Abstract
This study aimed to investigate the effects of polyunsaturated fatty acids (PUFAs) on patients with colorectal cancer (CRC). Electronic databases such as PubMed and Web of Science were searched. Studies on the application of PUFAs in patients with CRC, published up to January 2022, were conducted. Twelve studies involving 702 CRC patients were included. For patients undergoing surgery, subgroup analyses indicated that preoperative supplementation with PUFAs improved total postoperative infectious complications (RR: 0.37, p = 0.02). Furthermore, the supplementation of PUFAs in preoperative (WMD: −2.27, p < 0.001) and postoperative (WMD: −2.66, p = 0.01) groups was effective in shortening the postoperative hospital stay for patients with CRC. Tumor necrosis factor-α (TNF-α) (SMD: −0.56, p = 0.007) and interleukin-6 (IL-6) (SMD: −0.54, p = 0.004) levels were lower in all CRC patients receiving PUFAs intervention than in the control group. Moreover, supplementation with PUFAs in chemotherapy patients significantly increased albumin (WMD: 0.48, p = 0.03) and decreased C-reactive protein (CRP) (WMD: −6.12, p = 0.02) compared to the control group. This study demonstrated that PUFAs intervention could diminish the total postoperative infection complications of CRC patients, shorten the postoperative hospital stay, and reduce inflammation.
Introduction
According to the World Health Organization 2020 Cancer Burden Rises Report, the overall incidence of colorectal cancer (CRC) ranks third, and second only to lung and prostate cancers. Meanwhile, the mortality rate of CRC has risen to the second highest globally, reaching more than 930,000 cases in 2020 (Citation1). Recently, surgery has become the primary treatment for resectable CRC, whereas chemotherapy is generally predominant in patients with advanced CRC. For surgical treatment, although new technologies such as laparoscopy and robotics have been invented in recent years, the reduction in the incidence of postoperative complications remains an urgent problem (Citation2). Meanwhile, the quality of life of patients undergoing chemotherapy is affected to various degrees.
Polyunsaturated fatty acids (PUFAs), as a type of immune nutrient, can be divided into n-3 and n-6 PUFAs, and their functions have recently attracted much attention. A study on the effects of PUFAs in patients with advanced-stage lung cancer found that PUFAs supplementation increased the body weight of patients with lung cancer and significantly decreased the inflammation index (Citation3). In addition, among the gastrointestinal cancer patients receiving PUFAs intervention (Citation4), it was found that PUFAs could effectively enhance the immunity of surgical patients. However, there is no clear consensus on the effects of PUFAs on CRC (Citation4, Citation5). A previous meta-analysis of CRC patients mentioned that supplementation with PUFAs was beneficial in regulating inflammatory factors. However, its sample size was limited, and did not refer to the relationship between PUFAs and postoperative complications (Citation6). Consequently, the effects of PUFAs in patients with CRC must be improved. Therefore, we attempted to investigate the effects of PUFAs supplementation on postoperative complications, immune regulation, inflammatory factors, weight and body mass index (BMI) in CRC patients by reviewing published randomized controlled trials (RCTs).
Methods
Search Strategy
All relevant studies on the application of PUFAs in patients with CRC published until January 2022 were searched in PubMed, Web of Science Core Collection (Science Citation Index Expanded), Cochrane Library, and EBSCO. PUFAs were classified into two categories: n-3 PUFAs and n-6 PUFAs. In addition, the preferred reporting items for systematic reviews and meta-analyses (PRISMA) were followed (Citation7). Finally, to avoid missing potentially related studies, relevant literature in previously published meta-analyses and reviews was manually indexed. The retrieval formula was as follows: (Intestinal Neoplasm OR Neoplasm, Intestinal OR Neoplasms, Intestinal OR Intestines Neoplasms OR Intestines Neoplasm OR Neoplasm, Intestines OR Neoplasms, Intestines OR Cancer of Intestines OR Intestines Cancers OR Cancer of the Intestines OR Intestines Cancer OR Cancer, Intestines OR Cancers, Intestines OR Intestinal Cancer OR Cancer, Intestinal OR Cancers, Intestinal OR Intestinal Cancers OR Colorectal Neoplasm OR Neoplasm, Colorectal OR Neoplasms, Colorectal OR Colorectal Tumors OR Colorectal Tumor OR Tumor, Colorectal OR Tumors, Colorectal OR Colorectal Cancer OR Cancer, Colorectal OR Cancers, Colorectal OR Colorectal Cancers OR Colorectal Carcinoma OR Carcinoma, Colorectal OR Carcinomas, Colorectal OR Colorectal Carcinomas) AND (Acids, Unsaturated Fatty OR Unsaturated Fatty Acids OR Unsaturated Fatty Acid OR Acid, Unsaturated Fatty OR Fatty Acid, Unsaturated OR Polyunsaturated Fatty Acids OR Acids, Polyunsaturated Fatty OR Fatty Acids, Polyunsaturated OR Polyunsaturated Fatty Acid OR Acid, Polyunsaturated Fatty OR Fatty Acid, Polyunsaturated OR Fatty Acids, Unsaturated OR n-3 polyunsaturated fatty acids OR omega-3 fatty acids).
