Abstract
Endometrial cancer (EC) is becoming more common worldwide, primarily due to an increase in life expectancy and obesity. As several modifiable factors may affect EC incidence and progression, we aimed to elucidate how dietary habits and daily routines influence recurrence and survival among women with EC, using a Random Survival Forest (RSF) approach. 481 women who previously underwent hysterectomy due to EC completed two extensive questionnaires on dietary habits and daily routines, and we used RSF to identify risky or protective variables. Among the 186 variables considered, consumption of sugar-sweetened beverages and fried potatoes increased the risk of EC recurrence and death, while physical activity decreased the risk of death. We conclude that RSF is a suitable approach to study survival in multivariable datasets.
Introduction
Endometrial cancer (EC) arises from the inner layer of the uterus and accounted for 400,000 cases and 90,000 deaths worldwide in 2018 (Citation1). According to the World Health Organization, more than one-third of cancer-related deaths are associated with modifiable factors (Citation2), and EC is no exception. Increasing EC rates have been observed during the last decades, especially in developed countries, and have been linked to an increased incidence of obesity, chronic hyperinsulinemia, and western dietary patterns (Citation3). Most EC cases are diagnosed at an early stage, so the prognosis is usually favorable. Indeed, EC incidence ranks 6th among all female cancers, but EC mortality ranks 14th. EC progression is driven primarily by tumor characteristics, but it may fluctuate depending on environmental factors.
Medical science can now generate large amounts of data, the analysis of which requires the consideration of many interrelated variables. Classical statistical methods are often unable to process this kind of high- and ultra-high-dimensional data. One alternative approach is machine learning, which enables the automatic creation of analytical models. A comprehensive review of machine learning approaches is beyond the scope of the current study. However, dietary patterns are a good example of a machine learning application, since nutrition is usually multifactorial and complex. Selya and Anshutz recently investigated dietary and physical patterns in relation to obesity and concluded that machine learning is superior to conventional statistics for the prediction of risks regarding health outcomes (Citation4). Chatterjee et al. applied different machine learning algorithms to publicly-available health datasets and identified risk factors that may contribute to overweight/obesity (Citation5). Attempts have also been made to create an algorithm (based on a combination of machine and deep learning) that allows researchers to control for different confounding variables, such as age, gender, presence of disease, etc. (Citation6). In the current study, we aimed to elucidate how dietary habits and daily routines influence recurrence and survival among women with EC.
Material and Methods
Study Population
We used the Karolinska University Hospital’s database (TakeCare) to identify patients referred due to EC in 2007–2012 (Citation7). All patients underwent hysterectomy followed by collection of tumor tissue samples. Pathologists examined the samples, performed immunohistochemical analyses, and summarized the results into a pathologic-anatomic diagnosis. Some patients, most commonly those with advanced disease, received adjuvant treatment following hysterectomy.
We sent identified patients an invitation letter and two questionnaires via regular post. The questionnaires covered dietary habits and daily activities, including alcohol consumption and smoking status. The questionnaires have been described previously in detail (Citation7) and can be found in the Supplements. We collected information on a total of 186 variables from the questionnaires and medical records; however, for the sake of brevity, we address only a subset of these variables in this report ().
Statistical Analysis
We used Random Survival Forest (RSF) for time-to-event (recurrence or death) analyses. RSF is an extension of Breiman’s learning method for right-censored data (Citation8). We chose this method because we had a large number of potentially cross-correlating variables, which could create issues of multiple testing and multicollinearity. RSF has been shown to be reliable in evaluating the influence of variables on outcomes in different clinical settings, including patients with malignancies (Citation9–13). It has also been applied to classify EC (Citation14, Citation15). However, to the best of our knowledge, RSF has never been used to assess survival in EC patients. RSF is an entirely data-driven method; it does not rely on the assumptions of traditional survival analyses (Citation16, Citation17). This makes it an attractive alternative, especially in complex multivariable survival datasets (Citation18). We utilized the RandomForestSRC (Citation19) and ggRandomForest packages (Citation20) for additional visualization of the data. RSF grows a number of decision trees (1000 trees in our study) for prediction purposes, and this approach was previously used successfully to assess risk factors for several diseases (Citation10, Citation11, Citation13). Once a model is built, two primary measurements, variable importance (VIMP) and minimal depth, can be used to determine the informative value of each individual variable. The final score is calculated by averaging the scores of all trees. VIMP evaluates the change in prediction accuracy if a variable is excluded from the model: the highest-ranked variable is defined as the one that reduces the predictive power of the model the most. Minimal depth ranks the variables in terms of the proximity of the first split in nodes to the root node. Since VIMP and minimal depth are calculated differently, we ranked our variables using both. The RandomForestSRC package handles missing values by imputing them through adaptive tree imputation. An exploratory data analysis revealed a proportion of missing data of less than 1% for most of our variables.
