Abstract
Objective
The impact of pre-immunotherapy sarcopenia in patients with non-small cell lung cancer (NSCLC) receiving immune checkpoint inhibitors (ICIs) is elusive. We performed a meta-analysis to investigate the association between sarcopenia and clinical outcomes of ICIs.
Methods
PubMed, EMBASE, and the Cochrane Library were searched.
Results
Thirteen clinical trials were selected. The 1,2-year overall survival rate was lower in the sarcopenia group (odds ratio (OR) = 2.44, 95% confidence interval (CI), 1.78–3.35, P < 0.00001; OR = 1.60, 95% CI, 1.08–2.37, P = 0.02), with I2 = 34%, P = 0.15, and I2 = 41%, P = 0.12. The 1,2-year progression-free survival (PFS) was the same (OR = 3.43, 95% CI, 1.86–6.33, P < 0.0001; OR = 2.06, 95% CI, 1.19–3.58, P < 0.0001), with I2 = 31%, P = 0.17 and I2=31%, P = 0.17. Sarcopenia reduced the overall response rate (OR = 2.22, 95% CI, 1.01–4.84, P = 0.02), with I2= 56%, P = 0.02, and disease control rate (OR = 3.15, 95% CI, 2.10–4.72, P < 0.0001) with I2 = 33%, P = 0.18.
Conclusion
Pre-immunotherapy sarcopenia was associated with poor clinical outcomes in patients with advanced NSCLC who received ICIs.
Introduction
Lung cancer is the leading cause of cancer-related mortality worldwide and the second-leading cause of new cancer cases (Citation1). Non-small-cell lung cancer (NSCLC) is one of the main forms of lung cancer, and most patients with NSCLC are already in an advanced stage at the time of diagnosis (Citation2,Citation3). For patients with advanced-stage NSCLC, recurrent or metastatic diseases, molecular-targeted drugs, immune checkpoint inhibitors (ICIs), and cytotoxic anticancer drugs are the main treatments (Citation4–6). Recently, research on ICIs has rapidly progressed. Programmed cell death protein 1 (PD-1) and programmed cell death protein ligand 1 (PD-L1) monoclonal antibodies are the most noteworthy clinical improvements in advanced and metastatic NSCLC (Citation7,Citation8).
High efficacy of ICI monotherapy or platinum-combination chemotherapy plus ICIs such as PD-1/PD-L1 inhibitors, has been noted in patients with driver mutation-negative disease (Citation5,Citation6). However, different patients with NSCLC presenting the same basic characteristics respond differently to the same ICI-based treatment. A study reported that only 18%–20% of patients with NSCLC achieved durable responses after salvage anti-PD-1 therapy and most had primary resistance to anti-PD-1 therapy (Citation9). Therefore, it is important to understand the key mechanisms of response heterogeneity, and predictive markers of ICIs efficacy are important (Citation10). Although the tumor proportion score is used to measure the expression of PD-L1 in tumor cells, its validity in predicting the effects of combined ICIs and ICIs therapy is insufficient (Citation11). Therefore, the identification of new biomarkers to evaluate the extent of tumor regression upon ICI-based therapies is urgently required.
Sarcopenia is a systemic skeletal muscle disease characterized by the loss of skeletal muscle mass and function, primarily caused by malnutrition, chronic diseases, and cancer (Citation12), although cancer itself can also cause a considerable loss of muscle mass (Citation13). Indeed, sarcopenia occurs in approximately 50% of patients with advanced cancer, owing to malnutrition, and treatments (Citation14). Furthermore, sarcopenia is associated with worse outcomes and can be considered a negative prognostic factor for several cancers (Citation15). Computed tomography (CT) is the gold standard for quantifying muscle mass. Measurements of the cross-sectional area of skeletal or bilateral psoas muscles at the level of the third lumbar vertebra (L3) are generally used to diagnose sarcopenia (Citation16). Currently, little is known about the influence of skeletal muscle loss in patients with NSCLC treated with ICIs therapy. Therefore, we performed a meta-analysis to investigate the correlation between the loss of skeletal muscle mass and clinical outcomes of patients with advanced NSCLC after ICIs treatment.