Selection Criteria
Inclusion criteria:
Patients diagnosed with CRC who underwent surgery or chemotherapy;
Intervention with PUFAs during treatment (co-intervention including immune nutrients, such as arginine and nucleotides);
Control with placebo or reagents without PUFAs;
Studies including, but not limited to, the outcomes of postoperative complications, immune regulation, inflammatory factors, weight, and BMI;
Studies with RCTs.
Exclusion criteria:
Participants included patients with other cancers (e.g., esophageal cancer and gastric cancer);
Studies with unavailable outcome indicators;
Updated studies (largest sample size and/or latest studies included);
Articles with only a summary and no full text;
Articles not written in English.
Data Extraction
All included studies were independently reviewed by two investigators. A consensus was reached after discussion if there were any disputes. In addition, two investigators independently extracted all data, including the author, publication year, research country, sample number, study design, intervention time, patient recruitment time, age, and related outcome indicators, such as postoperative complications, immune regulation, inflammatory factors, weight, and/or BMI.
Quality Assessment
As all incorporated articles were RCTs, the Cochrane bias risk assessment tool was used to fully evaluate the literature (Citation8). Each study was systematically evaluated in seven aspects: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete data, selective reporting of research results, and other sources of bias. Each aspect was judged as “low risk of bias”, “high risk of bias”, and “unclear”.
Outcome Measures
This study aimed to investigate the effects of PUFAs supplementation on postoperative complications, immune regulation, inflammatory factors, weight, and/or BMI in patients with CRC. The leading observational indicators were postoperative complications, CD4/CD8 ratio, albumin, and C-reactive protein (CRP) levels.
Moreover, a subgroup analysis was performed. According to the timing of supplementation, the outcomes related to postoperative complications were divided into preoperative, perioperative, and postoperative supplement groups. What’s more, due to differences in treatment methods, CRC patients were divided into supplement groups (during surgery and chemotherapy) for outcomes related to immune regulation, inflammatory factors, weight, and BMI.
Statistical Analysis
RevMan software (version 5.3) was used for statistical analysis, with a unified use of risk ratios (RR) and 95% confidence intervals (95%CI) to assess dichotomous variables. Regarding continuous variables, if the difference between the data was not noticeable, the weighted mean difference (WMD) and 95%CI were used. Otherwise, the standardized mean difference (SMD) and 95%CI were used for the evaluation (Citation9). The I2 test was applied to heterogeneity analysis; I2 less than 25%, 25%–50%, 50%–75%, and more than 75% were used to identify no, low, medium, and high heterogeneity (Citation10). Meanwhile, since CRC patients receiving different treatments were included in this study, the random-effects model was adopted. When the number of studies was more than 10, Egger’s test was applied to evaluate publication bias (Citation11). Finally, a sensitivity analysis was performed to evaluate the stability of the results (Citation12). The above results were statistically significant (p < 0.05).
Results
Description of Selected Trials
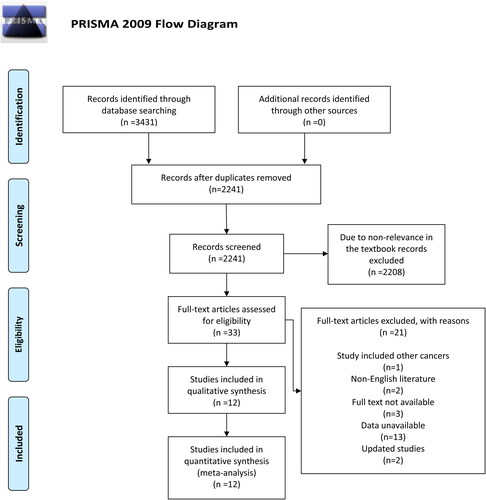
Based on the retrieval formula, 3431 potential studies were initially retrieved from relevant electronic databases. Then removing redundant articles, there were 2241 studies left. After reading the titles and abstracts, 2208 studies were excluded because they were irrelevant. Of the remaining 33 studies, one study was excluded due to involving other cancer patients, two articles not written in English were excluded, three studies were excluded because of the lack of full-text access, 13 studies were excluded because data were not reported. On account of priority given to studies with the largest and/or latest sample size, two studies were excluded on account of updating. Twelve studies met the inclusion criteria and were analyzed (Citation13–24) ().
