ABSTRACT
The majority of animals possess claws on their legs and they are mainly used for keeping attached to the substrate. Recent studies on claw shapes and function suggest that claws are not just simple attachment devices but highly specialized morphological traits bearing information about lifestyle, ecology and evolution. In that respect, knowledge about claws is extremely scarce, especially in highly diverse groups, as for example the oribatid mites, with more than 11000 named species occurring in every environment all over the world. This review summarizes all information about claws and relevant aspects for this large group of arthropods. There is a huge variety of ambulacral claw morphologies present in oribatid mites and this diversity does not follow a strict phylogenetic or systematic pattern. Lifestyle and ecology apparently play an important role in shaping claw morphologies. Intertidal oribatid mites are mostly characterized by having single large tarsal claws to withstand tidal flooding, a similar tendency can be found in terrestrial limnic species where monodactylous species prevail. Additionally, fresh-water Oribatida often show scaliform and barbed distal tarsal setae which cooperate with the claws helping the mites to stick to water plants. Claws of arboreal mites are often equipped with adhesive pads “pulvilli” allowing them to walk on smooth plant surfaces. Suction pads are also found in a few mites showing epilithic lifestyles and in mites being able to perform sudden evasive jumps. Claws of phoretic oribatid mites show in most cases no apparent adaptations but certain groups are equipped with highly modified claws allowing them to attach to specific structures of their hosts. Finally, this review gives an overview of all claw morphologies present in each larger phylogenetic oribatid mite group.
Introduction
Claws are morphological structures that are present in nearly every animal group from the tiniest arthropods to the larger vertebrates. They are mainly used for attachment but can also fulfil other functions, like digging, fighting, or catching prey (Tinius and Russell Citation2017). Despite their frequent occurrence in the animal kingdom, relatively little is known about the details of their shape, function, and correlation with other factors. This becomes especially evident in taxa with high species numbers and highly diverse ecologies, like the Acari (mites). There are more than 55,000 species known worldwide from nearly every environment (Walter and Proctor Citation1999) but there are only a handful of studies focusing specifically on claws and related aspects.
First insights into the basic anatomy and function of the claws of mites were given in the first half of the twentieth century (Grandjean Citation1941, Citation1943) and included information on Oribatida, some Prostigmata, and Astigmata. These studies demonstrated that tarsi of mites can basically bear 1–3 claws and that these are moved through the action of only two muscles. In Prostigmata, the central claw is lacking and replaced by other homologous structures, like pads or discs, whereas Astigmata are characterized by a single claw associated with a suction pad (Grandjean Citation1943). Decades later, another study focused only the latter group (Atyeo Citation1979), highlighting the unusual variety of single pretarsal claws and their associated fleshy suction pads. In the following years, considerable modifications of the supposed ancestral paired claw and median pad-like structures in prostigmatid plant mites (Tetranychoidea) were reported (Lindquist Citation1985), and the Opilioacariformes and Parasitiformes were shown to also possess cushion-like pads between paired lateral claws (Evans Citation1992). A subsequent review of the character states and evolution of claws among all chelicerates (Dunlop Citation2002) demonstrated that mites (Acari) show the largest number of empodial claw characteristics among Chelicerata and concluded that there is no “typical” claw apparatus for all mites and that it is extremely difficult to homologize all the various structures present in this taxon. These few fundamental studies laid the foundation of what we presently know about claws in mites, but afterwards, research on mite claws came to a halt.
However, claw features are supposed to be important parameters related to diverse ecological aspects (Ribas et al. Citation2004) and thus should be investigated. Numerous studies demonstrated a correlation between ecology and claw shape in vertebrates, like non-avian dinosaurs, birds, and lizards (e.g. Feduccia Citation1993; Zani Citation2000; Manning et al. Citation2006). In mites, on the other hand, there were only two recent studies (Karasawa and Hijii Citation2004; Pfingstl et al. Citation2020) demonstrating that claw number, length, and shape are correlated with habitat and lifestyle, indicating that ecology may play a major role in shaping these structures. According to these studies, claws of mites are more than just simple morphological structures for attachment, they are highly specialized traits bearing information about the lifestyle, ecology, and evolution of these animals. However, these studies included only a few oribatid mite species and none of the other mite groups, and therefore may be of limited significance.