We used median and interquartile range (IQR) to describe central tendency, due to the predominantly non-normal distribution of the continuous variables. The Mann-Whitney U test was used to compare continuous variables between unpaired samples, while the χ2 test was used for categorical variables. A Cox proportional hazards model was run to evaluate the association between recurrence or survival and the selected subset of variables. P-value was set at 0.05. Analyses were performed using RStudio 1.2 and Anaconda for Mac OS (Citation21, Citation22).
Results
Background Information
Of the 890 questionnaires sent; 481 were returned (participation rate: 54%). Median age and body mass index (BMI) among respondents was 67.00 years [IQR 61.75 − 75.00] and 26.50 kg/m2 [IQR 23.47 − 30.45], respectively. The majority of respondents were multigravidas and multiparous [mode: two pregnancies and two children, respectively] ().
Table 1. Background demographic, clinical, and tumor characteristics of endometrial cancer patients by recurrence status.
Women who had recurrence or died had higher median age (). By 1 February 2019, 54 women had recurrence (13.0%), with a median time-to-recurrence of 20 mo, [IQR 12.75-29.00]. As expected, the higher recurrence rate was associated with advanced stage, non-endometrioid histological type, poor differentiation, and aneuploidy (). This was also true for overall survival (OS).
Figure 2. Comparison of the age distribution between women who had a relapse or died and those who did not (p = 0.02 and p < 0.0001 respectively). Median is marked with white dot, while interquartile range – with black bars around.
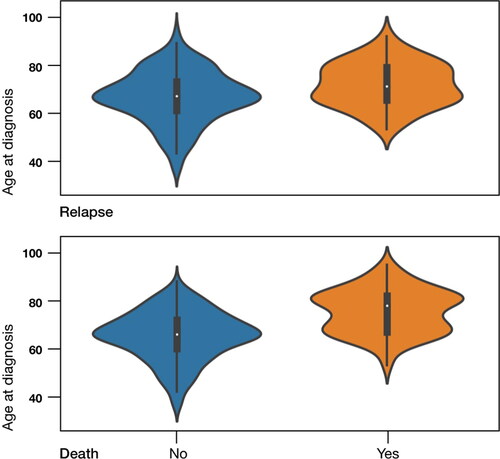
Table 2. Recurrence and death among EC patients by tumor characteristics.
Overall, 83 patients died (20.0%); median OS among them was 45 mo, [IQR 29.00-74.00]. The progression-free survival (PFS) model included all 186 variables. Recurrence and all-cause death were labeled as the main outcomes, while time-to-recurrence and OS were labeled as time-to-event characteristics. Death and OS period were excluded from the model with recurrence labeled as the outcome (and vice versa), to ensure that the model was not skewed toward these significant variables. We then calculated VIMP and minimal depth for all variables and ranked the top 10 in descending order ().
Table 3. Top 10 variables in descending order of importance based on VIMP and minimal depth assessments.
Several of the variables had high rankings according to both VIMP and minimal depth. For recurrence, these included stage, ploidy, tumor type, prunes, and fried potatoes; while for survival they included age, stage, tumor type, ploidy, sugar-sweetened beverages (SSBs), physical activity, and age at cessation of alcohol consumption (). As age and tumor characteristics are well-known modifiers of PFS and OS, something we also observed in our exploratory analysis, we chose to focus on the variables related to dietary habits, alcohol consumption, and physical activity.
