostate cancer without bone metastases: a randomized controlled trial. JCO. 2010;28(2):340–347. doi:https://doi.org/10.1200/JCO.2009.23.2488
- Joseph JS, Lam V, Patel MI. Preventing osteoporosis in men taking androgen deprivation therapy for prostate cancer: a systematic review and meta-analysis. Eur Urol Oncol. 2019;2(5):551–561. doi:https://doi.org/10.1016/j.euo.2018.11.001