There are approximately 11,000 named oribatid mite species (Subías Citation2022) occurring all over the world. They inhabit almost all terrestrial environments and they are often the dominant or most abundant arthropods in soils (Behan-Pelletier and Lindo Citation2023). Due to their small size, oribatid mites are able to occupy nearly every ecological niche. They can be found in leaf litter, mosses, lichens, grass, soil, on tree trunks, in the canopy, on rocks, in swamps, etc. and therefore, their claws interact with a variety of substrates. The large differences in the superficial physical characteristics of these terrestrial habitats probably pose very specific demands on claw morphology. Consequently, research on these attachment devices in oribatid mites could reveal important insights into their ecologies and interaction with their environment.
Our present knowledge about claws and related aspects is largely incomplete, not just in oribatid mites, but in any given mite group, and we are far from understanding the causes for all the various modifications of claws found in nature. Claw characteristics and possible implications have been neglected for a long time and many species descriptions completely lack information about this morphological feature. Numerous authors described claws with just a few words but did not provide any depiction regarding the specific shape of the claw. Others provided excellent and detailed descriptions, but did not give any information about the specific habitat of the species, making it difficult to correlate claw characteristics with the environment.
The present review intends to give an overview of claw morphologies found in Oribatida, as well as to summarize all the existing literature containing information about oribatid claws and their possible function and correlation with environmental aspects. This work should be a step towards increasing research on these important morphological features and should serve as a useful basis for future studies.
Material and methods
All data presented in this paper are based on published literature only. The respective literature is given to the best of my ability but considering the more than 11,000 known oribatid species (Subías Citation2022), I might have missed specific claw modifications or specific studies. The classification given herein follows that of Norton and Behan-Pelletier (Citation2009) and Schatz et al. (Citation2011). As this review contains the names of many species and as the text should be easy to read, I refrain from providing species author and date when the species is listed for the first time in the text.
All images of claws were created with the free open-source vector graphics editor Inkscape (https://inkscape.org) and the arrangement and labelling of figure plates was done with Adobe Photoshop 7.0. Several claw depictions were modified after original descriptions and in these cases the authors of the original work are indicated in the figure caption. Tarsal setae were omitted from the drawings to allow a better view of the claw structures. Morphological terminology for leg setal nomenclature used herein follows that of Norton (Citation1977).
Short glossary
ambulacrum – consists of claws and the empodium
apotele – the terminal element of the postcheliceral limbs
condylophore – joint, articulation
empodium – membranous region between the claws; basilar piece
heterodactylous – (I) claws of a single leg differ in appearance; (II) claw number varies among legs (in some cases referred to as polydactyly)
homodactylous – (I) all claws of a single leg with the same appearance; (II) claw number is constant among legs
mono-, bi-, tridactylous – one, two, three claws on a single leg
pulvillus – a small fleshy pad between the claws, typically eversible
subunguinal – at the base of the claw(s)
The basic structure of the ambulacrum (claw apparatus)
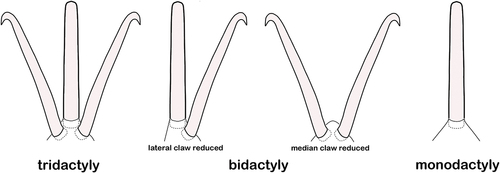
Among arachnids, Palpigradi retain the most plesiomorphic apotele morphology with three claws on all postcheliceral limbs, including the pedipalp (Dunlop Citation2002). In mites, tarsal claws of the pedipalp are generally lost, only the Opilioacariformes are known to have retained two claws on this limb (Dunlop Citation2002). In Oribatida, only the legs are equipped with clawed apoteles and mono-, bi- and tridactylous tarsi can be found in the adults (Evans Citation1992). Although the number of claws ranges from one to three in adult Oribatida, almost all juvenile stages are monodactylous, possessing only the median claw (Evans Citation1992), except for immatures of some Palaeosomata and Enarthronota, which may show more than one claw during their development (Grandjean Citation1954a; Travé Citation1967; Norton and Fuangarworn Citation2015).