The Cox proportional hazards model revealed that prune consumption did not influence the risk of EC recurrence or death, but each additional serving of fried potatoes increased both of these risks. These hazards persisted after adjustment for BMI, age, and smoking status. An additional serving of SSB increased the risk of death [hazard ratio, HR = 3.262; 95% confidence interval, CI 1.834-5.800], which persisted after adjustment for confounding variables. In contrast, physical activity decreased the risk of death. More precisely, the risk of death was reduced by 7.3% [95% CI 0.892-0.964] with each additional unit of metabolic equivalent (MET/day) of physical activity. The beneficial effect of physical activity persisted after adjustment for age and BMI, but not stage ().
Table 4. Hazard ratios (HRs) and 95% confidence intervals (CIs) of recurrence or death according to selected dietary habits and physical activity.
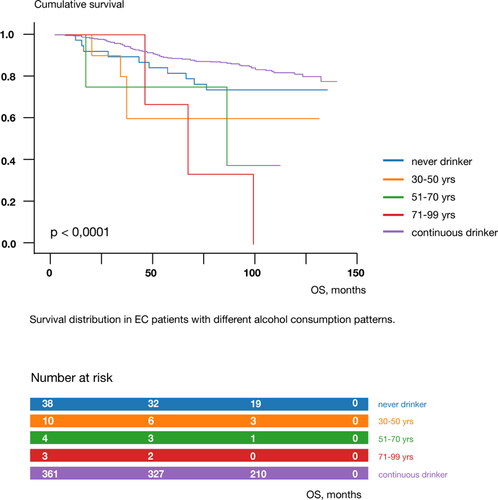
We stratified age at cessation of alcohol consumption into «never drinker», «30-50 years», «51-70 years», «71-99 years», and «continuous drinker», and Kaplan-Meier survival curves for these five categories differed significantly (log-rank test, chi2 = 24.237; p < 0.0001) (). Survival decreased from never drinkers to cessation at age 71-99 years, while continuous drinkers had a better prognosis. The amount of alcohol consumed did not significantly modulate the risk of EC recurrence or death (p = 0.527 and p = 0.137, respectively).
Consumption of fried potatoes was significantly correlated with consumption of bologna sausage and bacon [Spearman’s rho = 0.339, p < 0.001 and Spearman’s rho 0.285, p < 0.001, respectively]. At the same time, consumption of SSBs was positively correlated with that of jam and white bread [Spearman’s rho = 0.212, p < 0.001 and Spearman’s rho 0.199, p < 0.001, respectively].
Discussion
In our study, we utilized RSF, a machine learning approach, to study dietary habits and daily routines among patients with EC. We found that consumption of fried potatoes and SSBs, which may be indicative of specific dietary habits, increased the risk of EC recurrence and death, while physical activity decreased the risk of death.
EC recurrence depends on well-known individual and clinical characteristics, such as age, obesity, tumor type. However, we observed that dietary habits and daily routines also influence the ultimate risk of recurrence.
Starch-rich foods cooked at high temperatures, such as those used when frying or roasting, are known to have high concentrations of acrylamide (Citation23, Citation24). Acrylamide is a vinyl monomer, which was labeled ‘probably carcinogenic to humans’ by the International Agency for Research on Cancer Working Group in 1994. This conclusion mainly relied on experimental studies, in which animals were given a much higher doses of acrylamide than those contained in regular meals. So far, observational studies have not confirmed that acrylamide from foods directly causes cancer in humans; however, authorities recognize the need for more reliable data. In 2019, the United States Food and Drug Administration confirmed that acrylamide is still present in many products, although its levels have decreased somewhat in certain crackers and potato chips (Citation25). According to the National Food Agency, average acrylamide exposure in Sweden is about 25-40 μg/day per person, which is below the hazard level (Citation26). However, it has been shown that acrylamide may act as an endocrine disruptor, and that even minor doses can alter one’s hormonal balance (Citation27–29). Female rats exposed to glycidamide (epoxide metabolite of acrylamide) have been reported to develop endometrial hyperplasia and uterine adenocarcinoma (Citation30). Importantly, acrylamide was associated with an increase in estradiol levels in pre- and postmenopausal women (Citation31). This is important, given the role that estrogens play in endometrium proliferation. According to the most recent dose-response meta-analysis, acrylamide intake is associated with a small increase in the risk of EC, and this association was linear and stronger among never smokers (Citation32). In our study, the risk of death persisted even after adjustment for age, tumor stage, and smoking status, which we adjusted for due to the well-known antiestrogenic properties of smoking (Citation33, Citation34). Of note, smoking status per se did not affect recurrence or survival in our study sample. The negative effect of starch-rich foods was absent when analyzing boiled and mashed potatoes, which indicates that the cooking method itself might have an influence on EC risk. This finding is consistent with the knowledge that boiled or steamed foods contain less acrylamide than deep-fried or roasted foods.