Material and Methods
Literature Search Strategy
Literature searches of PubMed, EMBASE, and the Cochrane Library were performed from the date of inception to August 2022. The search was limited to human studies. The main keywords used for the search were “immunotherapy,” “immunotherapies,” “ipilimumab,” “PD-1 blockade,” “PD-1 checkpoints inhibitors,” “nivolumab,” “Opdivo,” “pembrolizumab,” “lambrolizumab,” “Keytruda,” “sintilimab,” “camrelizumab,” “cemiplimab,” “PD-L1 blockade,” “PD-L1 checkpoints inhabitors,” “PD-L1,” “atezolizumab,” “durvalumab,” “avelumab,” “non-small-cell lung cancer,” “non-small cell lung cancer,” “sarcopenia,” “muscle loss,” “decreased muscle,” “muscle depletion,” “muscle wasting,” and “muscle reduction.”
Inclusion and Exclusion Criteria
The inclusion criteria were as follows: (1) trials including tumors of advanced stage, metastatic, or recurrent NSCLC and treated with ICIs therapy; (2) studies of the effect of sarcopenia and non-sarcopenia on survival outcomes before ICIs therapy; (3) studies on sarcopenia assessed by CT or other imaging modalities; (4) detailed original data available from the research; and (5) survival data, such as overall survival (OS), progression-free survival (PFS), overall response rate (ORR), and disease control rate (DCR).
The exclusion criteria were as follows: (1) the histopathology of patients was small-cell lung cancer; and (2) tumors were not in an advanced advanced stage, metastatic, or recurrent. (3) methods of sarcopenia assessment were not CT or other imaging modalities; (4) detailed original data and survival data could not be obtained.
Quality Assessment
The Newcastle-Ottawa Scale (NOS) was used to assess the data included in the cohort studies. The total score was nine points, including three items: comparability (two points), selection (four points), and outcome (three points). A score of <6 points indicated a low-quality study, 6–7 points a medium-quality study, and 8–9 points a high-quality study. For one randomized controlled trial, the risk of bias was determined using the Cochrane risk of bias tool.
Statistical Methods
Two authors independently extracted data to rule out subjective effects. The following details were extracted: study period, number, and stage of patients, ICI type, diagnostic method of sarcopenia, site of measurement, and cutoff value for sarcopenia. The outcomes were the 1,2-year OS, PFS, DCR, and ORR. DCR-represented lesions included complete response, partial response or stable disease. ORR-represented lesions showed complete response and partial response. When 1,2-year survival or ORR and DCR were not reported in the text, they were independently calculated from the survival curves. The relative frequencies of OS, PFS, DCR, and ORR between the sarcopenia and non-sarcopenia groups were expressed as odds ratios (OR) and 95% confidence interval (CI). Data were extracted from primary publications and entered into the meta-analysis using RevMan 5.3 and Stata 15.0 software. The level of heterogeneity among studies was evaluated using the Cochrane Q test and I2 statistics. Egger’s regression test was used to assess the publication bias.
Results
Search Results
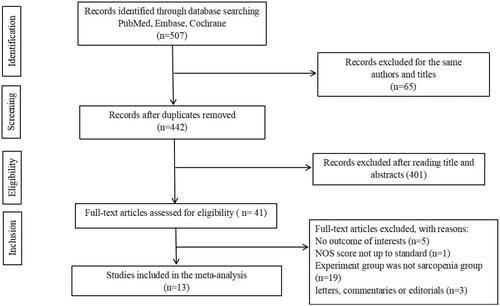
In total, 507 studies were retrieved. Following removal of duplicates, 442 studies were screened. A total of 401 studies were excluded after reading titles and abstracts. Among the remaining 41 articles, 28 studies were eventually excluded, 19 of which did not include a sarcopenia group, five did not report the outcome of interests, three were letters, commentaries, or editorials, and one had NOS scores not up to standard (). Finally, 13 studies were included in our research (Citation14,Citation17–25,Citation26–28).