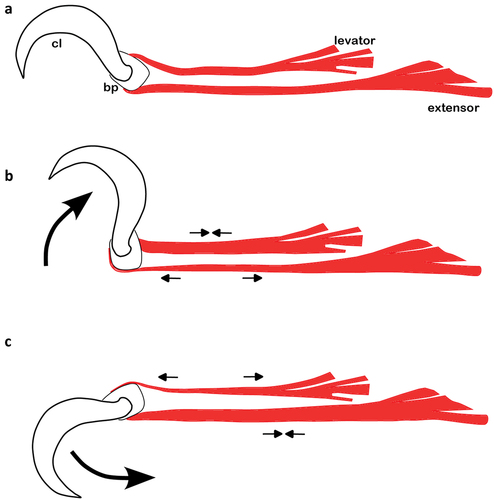
The fundamental structure of the ambulacrum is the same in all Oribatida, the claws insert on a mobile basilar piece that articulates with the tarsus via a pair of bilateral condylophores (Grandjean Citation1941). These condylophores are suggested to represent the extremity of the tarsus, accordingly the articulation between the basilar piece and the condylophores should correspond to the articulation between tarsus and apotele (Grandjean Citation1941). The basilar piece is a membranous, deformable structure and the claws are formed of modified setae (Evans Citation1992). The median claw is partially or completely fused with the basilar piece, whereas the lateral claws are only accessory structures with a more or less loose connection (Grandjean Citation1941). The lack of the lateral claws results in the monodactylous condition, with the single remaining claw being homologous to the median or empodial claw of a tridactylous apotele (). The only difference between a mono- and a tridactylous species is that the basilar piece is less wide and large in the former (Grandjean Citation1941). In bidactylous species, either one of the lateral claws is suppressed, e.g. in Nothrus, or the median claw may be partially or completely reduced, as for example in Eulohmannia ribagai or Gehypochthonius rhadamantus (Grandjean Citation1943).
Figure 1. Types of ambulacra in dorsal view showing possible configurations from three to one tarsal claws.
Movements of the claws are affected through the action of two muscles, the levator (flexor) and the extensor. The tendon of the levator is connected to the dorsal surface of the basilar piece and the muscle originates in the tarsus, whereas the tendon of the extensor is fused with the ventral surface of the basilar piece and extends across large parts of the tarsus or the whole tarsus while the muscle originates in the tibia (Grandjean Citation1941). Contraction of the respective muscle results in either lifting or lowering the claws, whereas the ambulacrum can generally rotate 90° (), in extreme cases even 180°, but no lateral movement is possible (Grandjean Citation1943).
Function of tarsal claws
Tarsal claws are the primary morphological feature that makes contact with the substrate and thus it seems apparent that they serve as typical attachment devices. Their mostly hook-like appearance suggests that they are used in a simple “hook and pull” fashion. This rationale cannot be discounted, however, observational or experimental studies investigating the function and operation of claws are largely lacking, at least in oribatid mites. Only a single study (Heethoff and Koerner Citation2007) demonstrated that the oribatid Archegozetes longisetosus produces exceptionally high relative claw forces. Due to the lack of other studies, it is unknown how these structures work in detail. For example, ambulacral adhesive pads are present in several arboreal taxa (e.g. Behan-Pelletier and Walter Citation2007) and it is assumed that they facilitate attachment to smooth surfaces, but how they make contact with the substrate or how they interact with different kinds of surfaces has never been observed or studied in oribatid mites. The diverse morphologies of tarsal claws and their accessory structures indicate that they often might be used in more than just a simple “hook and pull” function.