We also observed that consumption of SSBs increased the risk of death among patients with EC. Indeed, SSBs cause a rise in insulin, which has several known effects in relation to EC (Citation35, Citation36). First, insulin can inhibit the production of sex hormone-binding globulin (SHBG), which binds to circulating sex steroids (Citation37). Lack of SHBG, in turn, promotes higher levels of bioactive free estrogen, which induces the proliferation of endometrium (Citation38). Furthermore, insulin might exert antiapoptotic and mitogenic properties (Citation39, Citation40) by increasing blood levels of insulin growth factor-1 (Citation41, Citation42). Inoue-Choi et al. evaluated the consumption of sugar-containing foods and SSBs among 23,039 postmenopausal women and found that SSB intake was positively associated with an increased risk of type I EC. The association persisted after adjustment for BMI, physical activity, smoking status, and history of diabetes (Citation43). To conclude, our results on SSB consumption are in agreement with several previous studies. However, it should be noted that the ultimate effect differs, primarily because of different study designs and the various sugar levels in SSBs. A recent dose-response meta-analysis revealed that carbohydrate intake is associated with EC if large (>50,000 participants) and prolonged (≥10 years follow-up) studies are included (Citation44).
In our study, SSB consumption significantly increased the risk of death but not EC recurrence. Of note, light soda drinks, in which sugar is reduced or replaced with artificial sweeteners, did not affect recurrence or death. One explanation for this might be that our OS analyses included patients who died from any cause, not only EC. SSB consumption might influence the incidence of other conditions that are also common in older individuals, such as metabolic syndrome, and ultimately contribute to increased mortality through this path. Thus care should be taken when speculating on the influence of our selected variables on cancer-specific mortality. Our use of questionnaire data might lead to inaccuracies due to the self-reported nature of the information. Still, patients had unlimited time to complete the questionnaires, and we observed a low percentage of missing data, which testifies to the reliability of this approach. Many studies have focused on whether any specific food compound or activity influences the risk of EC recurrence. In contrast, our study focused on patients who had already undergone surgical treatment for EC. As the perspectives differ, so might the effects. However, our results are in accordance with previous major findings, implying that EC incidence and progression share at least some of the same pathways.
Apart from its beneficial effects on obesity, diabetes, and cardiovascular disorders, regular physical activity reduces the risk of several malignant tumors, including EC (Citation45). According to the meta-analysis by Moore et al., active women had a 30% lower risk of EC compared with non-active women (Citation46). Similar results were obtained in the systematic review, which reported that women who engaged in regular physical activity had a 20-40% lower risk of EC (Citation47). In our study, physical activity did not affect the risk of the EC recurrence, but decreased the risk of death. Again, since older patients usually have concurrent health conditions, the ultimate effect of physical activity is probably the result of various mechanisms. Some examples include the normalization of insulin levels, a decrease in inflammatory markers and reactive oxygen species, modulation of the immune system, and, the most obvious, weight reduction (Citation48–50). The latter usually coincides with the depletion of adipose tissue, which leads to less aromatization, ie., the converting of androgens into bioactive estrogens, which promotes endometrium proliferation.
Unfortunately, we cannot draw a robust conclusion on how the cessation of alcohol consumption affected PFS and OS among patients with EC, since the distribution was unequal. When we evaluated average alcohol consumption, we still found no statistically significant difference in the outcomes. However, there were significantly fewer people in the groups that stopped drinking alcohol than in the group that never stopped drinking, which in our opinion may affect the results. Furthermore, neither the amount nor the frequency of alcohol consumption among those who never stopped drinking influenced the likelihood of EC recurrence or death. More alcohol-related parameters should be evaluated to reveal any hidden patterns.