Study and Patient Characteristics
A total of 702 CRC patients were reported in 12 studies from 1994 to 2020. Five hundred and thirty-three patients received surgical treatment, of which 83 patients in two studies were supplemented with PUFAs 5 day before surgery (Citation14, Citation17), and 156 patients in four studies received perioperative supplementation (Citation13–15, Citation23), including preoperative and postoperative supplementation throughout the surgery. The shortest perioperative supplement was the night before surgery and the morning after surgery (Citation13). The longest supplementation time lasted for seven days before and after surgery (Citation23). In two studies (Citation18, Citation24), fifty patients were supplemented seven days after surgery, and the remaining 244 patients were included in the control group without PUFAs supplementation. A total of 169 patients with CRC received chemotherapy, with 87 and 82 patients in the experimental and control groups, respectively. The supplementation time was concentrated in the range of 6–9 weeks. Among the 12 studies, nine were supplemented with PUFAs by intravenous injection (Citation14–17, Citation19–23), and three were orally administered (Citation13, Citation18, Citation24). The study reports came from several countries, comprising 1 in the Netherlands (Citation13), 2 in Italy (Citation14, Citation15), 2 in Iran (Citation16, Citation20), 1 in Japan (Citation17), 2 in China (Citation18, Citation24), 2 in Brazil (Citation19, Citation22), 1 in Britain (Citation21), and 1 in Denmark (Citation23). Outcome indicators included postoperative complications such as total infection complications, surgical incision infection, urinary tract infection, pneumonia, abscess, anastomotic leakage, non-infection complications, intestinal obstruction, wound dehiscence, deep vein thrombosis, length of hospital stay, and the number of deaths. Immunomodulatory classes included CD4, CD8, and the ratio of CD4/CD8. Inflammatory factors included albumin, CRP, tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), interleukin-1β (IL-1β), as well as weight and BMI. A summary of the characteristics of studies included in this meta-analysis is presented in and .
Table 1. Characteristics of included studies in the meta-analysis.
Study on Internal Bias Risk
Of the 12 included studies, eight used random sequences, six implemented allocation concealment, and six reported the simultaneous use of blinding to participants, investigators, and evaluators. Consequently, a low risk of bias was considered. In addition, 12 studies were rated as “low risk” in terms of completeness of results, selective reporting of research results, and other biased sources ( and ).
Effect of Intervention Timing of PUFAs on Postoperative Complications
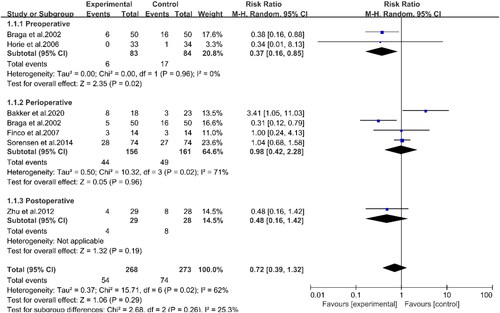
Six studies involving 491 patients reported the outcomes of total infectious complications (Citation13–15, Citation17, Citation23, Citation24) (). The data showed that PUFAs supplementation did not show a statistical difference between the experimental and control groups for total infectious complications (RR: 0.72, 95%CI: 0.39, 1.32, p = 0.29). Through subgroup analyses, two studies revealed that preoperative supplementation of PUFAs could effectively reduce total infectious complications in patients with CRC (RR: 0.37, 95%CI: 0.16, 0.85, p = 0.02) (Citation14, Citation17). However, the results of four studies with perioperative intervention indicated that PUFAs supplementation did not affect the total infectious complications (RR: 0.98, 95%CI: 0.42, 2.28, p = 0.96) (Citation13–15, Citation23). The indicators of postoperative complications were summarized in . There was no significant difference between the two groups in infectious complications, such as surgical site infection, urinary tract infection, pneumonia, abscess, and anastomotic leakage. Simultaneously, there was no statistically significant difference between the experimental and control groups in noninfectious complications, intestinal obstruction, wound dehiscence, deep vein thrombosis, and the number of deaths. Regarding hospitalization time (), PUFAs supplementation did not affect the postoperative hospital stay (WMD: −1.19, 95%CI: −2.62, 0.24, p = 0.10). However, subgroup analyses demonstrated that preoperative supplementation (WMD: −2.27, 95%CI: −3.58, −0.97, p < 0.001) and postoperative supplementation (WMD: −2.66, 95%CI: −4.70, −0.62, p = 0.01) significantly shortened the hospital stay in the experimental group compared with the control group. However, perioperative supplementation with PUFAs had no effect on the length of hospital stay (WMD: −0.08, 95%CI: −1.97, 1.80, p = 0.93). This result was highly heterogeneous and therefore would require further study.
Table 2. Analysis of postoperative complications outcomes.
Effect of PUFAs on Immune Regulation
PUFAs supplementation improved the CD4/CD8 ratio in surgical patients compared to controls (WMD: 0.32, 95%CI: −0.03, 0.68, p = 0.07), although the difference was not statistically significant (). However, there were no statistically significant differences in CD4 and CD8 between the two groups of surgically treated patients ().
Table 3. Analysis of immune regulation, inflammatory and nutritional status outcomes.