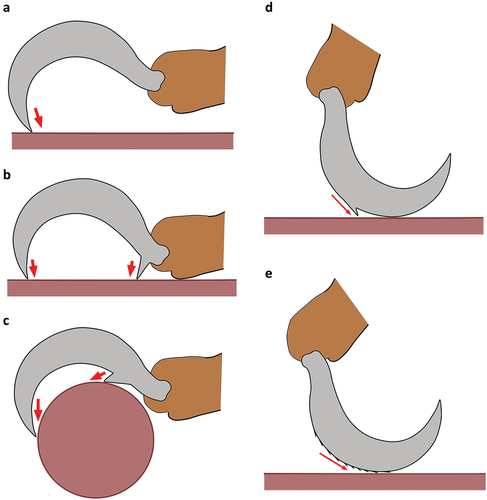
Claw curvature in monodactylous taxa, for example, ranges from strongly curved to hardly curved (), and the latter will clearly not work the exact same way as the strongly hooked claw. Teeth like structures at the base or the proximoventral part of a claw may allow two or multi-point fixations and thus a stronger grip to the substrate (). Different positions of the teeth may be related to walking and living on different surfaces. The presence of spines or barbs on the dorsal edge of claws, on the other hand, suggest that mites may occasionally walk with completely lowered claws (), in that case the spines and barbs would increase friction and prevent slipping on smooth surfaces. Another option would be that claws could be used for digging, then additional dorsal structures would also make sense.
Figure 3. Claws of selected monodactylous oribatid mites highlighting the wide range of different curvatures that can be found. The intertidal F. rotunda (Ameronothroidea) shows strongly curved claws (left) while the soil dwelling H. rufulus (Hypochthonioidea) possesses only weakly curved claws (right).
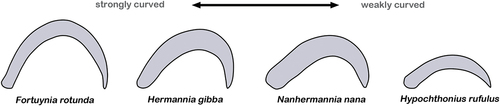
Figure 4. Basic function of claws and possible operation of additional claw structures. (a) Claw tip used for a simple “hook” movement. (b) Additional basoventral tooth allowing a two-point fixation. (c) Proximoventral tooth allowing two-point fixation on uneven or rounded surfaces. (d) Dorsal tooth prevents slipping when mite walks on dorsal edge of claw. (e) Barbed or serrated dorsal aspect increases friction when mite walks on dorsal edge of claw.
Mites mainly move forward and considering that legs I–II are oriented forwards and legs III–IV backwards, claws of the anterior legs work in a “hook and pull” fashion while claws of the posterior legs work mainly in a “hook and push” fashion. Although most mites show no apparent difference in shape and size between anterior and posterior leg claws, there are several taxa with different claw numbers, e.g. one claw on leg I–II, three claws on leg III–IV, or different claw features indicating that the function of the claw may indeed slightly diverge depending on which leg they are borne. In Phylleremus species, for example, leg I and II have large claws and small pulvilli while leg III and IV have large pulvilli but small claws (Behan-Pelletier and Walter Citation2007), consequently the anterior legs may be primarily used for moving forward and the posterior legs for keeping attached to the ground. In other species, e.g. Aleurodamaeus, the size of the claws becomes progressively larger from leg I to IV (Hugo-Coetzee Citation2013) indicating slightly divergent functionalities of each leg.
However, these are all just theories and require confirmation by observation and experiments on living specimens. Moreover, claws may also be used for other purposes than simple attachment, but presently, we are far from knowing all possible functions and far from understanding how tarsal claws operate in detail.