Characteristics of Selected Studies
Thirteen studies involving 970 participants were included. The publication dates ranged from 2019 to 2021 and the sample size ranged from 23 to 156 participants. All studies included the sarcopenia diagnostic method, site of measurement, and cutoff value definition of sarcopenia ().
Table 1. Characteristics of the included studies.
Effect of Sarcopenia on 1,2-Year OS
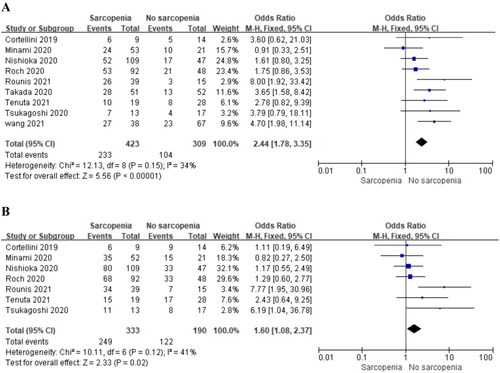
Nine and seven studies separately reported the impact of pre-immunotherapy sarcopenia on the 1,2-year OS in patients with NSCLC treated with ICIs therapy. The results showed a significantly poorer 1,2-year OS in the sarcopenia group compared to the group without sarcopenia () (OR = 2.44, 95% CI, 1.78–3.35, P < 0.00001; OR = 1.60, 95% CI, 1.08–2.37, P = 0.02). No significant heterogeneity existed among the included studies in the 1,2-year OS analysis (I2 = 34%, P = 0.15; I2 = 41%, P = 0.12), and a fixed-effects model was used.
Effect of Sarcopenia on 1,2-Year PFS
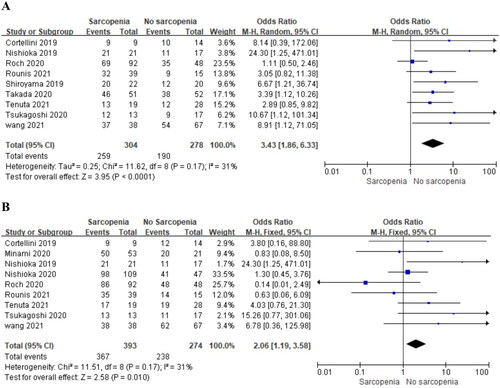
Nine studies separately reported the 1,2-year PFS of patients with NSCLC and pre-immunotherapy sarcopenia. The analyses showed a significantly poorer 1,2-year PFS with pre-immunotherapy sarcopenia (OR = 3.43, 95% CI, 1.86–6.33, P < 0.0001, ; OR = 2.06, 95% CI, 1.19–3.58, P < 0.0001, , respectively). No significant heterogeneity was observed among the included studies (I2 = 31%, P = 0.17; I2 = 31%, P = 0.17).
Effect of Sarcopenia on ORR
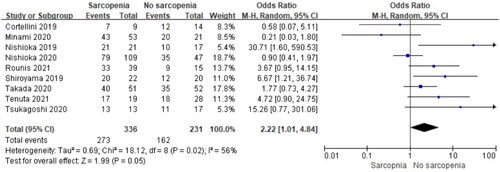
Nine studies reported the influence of pre-immunotherapy sarcopenia on the ORR. Subsequent analysis of these studies revealed that sarcopenia reduced ORR (OR = 2.22, 95% CI, 1.01–4.84, P = 0.02, ). Significant heterogeneity was found among the included studies (I2, 56%, P = 0.02); thus, a random effects model was used. Studies by Nishioka et al. (Citation20), Minami et al. (Citation25), and Cortellini et al. (Citation28) reported the least unfavorable effects of sarcopenia and were the main contributors to this heterogeneity. Following exclusion of these three studies, the heterogeneity was not significant (I2, 21%, P = 0.28), and the effects of sarcopenia remained significant (OR = 3.55, 95% CI, 1.70–7.40, P = 0.0007).
