Abstract
Background
A meta-analysis was conducted to investigate the correlation between calcium intake and the risk of brain tumors (especially glioma).
Methods
The PubMed, Web of Science, and Embase databases were searched for relevant papers on the association between calcium intake and glioma as of August 22, 2021. The odds ratio (OR) with a 95% confidence interval (CI) was calculated using a random-effects model. Egger’s test was conducted to assess publication bias.
Results
The meta-analysis includes four studies. The meta-analysis showed that calcium intake and the risk of brain tumors have a significant negative relationship (OR = 0.28; 95% CI: 0.11 to 0.72; P = 0.008). Dose-response analysis showed that for every 100 mg/day increase in calcium intake, the risk of glioma decreased by 7% (OR = 0.93; 95% CI: 0.88 to 0.98). In addition, compared with humans without calcium intake, when calcium intake is 455 mg/day, 800 mg/day and 1000 mg/day, the risk of glioma is 0.65 (95% CI 0.43, 0.97), 0.55 (95% CI 0.37, 0.82) and 0.37 (95% CI 0.15, 0.86).
Conclusion
There is a significant negative association between calcium intake and brain tumors (especially gliomas), but more high-quality studies are needed to verify these results.
Introduction
Glioma and meningioma are the two most prevalent forms of primary central nervous system tumors, accounting for more than 80% of all cases (Citation1). Glioma is a tumor that originates from brain glial cells and is the most common in primary brain tumors (Citation2). Gliomas have a reasonably high incidence of 4–5/100,000 persons each year, with the highest occurrence in the sixth decade of life (Citation3, Citation4). Aside from ionizing radiation and some genetic abnormalities, the risk factors for brain cancer remain unknown. Furthermore, other potential risk factors include exposure to chemical carcinogens in the environment and exposure to n-nitroso compounds in dietary factors (Citation5–7). Despite the low frequency of adult brain cancer, the prognosis for brain cancer (particularly glioma) is dismal (Citation8). Therefore, preventing the progression of glioma has become an important strategy to prevent and treat glioma.
Studies have shown that some dietary components may have a role in the etiology of glioma (Citation9–11). Dietary intake such as intake of fruits and vegetables, vitamins, tea and coffee, and poultry and eggs may affect the development of glioma (Citation9, Citation12–16). It is reported that calcium intake has a significant negative correlation with the risk of glioma, and the risk of glioma decreases significantly with the increase of calcium intake (Citation17). However, Tedeschi-Blok et al. explored the association between male and female calcium intake and glioma, and the results were not statistically significant (Citation18). Therefore, it is necessary to quantitatively assess the association between calcium intake and the risk of brain tumors (especially glioma) through meta-analysis and explore the impact of changes in intake doses on risk.
Methods
The meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines (Citation19) ().
Table 1. Characteristics of the included studies.
Search Strategy
A systematic search of the literature was conducted in PubMed, Embase, and Web of Science to identify studies that assess calcium intake and the risk of glioma. The last search date was August 22, 2021, with no language restrictions. Search strategies were developed using a variety of text words and indexed terms linked to "calcium", "glioma", "meningioma", "brain neoplasms", "brain cancer", "case control studies" and "cohort studies". Besides, manual searches were performed on paper-based documents, and relevant reviews and references included in the literature were screened to obtain more studies that can be used for meta-analysis.
Study Selection
The studies that are included must fulfill the following criteria: (Citation1) The study was an observational study such as a controlled study and a cohort study; (Citation2) Patients with brain tumors diagnosed by neurological or histological examinations; (Citation3) The study reported the association between calcium intake and the risk of brain tumors.
The exclusion criteria were as follows: (Citation1) Non-authoritative study such as reviews, conference abstract, and case reports; (Citation2) Lack of sufficient data to obtain OR/RR and 95% CI; (Citation3) For repeated publication studies, only the one with the complete research data will be included.
Data Exaction
Two investigators independently completed the literature screening based on the above study selection. Following the selection of the papers to be included in the study, the data was extracted independently using the pre-designed table. The following information was gleaned from the included studies: first author, year of publication, research type, research area and time, basic characteristics of the research object (sample size, age, gender, etc.), group of calcium intake, and study outcome. Case-control and cohort studies were evaluated using the Newcastle-Ottawa Scale (NOS) (Citation20). Scores of 7-9 points, 4-6 points and < 4 points were considered high-quality research, medium-quality and low-quality research, respectively. Any inconsistencies in the results were resolved through investigator consensus.