Claw development and ontogenetic patterns
Oribatid immatures show typically monodactylous legs, independent of the number of claws present in their adult stage (Evans Citation1992). Claws are already present in the earliest mobile developmental stage and the number generally does not change during the pre-adult phase. Only the Palaeosomata show unusual deviations from these common rules; in Palaeacarus hystricinus all immature stages are already bidactylous, just like the adults (which only possess the lateral claws and lack the central claw), with the number and the shape of claws differing between legs and ontogenetic stage (Grandjean Citation1954a). The first leg of the larva is monodactylous, the protonymph shows here a minute additional spine, and the deutonymph and tritonymph show two distinct claws. The other legs show two claws from the start and are clearly heterodactylous but they become finally homodactylous in the adult stage (Grandjean Citation1954a). In Acaronychus traegardhi, legs I, II and III of the larva and protonymph are monodactylous but become tri- and heterodactylous, in the deutonymphal stage. The development of the claws of the fourth leg is slightly retarded with the three claws being only present from the tritonymphal stage (Grandjean Citation1954a). In Aphelacarus acarinus, all the immature stages already possess three claws on each leg, only the shape of the median claw differs slightly between adult and juveniles (Grandjean Citation1954a). Apart from these Palaeosomata, there are two cases of non-monodactylous juveniles known in the Enarthronota, namely Phyllochthonius aouti, where the larva and protonymph are monodactylous, whereas the deuto- and tritonymph are bidactylous, like the adults (Travé Citation1967), and Nanohystrix hammerae where lateral claws are also absent in the protonymph, but appear in later stages, at least in the tritonymph (Norton and Fuangarworn Citation2015). The reasons for these specific changes during ontogeny are unknown. However, claws of juveniles and possible changes during ontogeny are clearly understudied in oribatid mites. In certain arachnids with maternal care, e.g. scorpions, whip-scorpions, and whip spiders, prenymphs lack claws but possess specific adhesive devices to hold on to their mother’s back. In the following free-living nymphal stages, these devices are reduced and claws appear (Wolff et al. Citation2015). Although such drastic changes are rather unlikely for oribatid mites, ecological shifts during the development may still result in significant differences between juvenile and adult claws. In Paraleius leontonycha, for example, the immatures dwell in the galleries of bark beetles and possess normally curved claws (Ermilov and Khaustov Citation2016), whereas the phoretic adults show specifically curved median claws () allowing them to attach to setae of the bark beetle for transport to another tree (Knee Citation2017). In Dometorina plantivaga, juvenile and adult stages show different feeding strategies, which is also coupled with a change in claw morphology. Immature stages are endophagous within lichens and show a robust, high, and strongly curved single claw, whereas the adult dwells outside the lichens and shows unremarkable normally curved median claws () (Grandjean Citation1950b; fig. 2, 3). There are several other taxa in which there is a similar clear ecological change between juvenile and adult stage, e.g. immatures of euphthiracaroid and phthiracaroid mites are exclusively endophagous in plant tissues, but in the adult stage they leave these tissues to forage outside (Norton and Behan-Pelletier Citation2009). Whether claw modifications occur in these cases is unknown because descriptions often lack features of the legs and there are no specific studies on their claws yet. The only study investigating the development of claw size and shape in an oribatid mite in detail, was that of Pfingstl and Kerschbaumer (Citation2022a). These authors demonstrated that the littoral habitat very early selects for a specific claw shape in monodactylous intertidal oribatid mites, and that juveniles show the same shape as the adults in the respective environment, i.e. immatures of species living on a rock, exhibit the same strongly curved claws like their adults, and juveniles of species living in mangroves already show the lower and less curved claws, typical for this environment. In each intertidal environment, claw curvature is almost static during development and claw length grows proportionally with increasing body size. The developmental changes in body size and weight are thus only compensated by a relative growth in size (Pfingstl and Kerschbaumer Citation2022a).
Figure 5. Possible developmental modifications of median claws range from no conspicuous change at all (L. caelatus/Licneremaeoidea) to extreme change of shape due to drastic change in lifestyle (P. leontonycha/Oripodoidea). Lateral claws of D. plantivaga (Oripodoidea) and P. leontonycha were omitted for clearer presentation. Depictions of D. plantivaga modified after Grandjean (Citation1950b) and of P. leontonycha after Ermilov & Khaustov (Citation2016)
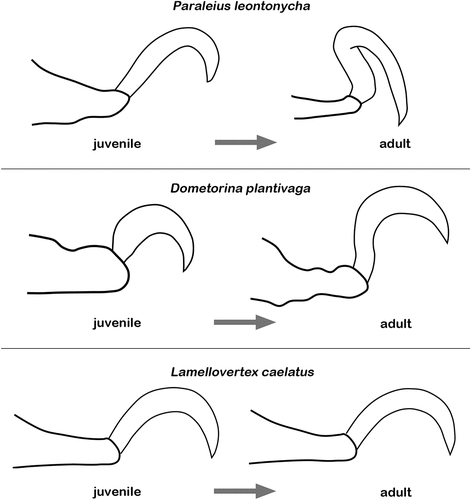
However, this was just a first study that investigated species without an ecological shift during development. Further studies are clearly needed to understand which ontogenetic changes in claw morphology might occur and what causes these changes.