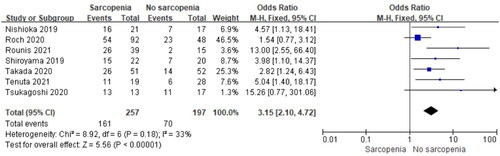
Effect of Sarcopenia on DCR
Seven studies reported the effect of pre-immunotherapy sarcopenia on DCR. Pooled data showed a significantly poor DCR in the sarcopenia group (OR = 3.15, 95% CI, 2.10–4.72, P < 0.0001, ). No significant heterogeneity was observed among included studies (I2 = 33%, P = 0.18).
Toxicities
In the included literature, two studies (Citation22,Citation28) reported the relationship between skeletal muscle mass loss and immune-related adverse event. Tsukagoshi’s study (Citation22) showed that three patients among 17 with normal muscle mass and two patients among 13 with skeletal muscle loss had immune-related adverse events, and the difference was not statistically significant (P = 1.00). Cortellini (Citation28) reported that three (33.3%) patients with low skeletal muscle mass and 11 (78.6%) with non-low skeletal muscle mass (P = 0.0771) experienced immune-related adverse events. Both studies reported that sarcopenia does not increase the rate of adverse effects of immunotherapy.
Literature Publication Bias Evaluation
The publication biases of included studies are shown in . The p-value for publication bias regarding the overall 1-year PFS and DCR was <0.05, suggesting the presence of publication bias, and the remaining comparisons had a p-value >0.05, indicating no significant publication bias. Egger’s regression test showed that publication bias existed in 1-year PFS and DCR. For publication bias of 1-year PFS, the scissor method showed that the combined results of effect indicators before and after clipping were 1.021 (95% CI, 0.556–1.486) and 2.215 (1.433–3.422), respectively using the fixed-effects model, and 1.225 (95% CI, 0.621–1.829) and 2.449 (1.328–4.514), respectively using the random-effects model. For publication bias of DCR, the scissor method before and after clipping was 1.096 (95% CI, 0.678–1.513) and 2.198 (1.520–3.179), respectively using the fixed effects model, and 1.250 (95% CI, 0.697–1.803) and 2.287 (1.294–4.044), respectively using the random effects model.
Table 2. Evaluation of publication bias in the included literature.
Discussion
This analysis included 13 studies involving 970 patients that examined the relationship between sarcopenia and immunotherapy, especially in relation to OS and PFS, in patients with advanced NSCLC. We found that patients with sarcopenia who received ICIs therapy showed significantly worse PFS, OS, ORR, and DCR compared to those of their non-sarcopenic counterparts. ICIs treatment is known to improve the survival of patients with advanced cancer. However, some patients with cancer fail to respond to ICIs therapy. To better evaluate the efficacy of ICIs therapy, a growing number of markers have been identified, such as the presence of CD8 tumor-infiltrating lymphocytes, PD-L1 expression, and tumor mutation burden (Citation29). An increasing number of studies have investigated the relationship between ICIs therapy and pretherapy skeletal mass loss. A meta-analysis conducted by Deng included nine cohort studies consisting of 740 patients with advanced cancer receiving ICIs. It was concluded that sarcopenia was an independent unfavorable prognostic factor in patients with advanced cancer receiving ICIs. They suggested that sarcopenia status and correction of sarcopenic status should be emphasized for patients with advanced cancer treated with ICIs (Citation30). Wang et al. performed a meta-analysis of the effects of sarcopenia on the clinical efficacy of ICIs in patients with NSCLC. This included nine studies consisting of 576 patients with NSCLC. They concluded that pre-immunotherapy sarcopenia was significantly associated with a worse OS (HR = 1.61, 95% CI = 1.24–2.10) and PFS (HR = 1.98, 95% CI = 1.32–2.97), lower DCR (RR = 0.70, 95% CI = 0.56–0.86) and ORR (RR = 0.54, 95% CI = 0.19–1.53) (Citation31). To some degree, the results of our study were consistent with the results of the described meta-analysis. However, our study included more studies and patients, and focused on the long-term (2- year) survival benefit of non-sarcopenia. Alternatively, our study emphasized that the lesions of patients with NSCLC were in an advanced stage, recurrent, and metastatic, and a prospective study was included to increase the robustness of the article.