Effect of PUFAs on Inflammatory Factors, Weight and BMI
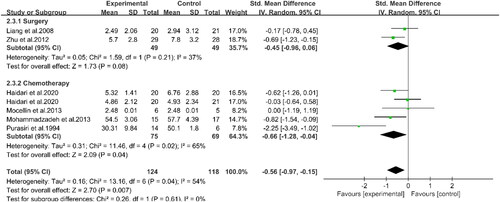
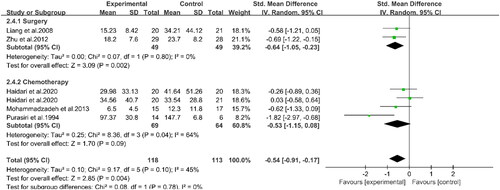
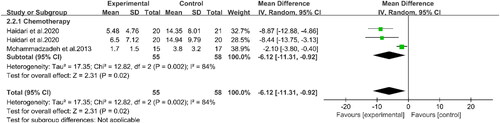
Three studies involving 100 patients reported the effects of PUFAs supplementation on albumin levels during chemotherapy (Citation16, Citation19, Citation22) (). The results demonstrated that the albumin levels in the experimental group were higher than those in the control group (WMD: 0.48, 95%CI: 0.04, 0.91, p = 0.03). This indicated that supplementation with PUFAs could increase the albumin levels of patients undergoing chemotherapy. Two studies indicated that supplementation with PUFAs during chemotherapy could effectively reduce CRP levels compared to the control group (WMD: −6.12, 95%CI: −11.31, −0.92, p = 0.02) (Citation16, Citation20) (). Compared to the controls, supplementation with PUFAs reduced TNF-α levels (SMD: −0.56, 95%CI: −0.97, −0.15, p = 0.007) (). Meanwhile, according to subgroup analyses, PUFAs supplementation decreased TNF-α levels in patients with chemotherapy (SMD: −0.66, 95%CI: −1.28, −0.04, p = 0.04) and surgery (SMD: −0.45, 95%CI: −0.96, 0.06, p = 0.08) compared with the control group. However, the latter group did not show statistical differences. Concerning IL-6 (), subgroup analyses displayed that PUFAs intervention reduced IL-6 levels in patients with CRC in the chemotherapy group (SMD: −0.53, 95%CI: −1.15, 0.08, p = 0.09) and the surgery group (SMD: −0.64, 95%CI: −1.05, −0.23, p = 0.002) compared to the control group. Nevertheless, the difference between the former was not significant. In summary, supplementation with PUFAs significantly reduced IL-6 levels (SMD: −0.54, 95%CI: −0.91, −0.17, p = 0.004). The other relevant indicators were summarized in . Chemotherapy in patients with PUFAs during treatment intervention for IL-1β, weight, and BMI was not statistically significant between the two groups.
Figure 3. Forest plot of the association between PUFAs supplementation and albumin in in patients with CRC.
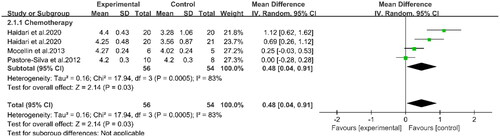
Figure 4. Forest plot of the association between PUFAs supplementation and CRP in in patients with CRC.
Discussion
This study aimed to investigate the effects of PUFAs supplementation on postoperative complications, immune regulation, inflammatory response, weight, and BMI in CRC patients undergoing surgery or chemotherapy. Twelve RCTs involving 702 CRC patients were included. Subgroup analyses showed that supplementation with PUFAs in the preoperative phase for CRC patients undergoing surgical treatment could reduce the incidence of total postoperative infectious complications. In addition, this study displayed that preoperative or postoperative intervention with PUFAs could significantly shorten the hospital stay of patients. In terms of immune regulation, the ratio of CD4/CD8 increased in patients undergoing surgery with PUFAs intervention. Furthermore, regarding anti-inflammatory ability, supplementation with PUFAs could improve the albumin levels of chemotherapy patients and reduce pro-inflammatory factors, such as CRP. Moreover, the findings of this study demonstrated that the levels of serum TNF-α and IL-6 were both decreased in patients with CRC who received supplementation during surgery and chemotherapy.
Due to anatomical and structural differences, tumors in the rectum usually have to be subjected to radiotherapy and chemotherapy before surgery to ease removal, whereas tumors in the colon do not usually require pretreatment (Citation25). Nevertheless, in terms of surgical techniques, open surgery, laparoscopic surgery, and robotic surgery can all be used for colon and rectal cancer resections. The Cancer Genome Atlas studied many tissue specimens of colon and rectal cancers and found that colon and rectal cancers were a single type of cancer (Citation26). The biological characteristics and behavior of the cancer cells were completely consistent, making the chemotherapy drugs for the two cancers completely consistent. Therefore, most of the studies included in this meta-analysis discussed colon and rectal cancers patients together. Unfortunately, this study could not strictly distinguish between colon cancer and rectal cancer patients and performed a statistical analysis. However, this aspect must be noted.
Effect of Intervention Timing of PUFAs on Postoperative Complications
Regarding the effects of PUFAs supplementation on postoperative complications in patients with cancer undergoing surgery, Okamoto et al. conducted a study on 60 patients with gastric cancer, and the results showed that preoperative supplementation of PUFAs could diminish the incidence of total infectious complications after gastrectomy, which was consistent with the results of this study. In addition, Okamoto et al. reported in their research results that preoperative supplementation with an immune-nutritional agent containing PUFAs could effectively shorten the duration of postoperative systemic inflammatory response syndrome in patients undergoing gastrectomy (Citation27). Interestingly, our study found that PUFAs supplementation reduced the levels of pro-inflammatory factors such as TNF-α and IL-6 in surgical patients. Therefore, it seems possible to speculate that PUFAs play a role in resisting inflammation by regulating the anabolism of different fatty acids in cancer patients, which will be beneficial for maintaining homeostasis of the body environment and reducing the total postoperative infectious complications (Citation27).