Statistical Analysis
All analyses were conducted with STATA 12.0 (College Station, Texas: STATA Corp LP). The Odd ratio (OR) and 95% CI was used as the effect size index. Cochran’s Q test and I2 statistic were used to assess the heterogeneity of the included studies. Significant heterogeneity among the studies was indicated by P < 0.05 or I2 > 50%, and the random-effects model was used for meta-analysis. Otherwise, a fixed model was used for analysis. A linear dose-response analysis was performed according to the method proposed by Greenland and Orsini et al. to examine the association between calcium intake and the risk of brain tumors (Citation21, Citation22). This method requires sample size and number of cases of brain tumors, OR and 95% CI of at least three quantitative exposure categories.
The Egger’s test was used to assess publication bias. A sensitivity analysis was performed by eliminating one study at a time to examine the robustness of the pooled results.
Results
Selection and Characteristics of Studies
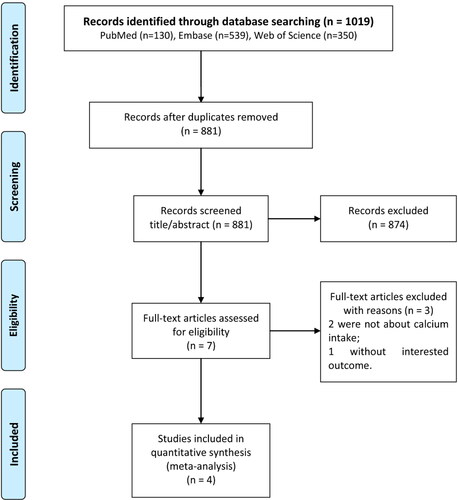
A total of 1019 records were retrieved through the preliminary search strategy. 881 articles remain after removing duplicates. 679 irrelevant studies that did not meet the inclusion criteria were removed by reading the titles and abstracts. Finally, four studies were included in the meta-analysis, involving 1942 patients (Citation17, Citation18, Citation23, Citation24). The study selection flow chart is shown in .
Of the four studies included in the final analysis, one was conducted in China, one was conducted in the United States and two were conducted in Iran (). These studies were published between 2001 and 2021, with sample sizes ranging from 384 to 787. The research subjects of Hu et al. included patients with glioma and meningioma (Citation24), and the other three studies only included patients with glioma (Citation17, Citation18, Citation23). These three studies involving patients with gliomas all show that calcium intake is significantly negatively correlated with the incidence of gliomas. The study of Hu et al. found that Calcium was inversely related to brain cancer risk, but it had no significant significance (Citation24). Besides, Three studies were divided into four categories according to the level of calcium intake and reported their respective OR and 95% CI.
The quality evaluation results are shown in . We ascertained that three studies (Citation17, Citation23, Citation24) had low risk of bias and one study had a moderate risk of bias (Citation18). The scores of the included studies are 6-8 points, and the methodological quality is good.
Table 2. Quality assessment (the Newcastle-Ottawa Scale) of the included studies.
Meta-Analysis
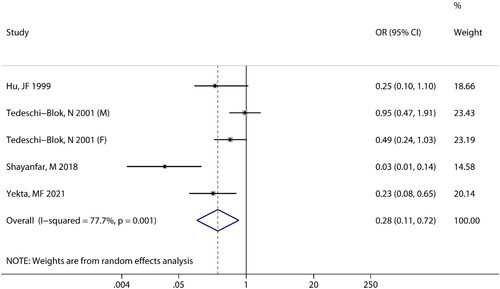
Four studies reported an association between calcium intake and the risk of brain tumors. There was heterogeneity between the two groups (I2 = 77.7%, P = 0.001), and a random-effects model was used. It was evident from that the combined effect size was (OR =0.28; 95% CI: 0.11 to 0.72; P = 0.008), which indicated that there is a significant negative correlation between calcium intake and the risk of brain tumors.