The strength of the study, in our opinion, is the relatively large number of participants, the long observation period, and the extensive set of variables evaluated. The RSF is also considered an efficient approach for data analysis in high-dimensional datasets with limited survival data. However, we acknowledge that unmeasured confounding may have influenced these findings. In addition, studies like ours that aim to conduct a comprehensive assessment of dietary habits and daily routines must inevitably be conducted using extensive questionnaires, the intricacy and length of which could contribute to a relatively low participation rate. Furthermore, the severity of EC might influence a patient’s desire to participate, as was shown in a recent meta-analysis by Cramer et al. (Citation51). The retrospective study of dietary habits always carries the internal risk of recall bias. To compensate for that, we utilized a machine learning approach, which is more capable of handling multiple interconnected variables and providing a holistic view of dietary patterns.
To conclude, we applied a machine learning algorithm - RSF - to study the effects of dietary habits and daily activities on EC recurrence and death. Of the 186 variables studied, the most significant impacts on recurrence or death from EC were found for the consumption of fried potatoes, which significantly increased the risk of EC recurrence and death, even after adjustment for age and BMI. SSB significantly reduced OS, but had a less pronounced effect on EC recurrence. Physical activity was associated with a lower risk of death among patients with EC, which persisted after adjustment for age and BMI. These results should be interpreted with caution, since each variable is an integral part of a complex system of dietary habits and daily activities. Mutually correlating elements can be combined into clusters that reflect an individual’s existing dietary preferences, which was also confirmed in our study. As a result, we propose to look at our results more holistically and consider the existing relationships. However, given these results and the available data, we would suggest that women treated for EC consider reducing their consumption of fried potatoes and SSBs, which might represent «fattier» or more «carbohydratic» dietary patterns. Instead, we would encourage them to enrich their diets with vegetables and fruits, which lower the odds of EC according to a previous meta-analysis, and to complement healthy dietary habits with a minimum level of physical activity (Citation52).
Ethics Approval
All patients were provided with a written study description and an informed consent form. The final database was anonymized before analysis. The ethical review board in Stockholm (Regionala etikprövningsnämnden i Stockholm) approved this project (dr nr 2006/649 and dr nr 2010/1536-31/2), and all methods were performed in accordance with relevant guidelines and regulations.
Consent to Participate
The patients were provided with a written study description and all participants signed an informed consent form. The final database was anonymized before analysis.
Availability of Data and Material
Most of the data are provided within the manuscript. Additional data can be provided upon reasonable request by contacting the corresponding author.
Authors’ Contributions
O.C.W.: designing the study, data collection; Z.R.: designing the study, data preparation; I.G.: statistical analysis, writing the primary draft; M.M.: designing the study, project administration, securing funding for data collection; All authors contributed to the final version of the manuscript.
Code Availability
Code is available upon request by contacting the corresponding author.
Disclosure Statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492.
- Danaei G, Vander Hoorn S, Lopez AD, Murray CJ, Ezzati M, Comparative Risk Assessment orating group (Cancers) Causes of cancer in the world: comparative risk assessment of nine behavioural and environmental risk factors. Lancet. 2005;366(9499):1784–93. doi: 10.1016/S0140-6736(05)67725-2.
- Saso S, Chatterjee J, Georgiou E, Ditri AM, Smith JR, Ghaem-Maghami S. Endometrial cancer. BMJ. 2011;343:d3954. doi: 10.1136/bmj.d3954.
- Selya A, Anshutz D, Griese E, Weber TL, Hsu B, Ward C. Predicting unplanned medical visits among patients with diabetes: translation from machine learning to clinical implementation. BMC Med Inform Decis Mak. 2021;21(1):111. doi: 10.1186/s12911-021-01474-1.