Furthermore, albumin levels increased in CRC patients who received PUFAs supplementation during chemotherapy. For cancer patients, there is often a problem of effusion, and the extraction of effusion is also accompanied by a large amount of albumin loss. The increase in albumin levels in the experimental group could prevent the loss of nutrition and electrolyte disorders, reducing more serious consequences in the body. Although data on albumin levels are lacking in the surgical supplementation group, changes in albumin levels are closely associated with postoperative complications. Thus far, decreased albumin levels have been generally believed to increase the probability of postoperative complications. This is because hypoalbuminemia impairs the innate immune response, leading to persistent wound healing and the risk of infection through decreased collagen synthesis and granuloma formation (Citation28). This study presented that preoperative supplementation with PUFAs could reduce the incidence of total postoperative infectious complications, which might be related to the increase in the albumin level of patients in the experimental group.
This study did reveal that compared with the control group, neither perioperative supplementation with PUFAs nor postoperative supplementation with PUFAs was associated with total infectious complications. Regrettably, the number of studies in this meta-analysis by intervention time subgroup was limited, so the reliability of the results would remain to be further discussed. However, a recent network meta-analysis reporting results in patients with gastrointestinal malignancies showed that preoperative, perioperative, or postoperative enteral immunonutrition with PUFAs reduced the overall incidence of postoperative infectious complications compared with the standard enteral nutrition group (Citation29). The discrepancy in our study results might be due to differences in the supplementation methods. This study’s intervention method for PUFAs was not limited to enteral nutrition. For instance, an included study on postoperative PUFAs supplementation was conducted with parenteral nutrition (Citation24). Except, another potential cause might be inconsistent supplementation time during the perioperative period, which was included in our study one night before surgery and the next morning after surgery (Citation13), six days before surgery and three days after surgery (Citation15), five days before and after surgery (Citation14) or seven days before and after surgery (Citation23).
Unfortunately, PUFAs intervention did not significantly improve postoperative complications in this study compared with the control group. The limited sample size in this study was a potential factor. Concerning surgical site infection, animal experiments by Karaud et al. demonstrated that using fish oil was beneficial in increasing the wound closure rate of skin tissues, which was believed to be due to the anti-inflammatory and antioxidant activities of fish oil (Citation30). However, in our study, PUFAs did not positively affect the prevention of surgical site infection. The significant difference in the subjects of the intervention might be the reason for the contradiction between our study and that of Karaud et al. In addition, in other postoperative infectious complications, including urinary tract infection, pneumonia, abscess and so on, we found no significant difference between experimental and control groups. Regarding noninfectious complications, Wehner et al. studied rodents and reported that PUFAs played an active role in postoperative intestinal inflammation and exercise, which were expected to become a choice for preventing intestinal obstruction (Citation31). However, owing to the differences between animals and humans, we did not find any relationship between PUFAs supplementation and the occurrence of intestinal obstruction. Furthermore, other noninfectious complications, including wound dehiscence and deep vein thrombosis, failed to show positive effects of PUFAs supplementation, which was supported by most studies (Citation23, Citation29, Citation32). Therefore, inflammatory injury may not be the leading cause of these noninfectious complications. Or the anti-inflammatory functions of PUFAs may not be found in these noninfectious complications.
Regarding the effects of PUFAs on hospital stay, Gianotti et al. (Citation33) and Bai et al. (Citation34) separately reported that the preoperative and postoperative supplementation groups had significantly shorter postoperative hospital stays than the control group. This was consistent with our findings. Thus, as per the above discussion, it is reasonable to believe that supplementation with PUFAs may assist CRC patients in obtaining strong resistance during surgical stress and reducing the inflammatory response, thereby effectively reducing the total postoperative infectious complications and promoting the shortening of hospital stay. However, this study found that perioperative supplementation with PUFAs did not affect the number of hospitalization days. The possible reason was that there were some differences in the definition of perioperative period in each included study, resulting in high heterogeneity of statistical results, so it would need to be further confirmed.
Effect of PUFAs on Immune Regulation
In terms of the immune response, one study mentioned that cancer or surgical pressure could inhibit the immune function of patients, easily impairing their immune status (Citation27). Acute stress, transient immunosuppression, and changes in related organ functions during surgery are important factors that affect the occurrence of postoperative complications. Interestingly, PUFAs supplementation increased the CD4/CD8 ratio in our meta-analysis, although no statistically significant difference was observed. Nevertheless, in a study of patients with gastrointestinal cancer by Yu et al., the CD4/CD8 ratio increased significantly (Citation4). The CD4/CD8 ratio is a clinically relevant biomarker for immune reconstitution. An increase in this ratio may improve immunosuppression in surgical patients, enhance their immune response, and reduce the possibility of total postoperative infectious complications. Simultaneously, a low CD4/CD8 ratio may increase the risk of non-AIDS-related malignant tumors (Citation35). Although no research on survival rate was involved in our research, the role of PUFAs, especially n-3 PUFAs, in anti-tumor immunity, has been increasingly affirmed, including the promotion of T cell activation and cytokine production. CD4 and CD8 T cells are crucial effector cells that mediate cellular immunity. Turbitt et al. conducted experiments on mice with breast cancer and found that fish oil feeding might promote T cells to synthesize and release protective cytokines, such as interleukin-2 (IL-2) and interferon-gamma (IFN-γ). Moreover, tumor immune infiltration increased (Citation36). The above experiments concluded that fish oil, as a PUFA, enhanced anti-tumor immunity.