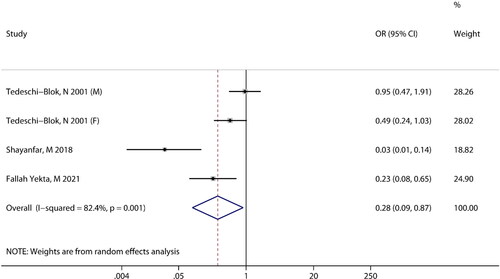
Three studies reported an association between calcium intake and the risk of glioma. Heterogeneity was observed among the included studies (I2 = 82.4%, P = 0.001), the random-effects model was used for meta-analysis. The pooled results revealed that there is a significant negative association between calcium intake and the risk of glioma (OR =0.28; 95% CI: 0.09 to 0.87; P = 0.028) ().
Dose-Response Analysis
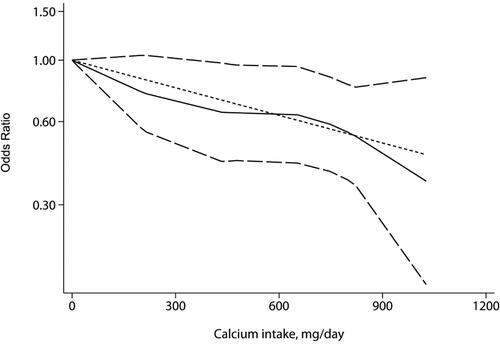
Three studies were analyzed for dose–response relationships. A linear relationship between calcium intake and risk of glioma was observed (, non-linear P = 0.672). For every 100 mg/day increased in calcium intake, the risk of glioma decreased by 7% (OR = 0.93; 95% CI: 0.88 to 0.98; P = 0.002). However, the results of non-linear fitting showed that the risk of gliomas decreased with the increase of the dose when the calcium intake was 0-433 mg/day, and the results were not statistically significant (P > 0.05). Besides, compared with humans without calcium intake, when calcium intake was 455 mg/day, 800 mg/day and 1000 mg/day, the risk of glioma was reduced by 35%, 45% and 63%, respectively (455 mg/day: OR = 0.65; 95% CI: 0.43 to 0.97; 800 mg/day: OR = 0.55; 95% CI: 0.37 to 0.82; 1000 mg/day: OR = 0.37; 95% CI: 0.15 to 0.86).
Publication Bias and Sensitive Analysis
The result of Egger’s test was P = 0.017, suggesting that there is a significant publication bias among the included studies. However, the sensitivity analysis results showed that excluding any one study did not have a major impact on the results, indicating that the combined results are stable.
Discussion
Our meta-analysis found that there is a significant negative correlation between calcium intake and the risk of brain tumors/gliomas. The dose-response meta-analysis showed that for every 100 mg/day increase in calcium intake, the risk of glioma decreased by 7%. To our knowledge, this is the first meta-analysis to assess the dose-response relationship between calcium intake and the risk of glioma.
Despite their rarity, gliomas are linked with significant morbidity and deaths in the adult population (Citation25). Glioma patients have a low chance of survival, with projected 5-year survival rates of 30% for males and 30% for women (Citation26). Therefore, it is important to identify the underlying factors that predict the risk of glioma. As an important dietary factor in the human diet, calcium has been reported to be related to many cancers, including breast cancer, prostate cancer, ovarian cancer and colorectal cancer (Citation27–32). However, there are currently few studies studying the relationship between calcium intake and the risk of glioma. Our meta-analysis found a significant negative association between calcium intake and the risk of brain tumors/gliomas. In addition, Tedeschi-Blok et al. found that the inverse association of calcium intake and glioma is confined to women, and this association was not observed in men (Citation18). This may be related to the level of estradiol, circulating estradiol might directly stimulate intestinal absorption of calcium. The specific reasons need more in-depth research and further exploration.
There are many explanations for the mechanism by which calcium reduces the risk of glioma. This may be related to the involvement of calcium in cell apoptosis. Juin et al. discovered that glutamate-induced apoptosis in cerebellar granule neurons is characterized by intracellular calcium increase (Citation33). Intracellular calcium may affect DNA repair through calmodulin (Citation34). Besides, higher levels of calcium will lead to down-regulation of parathyroid hormone production (Citation35). Parathyroid hormone has been considered as a tumor promoter and can act as a mitogen and anti-apoptotic factor. De Miguel et al. detected parathyroid hormone-related protein in human astrocytomas and proposed that the parathyroid hormonerelated protein, via the parathyroid hormone receptor, is associated with astrocytoma cell proliferation and dedifferentiation (Citation36). Our results support the evidence that calcium intake is negatively correlated with the risk of brain tumors, but the relationship between calcium intake and astrocytoma risk and serum parathyroid hormone levels needs further research to explore.