The mechanism by which sarcopenia leads to poor survival and prognosis in patients with cancer remains elusive. A better understanding of the underlying mechanisms of muscle loss in patients with cancer may help develop therapeutic strategies. Skeletal muscle atrophy is the combined result of an imbalance in protein synthesis and degradation, increased muscle cell apoptosis, and reduced regenerative capacity (Citation32). Several molecular mechanisms have been described as causes of sarcopenia, involving extremely different levels of muscle physiology. These mechanisms include the function of hormones, muscle fiber composition, neuromuscular drive, myo-satellite cell potential to differentiate and proliferate, inflammatory pathways, and intracellular mechanisms in proteostasis and mitochondrial function (Citation33). Skeletal muscle has another important function as an organ of cytokines that are extensively involved in inflammatory processes, and these cytokines have systemic effects on the antitumor immune response (Citation34). Decreased actin levels due to skeletal muscle loss may contribute to a poorer response to ICIs. For tumors, including NSCLC, checkpoint inhibitor pneumonitis significantly shortens the survival time of patients receiving immunotherapy (Citation35,Citation36). Kikuchi’s study included 74 patients with interstitial lung disease complicated NSCLC who received chemotherapy and concluded that sarcopenia group had a higher rate of interstitial lung disease exacerbation, together with shorter median OS, compared to nonsarcopenia group (Citation37). However, in our study, Tsukagoshi’s (Citation22) and Cortellini’s (Citation23) studies reported that sarcopenia does not increase the number of adverse effects of immunotherapy.
Of the 13 studies included in our meta-analysis, most used CT images to assess sarcopenia. The cross-sectional area of bilateral psoas muscles, or all muscles at the third lumbar vertebra (L3), were the measurement site. Different studies have reported different cutoff values for the diagnosis of sarcopenia. The most effective procedure to date is the use of dual-energy X-ray absorptiometry (DXA), which estimates lean mass (Citation38). In the included studies, Tenuta et al. assessed sarcopenia through DXA scans in a population of patients with advanced NSCLC prior to receiving PD-1/PD-L1 inhibitors. Moreover, their cutoff value to define sarcopenia were appendicular skeletal muscle mass/height2 <7.0 kg/m2 in men and <5.5 kg/m2 in women (Citation18).
No specific drug has been approved for the treatment of sarcopenia. Murphy et al. concluded that nutritional intervention with 2.2 g of fish oil per day appeared to provide a benefit over the no-intervention group, resulting in the maintenance of weight and muscle mass during chemotherapy (Citation39). An umbrella review of systematic reviews and meta-analyses focused on pharmacological interventions to improve muscle mass and identified ten pharmacological interventions: vitamin D, combined estrogen–progesterone, dehydroepiandrosterone, growth hormone, growth hormone-releasing hormone, combined testosterone growth hormone, insulin-like growth factor-1, pioglitazone, testosterone, and angiotensin-converting enzyme inhibitors (Citation40). A phase II, randomized, controlled, proof-of-concept study concluded that treatment with bimagrumab over 16 weeks increased muscle mass and strength in older adults with sarcopenia (Citation41). In the future, more research is required to explore the relationship between muscle gain and the efficacy of ICIs in patients with NSCLC.
Conclusion
This study has several limitations. First, most of the included studies were retrospective analyses and were influenced by selection bias and treatment variations during the study period. However, all these studies compared sarcopenia with no sarcopenia and evaluated the same endpoints. Thus, we believe that a meta-analysis is warranted to confirm the effects of sarcopenia on patients with advanced NSCLC receiving ICIs. Second, there is a potential publication bias: in the literature publication bias evaluation, we found that publication bias exists in the 1-year PFS and DCR. The result of 1-year PFS analysis consists of 2-year PFS, and the scissor method showed that before and after clipping, the result of DCR changed in the same direction; thus, publication bias may have no effect on the final results. Third, there was moderate heterogeneity in the ORR analysis. Therefore, we used a random-effects model to pool results, and three studies (Citation20,Citation25,Citation28) were the main contributors to this heterogeneity. Following exclusion of these three studies, the heterogeneity was not significant. Finally, the number of patients included in some studies was relatively small.