Effect of PUFAs on Inflammatory Factors, Weight and BMI
Regarding the role of PUFAs in inflammatory factors, we found that supplementation with PUFAs during chemotherapy improved albumin levels compared with the control group, while inflammatory factors such as CRP, TNF-α, and IL-6 were decreased. However, it is common for pro-inflammatory factor including CRP, TNF-α, and IL-6, to significantly increase cancer progression. A prospective cohort study showed that a high intake of PUFAs was associated with lower levels of CRP, suggesting that PUFAs inhibit the activity of δ-6 desaturase, δ-5 desaturase, and cyclooxygenase and reduce inflammation (Citation37). Through cell culture (Citation38) and experiments on healthy human volunteers (Citation39), it was found that eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) in PUFAs can not only affect G protein-coupled receptor-mediated signal transduction but also integrate into the lipid bilayer. Modifying lipid rafts could interrupt TLR-4, NF-κB, and other signaling pathways, thereby inhibiting the production of TNF-α and IL-6 by monocytes, showing anti-inflammatory effects, reducing inflammation, and improving the quality of life of patients undergoing chemotherapy. The other study reported that PUFAs regulated different white blood cell recruitment stages. For example, DHA could decrease the release of TNF-α, which was positively correlated with the expression levels of E-selectin in vascular endothelial cells (Citation40). Therefore, DHA led to a decrease in neutrophil recruitment from flowing blood, reducing the occurrence of inflammation.
In terms of weight and BMI, Wan et al. conducted a meta-analysis involving 977 patients with gastrointestinal cancer, consistent with our study (Citation5). And there was no significant difference in weight and BMI between patients treated with PUFAs intervention and those in the control group. Therefore, it may be speculated that PUFAs, as immune nutrients, cannot fully represent energy.
Strengths and Limitations
The main advantage of this study is that CRC patients were divided explicitly into the surgery supplement group and chemotherapy supplement group in terms of treatment modalities and divided into the preoperative, perioperative (preoperative and postoperative supplementation of PUFAs), and postoperative supplementation group in terms of the different periods of surgery supplementation for analysis. In addition, our included studies are from multiple countries and regions. Therefore, they represent a good population. Most importantly, this is the first RCT-based meta-analysis of the effects of PUFAs on postoperative complications, immune regulation, inflammatory factors, weight, and BMI in patients with CRC. Unfortunately, owing to limited data, multiple subgroup analyses cannot be conducted, such as a study of parenteral and enteral nutrition according to the mode of supplementation. Meanwhile, the number of studies on the effects of different intervention timings of PUFAs on postoperative complications is also limited, coupled with a certain degree of heterogeneity between each study conducted, which may potentially reduce the reliability of the study results. In addition, in three of the 12 included studies, the intervention factors contain components other than PUFAs, which, although supplemented in a limited amount and most of them belong to immune nutrition agents, may potentially interfere with the experimental results (Citation14, Citation15, Citation17). Therefore, large-scale, multi-center RCTs are necessary.
Conclusion
The present meta-analysis demonstrates that PUFAs are promising immunonutrition agents for improving total postoperative infectious complications and anti-inflammation of CRC patients. In addition, supplementation with PUFAs may be associated with an improved immune status. However, PUFAs have no positive effect on noninfectious complications, weight, or BMI.
Authors’ Contributions
All authors had read and approved the manuscript.
| Abbreviations | ||
| CRC | = | colorectal cancer |
| PUFAs | = | polyunsaturated fatty acids |
| BMI | = | body mass index |
| RCTs | = | randomized controlled trials |
| PRISMA | = | preferred reporting items for systematic reviews and meta-analyses |
| CRP | = | C-reactive protein |
| RR | = | risk ratios |
| 95%CI | = | 95% confidence intervals |
| WMD | = | weighted mean difference |
| SMD | = | standardized mean difference |
| TNF-α | = | tumor necrosis factor-α |
| IL-6 | = | interleukin-6 |
| IL-1β | = | interleukin-1β |
| IL-2 | = | interleukin-2 |
| IFN-γ | = | interferon-γ |
| EPA | = | eicosapentaenoic acid |
| DHA | = | docosahexaenoic acid. |
Supplemental Material
Download MS Word (14.8 KB)Supplemental Material
Download TIFF Image (607.1 KB)Supplemental Material
Download TIFF Image (1.1 MB)Availability of Data and Materials
The datasets supporting this article’s conclusions are included within the article and its additional files.
Conflict of Interest Statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
- van der Pas MH, Haglind E, Cuesta MA, Fürst A, Lacy AM, Hop WC, Bonjer HJ. Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol. 2013;14(3):210–8.