In fact, it is generally believed that there may be a u-shaped relationship between calcium intake and health status (Citation37). It has been reported that a high calcium intake (particularly via supplements) is linked to an elevated risk of cardiovascular disease and fracture (Citation38–41). The results of the dose-response analysis of our study showed that calcium intake has always been negatively correlated with the risk of gliomas when the intake is less than 1000 mg/day. However, considering the adverse effects of high calcium intake on health, humans should pay attention to the maximum daily calcium intake in diet and dietary supplements.
There are some limitations in the meta-analysis. First, due to the small number of included studies, it is impossible to explore the source of heterogeneity through quantitative methods such as subgroup analysis and meta-regression. Secondly, the included studies are retrospective cohort studies. Although the studies have carried out multivariate analysis to adjust for the influence of confounding factors on the results, the potential confounding factors adjusted in each study are different, which may affect the results to a certain extent. Third, the number of included literature is limited. Although significant results have been obtained, the 95% CI has a large range and low statistical accuracy. Therefore, more and larger sample studies are needed to verify the results.
Conclusion
In conclusion, this meta-analysis shows that calcium intake is significantly negatively associated with brain tumors (especially gliomas). More and better-designed prospective cohort studies are needed to explore the association between calcium intake and glioma risk.
Supplemental Material
Download PDF (75.5 KB)Disclosure Statement
No potential conflict of interest was reported by the authors.
Funding
The author(s) reported there is no funding associated with the work featured in this article.
References
- Barnholtz-Sloan JS, Ostrom QT, Cote D. Epidemiology of brain tumors. Neurol Clin. 2018;36(3):395–419. doi:10.1016/j.ncl.2018.04.001
- Ostrom QT, Gittleman H, Truitt G, Boscia A, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2011-2015. Neuro Oncol. 2018;20(suppl_4):iv1–iv86. doi:10.1093/neuonc/noy131
- Porter KR, McCarthy BJ, Freels S, Kim Y, Davis FG. Prevalence estimates for primary brain tumors in the United States by age, gender, behavior, and histology. Neuro Oncol. 2010;12(6):520–7. doi:10.1093/neuonc/nop066
- Stupp R, Brada M, van den Bent MJ, Tonn JC, Pentheroudakis G. High-grade glioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(Suppl 3):iii93–101. doi:10.1093/annonc/mdu050
- Bulnes S, Murueta-Goyena A, Lafuente JV. Differential exposure to N-ethyl N-nitrosourea during pregnancy is relevant to the induction of glioma and PNSTs in the brain. Neurotoxicol Teratol. 2021;86:106998. doi:10.1016/j.ntt.2021.106998
- Dubrow R, Darefsky AS, Park Y, Mayne ST, Moore SC, Kilfoy B, Cross AJ, Sinha R, Hollenbeck AR, Schatzkin A, et al. Dietary components related to N-nitroso compound formation: a prospective study of adult glioma. Cancer Epidemiol Biomarkers Prev. 2010;19(7):1709–22. doi:10.1158/1055-9965.Epi-10-0225
- Vienne-Jumeau A, Tafani C, Ricard D. Environmental risk factors of primary brain tumors: a review. Rev Neurol (Paris). 2019;175(10):664–78. doi:10.1016/j.neurol.2019.08.004
- Bellail AC, Hunter SB, Brat DJ, Tan C, Van Meir EG. Microregional extracellular matrix heterogeneity in brain modulates glioma cell invasion. Int J Biochem Cell Biol. 2004;36(6):1046–69. doi:10.1016/j.biocel.2004.01.013