The littoral environments
Oribatid mites living on the coast are often subject to splashing water, strong waves, and tidal inundation, which causes strong selection in terms of movement and attachment. In this extreme environment, claw characters strongly correlate with the substrate the animals walk on and dwell in (Pfingstl et al. Citation2020). A reduction in the number of claws represents one of the evolutionary adaptations to daily tidal flooding. Species living on the regularly flooded roots of a mangrove forest are monodactylous while species occurring in higher areas, like trunk and canopy, are dominated by tridactylous species (Karasawa and Hijii Citation2004). Indeed, all oribatid mite species that were recorded to live in the marine littoral zone of mangrove forests worldwide (Chatterjee et al. Citation2018), are exclusively monodactylous, except for one species, Sphaerochthonius litoralis, which shows heterotridactylous legs. Moreover, Haloribatula tenareae and Pontiobates denigratus, of the usually tridactylous Oribatulidae, have adapted to intertidal environments and show monodactylous legs (Schuster Citation1957; Luxton Citation1989). Members of Fortuyniidae and Selenoribatidae, all stenotopic inhabitants of the intertidal area, not only in mangrove forests but also in any other subtropical and tropical coastal environments, all possess a single tarsal claw on each leg (Pfingstl Citation2017). The marine associated Ameronothridae and Podacaridae, on the other hand, show both monodactylous and tridactylous species, but they also have both intertidal and non-intertidal representatives (Pfingstl Citation2017). However, the majority of littoral oribatid mites are monodactylous, indicating that a single claw is more favourable in this environment. The fast-changing littoral habitat requires the ability to move fast, and possessing a single claw may allow the mites to quickly react and evade tidal wave action (Pfingstl et al. Citation2020).
Apart from claw number, there is also a correlation between claw size and the environment. The claws of eulittoral Ameronothrus species, for example, are proportionally longer than those of congeneric supralittoral species (Pugh et al. Citation1987). The relative claw length of littoral oribatid mites was generally shown to be nearly twice as long as that of typical terrestrial species (Karasawa and Hijii Citation2004; Pfingstl et al. Citation2020). Accordingly, tidal flooding and wave action strongly select for single and extraordinarily long claws (Pfingstl et al. Citation2020).
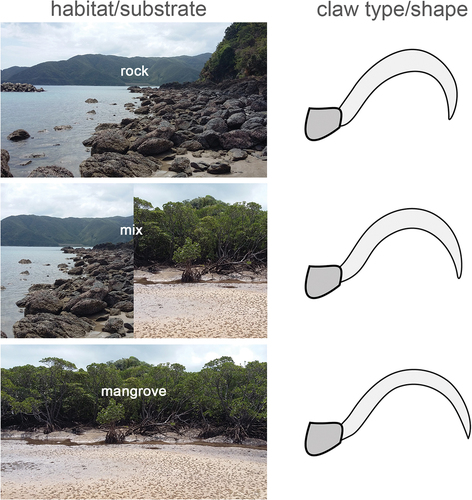
Within the intertidal environment, claw shape further strongly correlates with the respective microhabitat, where species living exclusively on rocky shores show remarkably high and strongly curved claws. Species dwelling in mangrove habitats possess lower and less curved claws and species occurring in a wide range of habitats show an intermediate claw type () (Pfingstl et al. Citation2020). Hard and soft substrates, apparently require different claw shapes and even in closely related groups different claw types can be found, indicating that microhabitat is the most important factor in shaping the claws of intertidal mites (Kerschbaumer and Pfingstl Citation2021).