- Chatterjee A, Gerdes MW, Martinez SG. Identification of risk factors associated with obesity and overweight-a machine learning overview. Sensors (Basel). 2020;20(9):2734. doi:10.3390/s20092734
- Iwendi C, Khan S, Anajemba JH, Mittal M, Alenezi M, Alazab M. The use of ensemble models for multiple class and binary class classification for improving intrusion detection systems. Sensors (Basel). 2020;20(9):2559. doi:10.3390/s20092559
- Razumova Z, Govorov I, Östensson E, Mints M. Cadmium intake as a prognostic factor in endometrial cancer: A Swedish cohort-based study. Nutr Cancer. 2022;74(1):175–184. doi:10.1080/01635581.2021.1883681
- Breiman L. Random forests. Machine Learning. 2001;45(1):5–32. doi:10.1023/A:1010933404324
- Akai H, Yasaka K, Kunimatsu A, Nojima M, Kokudo T, Kokudo N, Hasegawa K, Abe O, Ohtomo K, Kiryu S, et al. Predicting prognosis of resected hepatocellular carcinoma by radiomics analysis with random survival forest. Diagn Interv Imaging. 2018;99(10):643–51. doi: 10.1016/j.diii.2018.05.008.
- Datema FR, Moya A, Krause P, Bäck T, Willmes L, Langeveld T, Baatenburg de Jong RJ, Blom HM. Novel head and neck cancer survival analysis approach: random survival forests versus Cox proportional hazards regression. Head Neck. 2012;34(1):50–8. doi: 10.1002/hed.21698.
- Hsich E, Gorodeski EZ, Blackstone EH, Ishwaran H, Lauer MS. Identifying important risk factors for survival in patient with systolic heart failure using random survival forests. Circ Cardiovasc Qual Outcomes. 2011;4(1):39–45. doi: 10.1161/CIRCOUTCOMES.110.939371.
- Miao F, Cai YP, Zhang YX, Fan XM, Li Y. Predictive modeling of hospital mortality for patients with heart failure by using an improved random survival forest. IEEE Access. 2018;6:7244–53. doi:10.1109/ACCESS.2018.2789898
- Omurlu IK, Ture M, Tokatli F. The comparisons of random survival forests and Cox regression analysis with simulation and an application related to breast cancer. Expert Syst Appl. 2009;36(4):8582–8. doi:10.1016/j.eswa.2008.10.023
- López-Reig R, Fernández-Serra A, Romero I, Zorrero C, Illueca C, García-Casado Z, Poveda A, López-Guerrero JA. Prognostic classification of endometrial cancer using a molecular approach based on a twelve-gene NGS panel. Sci Rep. 2019;9(1):18093. doi: 10.1038/s41598-019-54624-x.
- Wang Q, Xu T, Tong Y, Wu J, Zhu W, Lu Z, Ying J. Prognostic potential of alternative splicing markers in endometrial cancer. Mol Ther Nucleic Acids. 2019;18:1039–48. doi: 10.1016/j.omtn.2019.10.027.
- Ishwaran H, Kogalur UB, Blackstone EH, Lauer MS. Random survival forests. Ann Appl Stat. 2008;2:841–60. doi:10.1214/08-Aoas169
- Mogensen UB, Ishwaran H, Gerds TA. Evaluating random forests for survival analysis using prediction error curves. J Stat Softw. 2012;50(11):1–23. doi:10.18637/jss.v050.i11
- Dietrich S. Investigation of the machine learning method Random Survival Forest as an exploratory analysis tool for the identification of variables associated with disease risks in complex survival data. 2016.
- Ishwaran H, Kogalur U. RandomForestSRC: Random forests for survival, regression and classification (RF-SRC). R Package Version. 2014;1.
- Ehrlinger J. ggRandomForests: Exploring random forest survival. arXiv preprint arXiv:161208974; 2016.
- Team R. RStudio: Integrated development for R. Boston, MA: RStudio, PBC; 2020. http://www.rstudio.com/.
- Anaconda Software Distribution. Anaconda Documentation. Anaconda Inc.; 2020. Avaliable from https://docs.anaconda.com/
- Dybing E, Farmer PB, Andersen M, Fennell TR, Lalljie SPD, Müller DJG, Olin S, Petersen BJ, Schlatter J, Scholz G, et al. Human exposure and internal dose assessments of acrylamide in food. Food Chem Toxicol. 2005;43(3):365–410. doi: 10.1016/j.fct.2004.11.004.
- Pelucchi C, Franceschi S, Levi F, Trichopoulos D, Bosetti C, Negri E, La Vecchia C. Fried potatoes and human cancer. Int J Cancer. 2003;105(4):558–60. doi: 10.1002/ijc.11118.