- Lv W, Zhong X, Xu L, Han W. Association between dietary vitamin A intake and the risk of glioma: evidence from a meta-analysis. Nutrients. 2015;7(11):8897–904. doi:10.3390/nu7115438
- Noorlag L, De Vos FY, Kok A, Broekman MLD, Seute T, Robe PA, Snijders TJ. Treatment of malignant gliomas with ketogenic or caloric restricted diets: a systematic review of preclinical and early clinical studies. Clin Nutr. 2019;38(5):1986–94. doi:10.1016/j.clnu.2018.10.024
- Xie L, Mo M, Jia H-X, Liang F, Yuan J, Zhu J. Association between dietary nitrate and nitrite intake and sitespecific cancer risk: evidence from observational studies. Oncotarget. 2016;7(35):56915–32. doi:10.18632/oncotarget.10917
- Sheweita SA, Sheikh BY. Can dietary antioxidants reduce the incidence of brain tumors? Curr Drug Metab. 2011;12(6):587–93. doi:10.2174/138920011795713733
- Huncharek M, Kupelnick B, Wheeler L. Dietary cured meat and the risk of adult glioma: a meta-analysis of nine observational studies. J Environ Pathol Toxicol Oncol. 2003;22(2):129–37. doi:10.1615/jenvpathtoxoncol.v22.i2.60
- Li Y. Association between fruit and vegetable intake and risk for glioma: a meta-analysis. Nutrition. 2014;30(11–12):1272–8. doi:10.1016/j.nut.2014.03.027
- Zhou S, Wang X, Tan Y, Qiu L, Fang H, Li W. Association between vitamin C intake and glioma risk: evidence from a meta-analysis. Neuroepidemiology. 2015;44(1):39–44. doi:10.1159/000369814
- Song Y, Wang Z, Jin Y, Guo J. Association between tea and coffee consumption and brain cancer risk: an updated meta-analysis. World J Surg Oncol. 2019;17(1):51. doi:10.1186/s12957-019-1591-y
- Fallah Yekta M, Soltani S, Shayanfar M, Benisi-Kohansal S, Mohammad-Shirazi M, Sharifi G, Esmaillzadeh A. A case-control study on dietary calcium intake and risk of glioma. Eur J Cancer Prev. 2021;30(4):322–7. doi:10.1097/CEJ.0000000000000629
- Tedeschi-Blok N, Schwartzbaum J, Lee M, Miike R, Wrensch M. Dietary calcium consumption and astrocytic glioma: the San Francisco Bay Area Adult Glioma Study, 1991-1995. Nutr Cancer. 2001;39(2):196–203. doi:10.1207/S15327914nc392_6
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi:10.1136/bmj.n71
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5. doi:10.1007/s10654-010-9491-z
- Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135(11):1301–9. doi:10.1093/oxfordjournals.aje.a116237
- Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol. 2012;175(1):66–73. doi:10.1093/aje/kwr265
- Shayanfar M, Vahid F, Faghfoori Z, Davoodi SH, Goodarzi R. The Association between Index of Nutritional Quality (INQ) and glioma and evaluation of nutrient intakes of these patients: a case-control study. Nutr Cancer. 2018;70(2):213–20. doi:10.1080/01635581.2018.1412469
- Hu J, La Vecchia C, Negri E, Chatenoud L, Bosetti C, Jia X, Liu R, Huang G, Bi D, Wang C, et al. Diet and brain cancer in adults: a case-control study in northeast China. Int J Cancer. 1999;81(1):20–3. doi:10.1002/(sici)1097-0215(19990331)81:1 < 20::aid-ijc4 > 3.0.co;2-2
- Ghouzlani A, Kandoussi S, Tall M, Reddy KP, Rafii S, Badou A. Immune checkpoint inhibitors in human glioma microenvironment. Front Immunol. 2021;12:679425. doi:10.3389/fimmu.2021.679425
- Holick CN, Giovannucci EL, Rosner B, Stampfer MJ, Michaud DS. Prospective study of intake of fruit, vegetables, and carotenoids and the risk of adult glioma. Am J Clin Nutr. 2007;85(3):877–86. doi:10.1093/ajcn/85.3.877