Figure 6. Graph highlighting the correlation between used substrate and claw shape of oribatid mites living in the intertidal environment (modified after Pfingstl et al. Citation2020).
Freshwater environments
Certain oribatid mites are found in freshwater aquatic and semiaquatic habitats, like springs, streams, ponds, lakes, and wetlands all over the world, but their diversity is comparatively low (Behan-Pelletier and Eamer Citation2007). These mites do not actively swim in the water and most are supposed to show no apparent modifications to these freshwater habitats (Behan-Pelletier and Eamer Citation2007). Still, there might be some adaptations for the movement in the water. Mucronothrus nasalis belongs to a small genus that inhabits riffles in streams, spring seepages, meltwaters at high altitude, cold riverbeds, and oligotrophic lakes (Norton et al. Citation1996) and is truly aquatic as it completes its life-cycle while submerged (Behan-Pelletier and Eamer Citation2007). This species shows a single robust well curved claw on each leg with a bilateral row of inconspicuous cilia on its basal half, which is nothing out of the ordinary, but leg I and II are commonly splayed outward so that the claws point more laterad than ventrad, and this flexibility is suggested to facilitate movement under water (Norton et al. Citation1988, Citation1996). Furthermore, the distal tarsal setae are mostly scaliform paired with a fringe of minute barbs (Norton et al. Citation1996), and these modified setae may be used in addition to the claw to anchor the legs. Members of the genus Trhypochthoniellus are also known to be associated with aquatic vegetation in ponds and swamps (Behan-Pelletier and Eamer Citation2007; Ojeda et al. Citation2020) but show, in contrast to M. nasalis, three equal and smooth claws on each leg, except for T. chilensis, which represents the only monodactylous species of the genus (Ermilov and Weigmann Citation2015). However, Trhypochthoniellus species also possess thickened or dilated barbed distal tarsal setae (e.g. Ermilov and Weigmann Citation2015; Ojeda et al. Citation2020). Species of the trhypochthoniid genus Mainothrus are also known to prefer aquatic or semiaquatic habitats, these species are homotridactylous but show no thickened or dilated distal tarsal setae at all (Choi Citation1996; Ermilov Citation2021). The Malaconothridae show a similar preference for semiaquatic and aquatic habitats, like Sphagnum bogs, moss in streams, and under waterfalls, but are either monodactylous or tridactylous (e.g. Colloff Citation2013). The number of claws was even used to separate the genera Malaconothrus and Trimalaconothrus, but monodactyly is supposed to be a convergent apomorphy with no taxonomic value and therefore, the genus Trimalaconothrus should be regarded as a junior synonym of Malaconothrus (Colloff Citation2013; Colloff and Cameron Citation2013). However, most monodactylous and tridactylous Malaconothridae with semiaquatic or aquatic lifestyles show no unusual claws, but often conspicuously thickened or dilated, and sometimes even barbed distal tarsal setae (e.g. Yamamoto and Aoki Citation1998; Colloff Citation2013; Colloff and Cameron Citation2013).