- US Food and Drug Administration (USFDA). 2022. Survey data on acrylamide in food, Washington, DC: USFDA. Available from https://www.fda.gov/food/chemical-contaminants-food/survey-data-acrylamide-food.
- Mucci LA, Sandin S, Bälter K, Adami H-O, Magnusson C, Weiderpass E. Acrylamide intake and breast cancer risk in Swedish women. JAMA. 2005;293(11):1326–7. doi: 10.1001/jama.293.11.1326.
- Hass U, Christiansen S, Andersen MD, Rosenberg SA, Mandrup K, Egebjerg SB, Nikolov NG, Holbech H, Morthorst JE. List of endocrine disrupting chemicals; DTUfood, National Food Institute; 2017.
- Kassotis CD, Klemp KC, Vu DC, Lin C-H, Meng C-X, Besch-Williford CL, Pinatti L, Zoeller RT, Drobnis EZ, Balise VD, et al. Endocrine-disrupting activity of hydraulic fracturing chemicals and adverse health outcomes after prenatal exposure in male mice. Endocrinology. 2015;156(12):4458–73. doi: 10.1210/en.2015-1375.
- Matoso V, Bargi-Souza P, Ivanski F, Romano MA, Romano RM. Acrylamide: A review about its toxic effects in the light of Developmental Origin of Health and Disease (DOHaD) concept. Food Chem. 2019;283:422–30. doi: 10.1016/j.foodchem.2019.01.054.
- Beland FA, Olson GR, Mendoza MC, Marques MM, Doerge DR. Carcinogenicity of glycidamide in B6C3F1 mice and F344/N rats from a two-year drinking water exposure. Food Chem Toxicol. 2015;86:104–15. doi: 10.1016/j.fct.2015.09.017.
- Nagata C, Konishi K, Tamura T, Wada K, Tsuji M, Hayashi M, Takeda N, Yasuda K. Associations of acrylamide intake with circulating levels of sex hormones and prolactin in premenopausal Japanese women. Cancer Epidemiol Biomarkers Prev. 2015;24(1):249–54. doi: 10.1158/1055-9965.EPI-14-0935.
- Adani G, Filippini T, Wise LA, Halldorsson TI, Blaha L, Vinceti M. Dietary intake of acrylamide and risk of breast, endometrial, and ovarian cancers: A systematic review and dose-response meta-analysis. Cancer Epidemiol Biomarkers Prev. 2020;29(6):1095–1106. doi:10.1158/1055-9965
- Felix AS, Yang HP, Gierach GL, Park Y, Brinton LA. Cigarette smoking and endometrial carcinoma risk: the role of effect modification and tumor heterogeneity. Cancer Causes Control. 2014;25(4):479–89. doi: 10.1007/s10552-014-0350-1.
- Tanko LB, Christiansen C. An update on the antiestrogenic effect of smoking: a literature review with implications for researchers and practitioners. Menopause. 2004;11(1):104–9. doi: 10.1097/01.GME.0000079740.18541.DB.
- Jenkins DJ, Wolever TM, Taylor RH, Barker H, Fielden H, Baldwin JM, Bowling AC, Newman HC, Jenkins AL, Goff DV, et al. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am J Clin Nutr. 1981;34(3):362–6. doi: 10.1093/ajcn/34.3.362.
- Kaaks R, Lukanova A, Kurzer MS. Obesity, endogenous hormones, and endometrial cancer risk: a synthetic review. Cancer Epidemiol Biomarkers Prev. 2002;11(12):1531–43.
- Strain G, Zumoff B, Rosner W, Pi-Sunyer X. The relationship between serum levels of insulin and sex hormone-binding globulin in men - the effect of weight-loss. J Clin Endocrinol Metabol. 1994;79(4):1173–6. doi:10.1210/jc.79.4.1173
- Filardo EJ, Thomas P. Minireview: G protein-coupled estrogen receptor-1, GPER-1: its mechanism of action and role in female reproductive cancer, renal and vascular physiology. Endocrinology. 2012;153(7):2953–62. doi: 10.1210/en.2012-1061.
- Coleman HG, Kitahara CM, Murray LJ, Dodd KW, Black A, Stolzenberg-Solomon RZ, Cantwell MM. Dietary carbohydrate intake, glycemic index, and glycemic load and endometrial cancer risk: a prospective cohort study. Am J Epidemiol. 2014;179(1):75–84. doi: 10.1093/aje/kwt222.