- Finocchiaro C, Segre O, Fadda M, Monge T, Scigliano M, Schena M, Tinivella M, Tiozzo E, Catalano MG, Pugliese M, et al. Effect of n-3 fatty acids on patients with advanced lung cancer: a double-blind, placebo-controlled study. Br J Nutr. 2012;108(2):327–33.
- Yu J, Liu L, Zhang Y, Wei J, Yang F. Effects of omega-3 fatty acids on patients undergoing surgery for gastrointestinal malignancy: a systematic review and meta-analysis. BMC Cancer. 2017;17(1):271.
- Wan GY, Zheng LY, Li HQ, Yuan H, Xue H, Zhang XY. Effects of enteral nutritional rich in n-3 polyunsaturated fatty acids on the nutritional status of gastrointestinal cancer patients: a systematic review and meta-analysis. Eur J Clin Nutr. 2020;74(2):220–30.
- Mocellin MC, Camargo CQ, Nunes EA, Fiates GMR, Trindade E. A systematic review and meta-analysis of the n-3 polyunsaturated fatty acids effects on inflammatory markers in colorectal cancer. Clin Nutr. 2016;35(2):359–69.
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700.
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
- Zhou L, Ding C, Wu J, Chen X, Ng DM, Wang H, Zhang Y, Shi N. Probiotics and synbiotics show clinical efficacy in treating gestational diabetes mellitus: a meta-analysis. Prim Care Diabetes. 2021;15(6):937–47.
- Czumbel LM, Kiss S, Farkas N, Mandel I, Hegyi A, Nagy Á, Lohinai Z, Szakács Z, Hegyi P, Steward MC, et al. Saliva as a candidate for COVID-19 diagnostic testing: a meta-analysis. Front Med (Lausanne). 2020;7:465.
- Lin L, Chu H. Quantifying publication bias in meta-analysis. Biometrics. 2018;74(3):785–94.
- Gu L, Khadaroo PA, Su H, Kong L, Chen L, Wang X, Li X, Zhu H, Zhong X, Pan J, et al. The safety and tolerability of combined immune checkpoint inhibitors (anti-PD-1/PD-L1 plus anti-CTLA-4): a systematic review and meta-analysis. BMC Cancer 2019;19(1):559.
- Bakker N, van den Helder RS, Stoutjesdijk E, van Pelt J, Houdijk APJ. Effects of perioperative intravenous ω-3 fatty acids in colon cancer patients: a randomized, double-blind, placebo-controlled clinical trial. Am J Clin Nutr. 2020;111(2):385–95.
- Braga M, Gianotti L, Vignali A, Carlo VD. Preoperative oral arginine and n-3 fatty acid supplementation improves the immunometabolic host response and outcome after colorectal resection for cancer. Surgery. 2002;132(5):805–14.
- Finco C, Magnanini P, Sarzo G, Vecchiato M, Luongo B, Savastano S, Bortoliero M, Barison P, Merigliano S. Prospective randomized study on perioperative enteral immunonutrition in laparoscopic colorectal surgery. Surg Endosc. 2007;21(7):1175–9.
- Haidari F, Abiri B, Iravani M, Ahmadi-Angali K, Vafa M. Randomized study design to test effects of vitamin D and omega-3 fatty acid supplementation as adjuvant therapy in colorectal cancer patients. Methods Mol Biol (Clifton, NJ). 2020;2138:337–50.
- Horie H, Okada M, Kojima M, Nagai H. Favorable effects of preoperative enteral immunonutrition on a surgical site infection in patients with colorectal cancer without malnutrition. Surg Today. 2006;36(12):1063–8.
- Liang B, Wang S, Ye YJ, Yang XD, Wang YL, Qu J, Xie QW, Yin MJ. Impact of postoperative omega-3 fatty acid-supplemented parenteral nutrition on clinical outcomes and immunomodulations in colorectal cancer patients. World J Gastroenterol. 2008;14(15):2434–9.
- Mocellin MC, Pastore e Silva JdA, Camargo CdQ, Fabre MEdS, Gevaerd S, Naliwaiko K, Moreno YMF, Nunes EA, Trindade EBSdM. Fish oil decreases C-reactive protein/albumin ratio improving nutritional prognosis and plasma fatty acid profile in colorectal cancer patients. Lipids. 2013;48(9):879–88.
- Mohammadzadeh M, Faramarzi E, Mahdavi R, Nasirimotlagh B, Asghari Jafarabadi M. Effect of conjugated linoleic acid supplementation on inflammatory factors and matrix metalloproteinase enzymes in rectal cancer patients undergoing chemoradiotherapy. Integr Cancer Ther. 2013;12(6):496–502.
- Purasiri P, Murray A, Richardson S, Heys SD, Horrobin D, Eremin O. Modulation of cytokine production in vivo by dietary essential fatty acids in patients with colorectal cancer. Clin Sci (Lond). 1994;87(6):711–7.
- Silva JdAP, Trindade EBSdM, Fabre MEdS, Menegotto VM, Gevaerd S, Buss ZdS, Frode TS. Fish oil supplement alters markers of inflammatory and nutritional status in colorectal cancer patients. Nutr Cancer. 2012;64(2):267–73.