The influence of sarcopenia in patients with NSCLC treated with ICIs is not well known. Based on the existing analysis, patients with advanced NSCLC with sarcopenia undergoing ICIs therapy have a poorer prognosis. Screening for sarcopenia could help identify patients with NSCLC who may benefit from ICIs in clinical settings. Larger prospective randomized controlled trials are required to further demonstrate the role of sarcopenia in patients with NSCLC treated with ICIs.
Acknowledgments
Bixin Ren, Jiucheng Shen, and Yajuan Qian conceived and coordinated the study, designed, performed, and analyzed the experiments, and wrote the manuscript. Bixin Ren, Jiucheng Shen, and Yajuan Qian collected and analyzed the data. Tongzhou revised the manuscript accordingly. All authors have reviewed the results and approved the final version of the manuscript.
Disclosure Statement
The authors have no conflict of interest to declare.
Funding
This study was funded by Suzhou Science and Technology Bureau, Science and Technology Development Program Medical Devices and New Medicines (Clinical Trials), SLJ202008.
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
- Bade BC, Dela Cruz CS. Lung cancer 2020: epidemiology, etiology, and prevention. Clin Chest Med. 2020;41(1):1–24.
- Rosell R, Karachaliou N. Large-scale screening for somatic mutations in lung cancer[J]. Lancet. 2016;387(10026):1354–6.
- Paik PK, Felip E, Veillon R, Sakai H, Cortot AB, Garassino MC, Mazieres J, Viteri S, Senellart H, Van Meerbeeck J, et al. Tepotinib in non-small-cell lung cancer with MET exon 14 skipping mutations. N Engl J Med. 2020;383(10):931–43.
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ, Powell SF, KEYNOTE-189 Investigators, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078–92.
- Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, Hermes B, Çay Şenler F, Csőszi T, Fülöp A, KEYNOTE-407 Investigators, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379(21):2040–51.
- Li X, Lian Z, Wang S, Xing L, Yu J. Interactions between EGFR and PD-1/pd-L1 Pathway:implications for Treatment of NSCLC. Cancer Lett. 2018;418:1–9.
- Mok TSK, Wu Y-L, Kudaba I, Kowalski DM, Cho BC, Turna HZ, Castro G, Srimuninnimit V, Laktionov KK, Bondarenko I, KEYNOTE-042 Investigators, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819–30.
- Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols MC, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255–65.
- Nakamura Y. Biomarkers for immune checkpoint inhibitor-mediated tumor response and adverse events. Front Med (Lausanne). 2019;6:119.
- Schildhaus HU. Predictive value of PD-L1 diagnostics. Pathologe. 2018;39(6):498–519.
- Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet. 2019;393(10191):2636–46.
- Bossi P, Delrio P, Mascheroni A, Zanetti M. The spectrum of malnutrition/cachexia/sarcopenia in oncology according to different cancer types and settings: a narrative review. Nutrients. 2021;13(6):1980.
- Shiroyama T, Nagatomo I, Koyama S, Hirata H, Nishida S, Miyake K, Fukushima K, Shirai Y, Mitsui Y, Takata S, et al. Impact of sarcopenia in patients with advanced non-small cell lung cancer treated with PD-1 inhibitors: a preliminary retrospective study. Sci Rep. 2019;9(1):2447.
- Shachar S, Williams GR, Muss HB, Nishijima TF. Prognostic value of sarcopenia in adults with solid tumours: a meta analysis and systematic review. Eur J Cancer. 2016;57:58–67.