- Hidayat K, Chen G-C, Zhang R, Du X, Zou S-Y, Shi B-M, Qin L-Q. Calcium intake and breast cancer risk: meta-analysis of prospective cohort studies. Br J Nutr. 2016;116(1):158–66. doi:10.1017/S0007114516001768
- Chen P, Hu P, Xie D, Qin Y, Wang F, Wang H. Meta-analysis of vitamin D, calcium and the prevention of breast cancer. Breast Cancer Res Treat. 2010;121(2):469–77. doi:10.1007/s10549-009-0593-9
- Rahmati S, Azami M, Delpisheh A, Hafezi Ahmadi MR, Sayehmiri K. Total calcium (dietary and supplementary) intake and prostate cancer: a systematic review and meta-analysis. Asian Pac J Cancer Prev. 2018;19:1449–56. doi:10.22034/APJCP.2018.19.6.1449
- Xu J, Chen K, Zhao F, Huang D, Zhang H, Fu Z, Xu J, Wu Y, Lin H, Zhou Y, et al. Association between vitamin D/calcium intake and 25-hydroxyvitamin D and risk of ovarian cancer: a dose-response relationship meta-analysis. Eur J Clin Nutr. 2021;75(3):417–29. doi:10.1038/s41430-020-00724-1
- Song X, Li Z, Ji X, Zhang D. Calcium intake and the risk of ovarian cancer: a meta-analysis. Nutrients. 2017;9(7):679. doi:10.3390/nu9070679
- Keum N, Aune D, Greenwood DC, Ju W, Giovannucci EL. Calcium intake and colorectal cancer risk: dose-response meta-analysis of prospective observational studies. Int J Cancer. 2014;135(8):1940–8. doi:10.1002/ijc.28840
- Juin P, Pelletier M, Oliver L, Tremblais K, Grégoire M, Meflah K, Vallette FM. Induction of a caspase-3-like activity by calcium in normal cytosolic extracts triggers nuclear apoptosis in a cell-free system. J Biol Chem. 1998;273(28):17559–64. doi:10.1074/jbc.273.28.17559
- Llor X, Jacoby RF, Teng BB, Davidson NO, Sitrin MD, Brasitus TA. K-ras mutations in 1,2-dimethylhydrazine-induced colonic tumors: effects of supplemental dietary calcium and vitamin D deficiency. Cancer Res. 1991;51(16):4305–9.
- McCarty MF. Parathyroid hormone may be a cancer promoter - an explanation for the decrease in cancer risk associated with ultraviolet light, calcium, and vitamin D. Med Hypotheses. 2000;54(3):475–82. doi:10.1054/mehy.1999.0880
- de Miguel F, Sarasa JL, López-Ferro O, Esbrit P. Immunohistochemical detection of parathyroid hormone-related protein in human astrocytomas. J Histochem Cytochem. 1998;46(2):277–9. doi:10.1177/002215549804600218
- Wang X, Chen H, Ouyang Y, Liu J, Zhao G, Bao W, Yan M. Dietary calcium intake and mortality risk from cardiovascular disease and all causes: a meta-analysis of prospective cohort studies. BMC Med. 2014;12(1):158. doi:10.1186/s12916-014-0158-6
- Xiao Q, Murphy RA, Houston DK, Harris TB, Chow W-H, Park Y. Dietary and supplemental calcium intake and cardiovascular disease mortality: the National Institutes of Health-AARP diet and health study. JAMA Intern Med. 2013;173(8):639–46. doi:10.1001/jamainternmed.2013.3283
- Bolland MJ, Barber PA, Doughty RN, Mason B, Horne A, Ames R, Gamble GD, Grey A, Reid IR. Vascular events in healthy older women receiving calcium supplementation: randomised controlled trial. BMJ. 2008;336(7638):262–6. doi:10.1136/bmj.39440.525752.BE
- Bischoff-Ferrari HA, Dawson-Hughes B, Baron JA, Burckhardt P, Li R, Spiegelman D, Specker B, Orav JE, Wong JB, Staehelin HB, et al. Calcium intake and hip fracture risk in men and women: a meta-analysis of prospective cohort studies and randomized controlled trials. Am J Clin Nutr. 2007;86(6):1780–90. doi:10.1093/ajcn/86.5.1780
- Zhao JG, Zeng XT, Wang J, Liu L. Association between calcium or vitamin D supplementation and fracture incidence in community-dwelling older adults: a systematic review and meta-analysis. JAMA. 2017;318(24):2466–82. doi:10.1001/jama.2017.19344