- Sorensen LS, Thorlacius-Ussing O, Schmidt EB, Rasmussen HH, Lundbye-Christensen S, Calder PC, Lindorff-Larsen K. Randomized clinical trial of perioperative omega-3 fatty acid supplements in elective colorectal cancer surgery. Br J Surg. 2014;101(2):33–42.
- Zhu MW, Tang DN, Hou J, Wei JM, Hua B, Sun JH, Cui HY. Impact of fish oil enriched total parenteral nutrition on elderly patients after colorectal cancer surgery. Chin Med J (Engl). 2012;125(2):178–81.
- Tamas K, Walenkamp AM, de Vries EG, van Vugt MA, Beets-Tan RG, van Etten B, de Groot DJ, Hospers GA. Rectal and colon cancer: Not just a different anatomic site. Cancer Treat Rev. 2015;41(8):671–9.
- Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487(7407):330–7.
- Okamoto Y, Okano K, Izuishi K, Usuki H, Wakabayashi H, Suzuki Y. Attenuation of the systemic inflammatory response and infectious complications after gastrectomy with preoperative oral arginine and omega-3 fatty acids supplemented immunonutrition. World J Surg. 2009;33(9):1815–21.
- Ge X, Dai X, Ding C, Tian H, Yang J, Gong J, Zhu W, Li N, Li J. Early postoperative decrease of serum albumin predicts surgical outcome in patients undergoing colorectal resection. Dis Colon Rectum. 2017;60(3):326–34.
- Song GM, Tian X, Zhang L, Ou YX, Yi LJ, Shuai T, Zhou JG, Zeng Z, Yang HL. Immunonutrition support for patients undergoing surgery for gastrointestinal malignancy: preoperative, postoperative, or perioperative? A Bayesian network meta-analysis of randomized controlled trials. Medicine (Baltimore). 2015;94(29):e1225.
- Karoud W, Ghlissi Z, Krichen F, Kallel R, Bougatef H, Zarai Z, Boudawara T, Sahnoun Z, Sila A, Bougatef A. Oil from hake (Merluccius merluccius): characterization, antioxidant activity, wound healing and anti-inflammatory effects. J Tissue Viability. 2020;29(2):138–47.
- Wehner S, Meder K, Vilz TO, Alteheld B, Stehle P, Pech T, Kalff JC. Preoperative short-term parenteral administration of polyunsaturated fatty acids ameliorates intestinal inflammation and postoperative ileus in rodents. Langenbecks Arch Surg. 2012;397(2):307–15.
- Guan H, Chen S, Huang Q. Effects of enteral immunonutrition in patients undergoing pancreaticoduodenectomy: a meta-analysis of randomized controlled trials. Ann Nutr Metab. 2019;74(1):53–61.
- Gianotti L, Braga M, Nespoli L, Radaelli G, Beneduce A, Di Carlo V. A randomized controlled trial of preoperative oral supplementation with a specialized diet in patients with gastrointestinal cancer. Gastroenterology. 2002;122(7):1763–70.
- Aragona F, Franco V, Rodolico V, Dardanoni G, Cabibi D, Melloni D, Pavone C, Campesi G, Pavone-Macaluso M. Interactive computerized morphometric analysis for the differential diagnosis between dysplasia and well differentiated adenocarcinoma of the prostate. Urol Res. 1989;17(1):35–40.
- Zhabokritsky A, Szadkowski L, Cooper C, Loutfy M, Wong A, McClean A, Hogg RS, Walmsley SL. Increased CD4 : CD8 ratio normalization with implementation of current ART management guidelines. J Antimicrob Chemother. 2021;76(3):729–37.
- Turbitt WJ, Black AJ, Collins SD, Meng H, Xu H, Washington S, Aliaga C, El-Bayoumy K, Manni A, Rogers CJ. Fish oil enhances T cell function and tumor infiltration and is correlated with a cancer prevention effect in HER-2/neu but not PyMT transgenic mice. Nutr Cancer. 2015;67(6):965–75.
- Muka T, Kiefte-de Jong JC, Hofman A, Dehghan A, Rivadeneira F, Franco OH. Polyunsaturated fatty acids and serum C-reactive protein: the Rotterdam study. Am J Epidemiol. 2015;181(11):846–56.
- Lo CJ, Chiu KC, Fu M, Lo R, Helton S. Fish oil decreases macrophage tumor necrosis factor gene transcription by altering the NF kappa B activity. J Surg Res. 1999;82(2):216–21.
- Trebble T, Arden NK, Stroud MA, Wootton SA, Burdge GC, Miles EA, Ballinger AB, Thompson RL, Calder PC. Inhibition of tumour necrosis factor-alpha and interleukin 6 production by mononuclear cells following dietary fish-oil supplementation in healthy men and response to antioxidant co-supplementation. Br J Nutr. 2003;90(2):405–12.
- Yates CM, Tull SP, Madden J, Calder PC, Grimble RF, Nash GB, Rainger GE. Docosahexaenoic acid inhibits the adhesion of flowing neutrophils to cytokine stimulated human umbilical vein endothelial cells. J Nutr. 2011;141(7):1331–4.