- Nakamura R, Inage Y, Tobita R, Yoneyama S, Numata T, Ota K, Yanai H, Endo T, Inadome Y, Sakashita S, et al. Sarcopenia in resected NSCLC: effect on postoperative outcomes. J Thorac Oncol. 2018;13(7):895–903.
- Wang Y, Chen P, Huang J, Liu M, Peng D, Li Z, Chen T, Hong S, Zhou Y. Assessment of sarcopenia as a predictor of poor overall survival for advanced non-small-cell lung cancer patients receiving salvage anti-PD-1 immunotherapy. Ann Transl Med. 2021;9(24):1801.
- Tenuta M, Gelibter A, Pandozzi C, Sirgiovanni G, Campolo F, Venneri MA, Caponnetto S, Cortesi E, Marchetti P, Isidori AM, et al. Impact of sarcopenia and inflammation on patients with advanced non-small cell lung cancer (NCSCL) treated with immune checkpoint inhibitors (ICIs): a prospective study. Cancers (Basel). 2021;13(24):6355.
- Petrova MP, Donev IS, Radanova MA, Eneva MI, Dimitrova EG, Valchev GN, Minchev VT, Taushanova MS, Boneva MV, Karanikolova TS, et al. Sarcopenia and high NLR are associated with the development of hyperprogressive disease after second-line pembrolizumab in patients with non-small-cell lung cancer. Clin Exp Immunol. 2020;202(3):353–62.
- Nishioka N, Naito T, Notsu A, Mori K, Kodama H, Miyawaki E, Miyawaki T, Mamesaya N, Kobayashi H, Omori S, et al. Unfavorable impact of decreased muscle quality on the efficacy of immunotherapy for advanced non-small cell lung cancer. Cancer Med. 2021;10(1):247–56.
- Rounis K, Makrakis D, Tsigkas A-P, Georgiou A, Galanakis N, Papadaki C, Monastirioti A, Vamvakas L, Kalbakis K, Vardakis N, et al. Cancer cachexia syndrome and clinical outcome in patients with metastatic non-small cell lung cancer treated with PD-1/PD-L1 inhibitors: results from a prospective, observational study. Transl Lung Cancer Res. 2021;10(8):3538–49.
- Tsukagoshi M, Yokobori T, Yajima T, Maeno T, Shimizu K, Mogi A, Araki K, Harimoto N, Shirabe K, Kaira K, et al. Skeletal muscle mass predicts the outcome of nivolumab treatment for non-small cell lung cancer. Medicine (Baltimore). 2020;99(7):e19059.
- Takada K, Yoneshima Y, Tanaka K, Okamoto I, Shimokawa M, Wakasu S, Takamori S, Toyokawa G, Oba T, Osoegawa A, et al. Clinical impact of skeletal muscle area in patients with non-small cell lung cancer treated with anti-PD-1 inhibitors. J Cancer Res Clin Oncol. 2020;146(5):1217–25.
- Roch B, Coffy A, Jean-Baptiste S, Palaysi E, Daures J-P, Pujol J-L, Bommart S. Cachexia - sarcopenia as a determinant of disease control rate and survival in non-small lung cancer patients receiving immune-checkpoint inhibitors. Lung Cancer. 2020;143:19–26.
- Minami S, Ihara S, Tanaka T, Komuta K. Sarcopenia and visceral adiposity did not affect efficacy of immune-checkpoint inhibitor monotherapy for pretreated patients with advanced non-small cell lung cancer. World J Oncol. 2020;11(1):9–22.
- Nishioka N, Uchino J, Hirai S, Katayama Y, Yoshimura A, Okura N, Tanimura K, Harita S, Imabayashi T, Chihara Y, et al. Association of sarcopenia with and efficacy of anti-PD-1/PD-L1 therapy in non-small-cell lung cancer. JCM. 2019;8(4):450.
- Magri V, Gottfried T, Di Segni M, Urban D, Peled M, Daher S, Stoff R, Bar J, Onn A. Correlation of body composition by computerized tomography and metabolic parameters with survival of nivolumab-treated lung cancer patients. Cancer Manag Res. 2019;11:8201–7.