- Cust AE, Slimani N, Kaaks R, van Bakel M, Biessy C, Ferrari P, Laville M, Tjønneland A, Olsen A, Overvad K, et al. Dietary carbohydrates, glycemic index, glycemic load, and endometrial cancer risk within the European Prospective Investigation into Cancer and Nutrition cohort. Am J Epidemiol. 2007;166(8):912–23. doi:10.1093/aje/kwm161
- Bruchim I, Sarfstein R, Werner H. The IGF hormonal network in endometrial cancer: Functions, regulation, and targeting approaches. Front Endocrinol (Lausanne). 2014;5:76. doi: 10.3389/fendo.2014.00076.
- Ogawa K, Sun CL, Horii A. Exploration of genetic alterations in human endometrial cancer and melanoma: Distinct tumorigenic pathways that share a frequent abnormal PI3K/AKT cascade. Oncol Rep. 2005;14:1481–5. doi:10.3892/or.14.6.1481
- Inoue-Choi M, Robien K, Mariani A, Cerhan JR, Anderson KE. Sugar-sweetened beverage intake and the risk of type I and type II endometrial cancer among postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2013;22(12):2384–94. doi: 10.1158/1055-9965.EPI-13-0636.
- Sadeghi A, Sadeghian M, Nasiri M, Rahmani J, Khodadost M, Pirouzi A, Maleki V, Sadeghi O. Carbohydrate quantity and quality affect the risk of endometrial cancer: A systematic review and dose-response meta-analysis. Clin Nutr. 2020;39(6):1681–1691. doi:10.1016/j.clnu.2019.08.001
- Lugo D, Pulido AL, Mihos CG, Issa O, Cusnir M, Horvath SA, Lin J, Santana O. The effects of physical activity on cancer prevention, treatment and prognosis: A review of the literature. Complement Ther Med. 2019;44:9–13. doi: 10.1016/j.ctim.2019.03.013.
- Moore SC, Gierach GL, Schatzkin A, Matthews CE. Physical activity, sedentary behaviours, and the prevention of endometrial cancer. Br J Cancer. 2010;103(7):933–8. doi: 10.1038/sj.bjc.6605902.
- Voskuil DW, Monninkhof EM, Elias SG, Vlems FA, van Leeuwen FE, Task Force Physical Activity and Cancer. Physical activity and endometrial cancer risk, a systematic review of current evidence. Cancer Epidemiol Biomarkers Prev. 2007;16(4):639–48. doi: 10.1158/1055-9965.EPI-06-0742.
- Bradley RL, Jeon JY, Liu F-F, Maratos-Flier E. Maratos-Flier E: Voluntary exercise improves insulin sensitivity and adipose tissue inflammation in diet-induced obese mice. Am J Physiol Endocrinol Metab. 2008;295(3):E586–94. doi: 10.1152/ajpendo.00309.2007.
- Marzatico F, Pansarasa O, Bertorelli L, Somenzini L, Della Valle G. Blood free radical antioxidant enzymes and lipid peroxides following long-distance and lactacidemic performances in highly trained aerobic and sprint athletes. J Sports Med Phys Fitness. 1997;37:235–9.
- Pedersen L, Idorn M, Olofsson GH, Lauenborg B, Nookaew I, Hansen RH, Johannesen HH, Becker JC, Pedersen KS, Dethlefsen C, et al. Voluntary running suppresses tumor growth through epinephrine- and IL-6-dependent NK cell mobilization and redistribution. Cell Metab. 2016;23(3):554–62. doi: 10.1016/j.cmet.2016.01.011.
- Cramer H, Haller H, Dobos G, Lauche R. Lauche R: A systematic review and meta-analysis estimating the expected dropout rates in randomized controlled trials on yoga interventions. Evid Based Complement Alternat Med. 2016;2016:5859729. doi: 10.1155/2016/5859729.
- Bandera EV, Kushi LH, Moore DF, Gifkins DM, McCullough ML. Fruits and vegetables and endometrial cancer risk: a systematic literature review and meta-analysis. Nutr Cancer. 2007;58(1):6–21. doi: 10.1080/01635580701307929.