- Cortellini A, Verna L, Porzio G, Bozzetti F, Palumbo P, Masciocchi C, Cannita K, Parisi A, Brocco D, Tinari N, et al. Predictive value of skeletal muscle mass for immunotherapy with nivolumab in non-small cell lung cancer patients: a "hypothesis-generator" preliminary report. Thorac Cancer. 2019;10(2):347–51.
- Hugo W, Zaretsky JM, Sun L, Song C, Moreno BH, Hu-Lieskovan S, Berent-Maoz B, Pang J, Chmielowski B, Cherry G, et al. Genomic and transcriptomic features of response to Anti-PD-1 therapy in metastatic melanoma. Cell. 2016;165(1):35–44.
- Deng H-Y, Chen Z-J, Qiu X-M, Zhu D-X, Tang X-J, Zhou Q. Sarcopenia and prognosis of advanced cancer patients receiving immune checkpoint inhibitors: a comprehensive systematic review an meta-analysis. Nutrition. 2021;90:111345.
- Wang J, Cao L, Xu S. Arcopenia affects clinical efficacy of immune checkpoint inhibitors in non-small cell lung cancer patients: a systematic review and meta-analysis. Int Immunopharmacol. 2020;88:106907.
- Argilés JM, Busquets S, Stemmler B, López-Soriano FJ. Cachexia and sarcopenia: mechanisms and potential targets for intervention. Curr Opin Pharmacol. 2015;22:100–6.
- Wiedmer P, Jung T, Castro JP, Pomatto LCD, Sun PY, Davies KJA, Grune T. Sarcopenia - Molecular mechanisms and open questions. Ageing Res Rev. 2021;65:101200.
- Pratesi A, Tarantini F, Di Bari M. Skeletal muscle: an endocrine organ. Clin Cases Miner Bone Metab. 2013;10(1):11–4.
- Suresh K, Psoter KJ, Voong KR, Shankar B, Forde PM, Ettinger DS, Marrone KA, Kelly RJ, Hann CL, Levy B, et al. Impact of checkpoint inhibitor pneumonitis on survival in NSCLC patients receiving immune checkpoint immunotherapy. J Thorac Oncol. 2019;14(3):494–502.
- Gomatou G, Tzilas V, Kotteas E, Syrigos K, Bouros D. Immune checkpoint inhibitor-related pneumonitis. Respiration. 2020;99(11):932–42.
- Kikuchi R, Takoi H, Ishiwari M, Toriyama K, Kono Y, Togashi Y, Abe S. Impact of sarcopenia on chemotherapy-triggered exacerbation of interstitial lung disease in patients with non-small cell lung cancer. Thorac Cancer. 2022;13(4):549–56.
- Buckinx F, Landi F, Cesari M, Fielding RA, Visser M, Engelke K, Maggi S, Dennison E, Al-Daghri NM, Allepaerts S, et al. Pitfalls in the measurement of muscle mass: a need for a reference standard. J Cachexia Sarcopenia Muscle. 2018;9(2):269–78.
- Murphy RA, Mourtzakis M, Chu QSC, Baracos VE, Reiman T, Mazurak VC. Nutritional intervention with fish oil provides a benefit over standard of care for weight and skeletal muscle mass in patients with Non-small cell lung cancer receiving chemotherapy. Cancer. 2011;117(8):1775–82.
- De Spiegeleer A, Beckwee D, Bautmans I, Petrovic M, the Sarcopenia Guidelines Development group of the Belgian Society of Gerontology and Geriatrics (BSGG). Pharmacological interventions to improve muscle mass, muscle strength and physical performance in older people: an umbrella review of systematic reviews and meta-analyses. Drugs Aging. 2018;35(8):719–34.
- Rooks D, Praestgaard J, Hariry S, Laurent D, Petricoul O, Perry RG, Lach-Trifilieff E, Roubenoff R. Treatment of sarcopenia with bimagrumab: results from a Phase II, randomized, controlled, proof-of-concept study. J Am Geriatr Soc. 2017;65(9):1988–95.