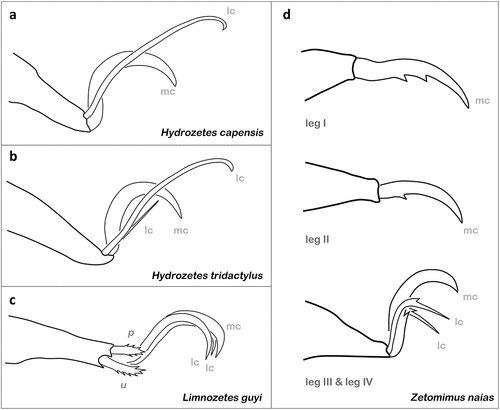
Among Brachypylina, the genus Hydrozetes represents the largest group of true freshwater limnic oribatid mites and can be found throughout the world in aquatic situations, mostly associated with water plants (Krantz and Baker Citation1982). The species of this genus all show monodactylous legs I–III, but the number of claws on leg IV varies from one claw to three claws (e.g. Grandjean Citation1948; Fernandez and Travé Citation1984). If there are two claws on leg IV, the median claw is normal and the paraxial claw is long, thin, and hooklike (), like in H. capensis for example (Engelbrecht Citation1974a). If there are three claws, which is the case in only one species, H. tridactylus, then the paraxial claw is long, thin, and hook-like, the median claw is normal, and the antiaxial claw is short, thin, and spine-like (), and the size ratio among them is 5:3:1 (Abd-El-Hamid Citation1964). The reason for this variation in claw number on leg IV is unknown, but the monodactyly on all the other legs of Hydrozetes might be related to their aquatic lifestyle. The confamilial Limnozetes are very common in semiaquatic habitats, like bogs, fens, and edges of lotic habitats, but little is known about their specific ecology or habitat requirements (Behan-Pelletier Citation1989). They show three claws on each leg, the median claw is strong and the laterals are of equal size but thinner. Despite the differences in claw number between Hydrozetes and Limnozetes, immatures and adults of both possess short, thick, and barbed distal tarsal setae (u, p) on each leg (), and these setae were observed to cooperate with the claws helping the mites to stick to aquatic plants (Seniczak and Seniczak Citation2020, Citation2021).
Figure 7. Examples of claw morphologies in aquatic oribatid mites. (a) Tarsus IV of H. capensis (Limnozetoidea) in paraxial view (modified after Engelbrecht Citation1974a). (b) Tarsus IV of H. tridactylous (Limnozetoidea) in paraxial view (modified after Abd-El-Hamid Citation1964). (c) Tarsus I of L. guyi (Limnozetoidea) in antiaxial view (after Behan-Pelletier Citation1989). (d) Tarsi of Z. naias (Ceratozetoidea) in paraxial view (after Behan-Pelletier Citation1998). mc = main or median claw; lc = lateral claw; p, u = tarsal setae (proral and unguinal).
The monogeneric genus Tegeocranellus is also mostly known from semiaquatic habitats and all species show monodactylous tarsi with quite large sickle-shaped claws, but modified distal tarsal setae are lacking in nearly all species (Behan-Pelletier Citation1997b). Their claws and associated structures strongly resemble that of their closely related marine littoral Fortuyniidae and Selenoribatidae. Only the South African T. knysnaensis and T. sacchareus, as well as the Vietnamese T. martinezi, have conspicuously thickened tarsal seta a´ and pv´´ with large spines (Kok Citation1968a; Ermilov and Anichkin Citation2014). As all these three species were found in typical terrestrial habitats (Kok Citation1968a; Ermilov and Anichkin Citation2014), it is questionable if these modified setae are also related to attachment under flooded conditions.
Members of the ceratozetoid family Zetomimidae are known to occur in semiaquatic and aquatic habitats (Behan-Pelletier and Eamer Citation2007; Schatz and Behan-Pelletier Citation2008). Naiazetes reevesi, for example, is a sexually dimorphic species dwelling at the edge of ponds and lakes in eastern North America, and shows heterotridactylous tarsi, whereas the lateral claws possess minute dens on their dorsal aspect, but distal tarsal setae are not specifically modified (Behan-Pelletier Citation1997a). Species of the genus Zetomimus complete their life-cycle underwater and have been collected from aquatic or semiaquatic environments (Ohkubo Citation1987; Schatz and Behan-Pelletier Citation2008). Zetomimus furcatus and Z. brevis are very lively mites that have the ability to run or walk skilfully on the surface of the water (Willmann Citation1931; Ohkubo Citation1987). They possess large strong median claws on all legs, but legs I and II are monodactylous and legs III and IV are heterotridactylous with the lateral claws being only setiform (Ohkubo Citation1987). Zetomimus naias, found on vegetation on the surface of water in a swamp in Costa Rica, has not been observed yet to walk on water, but shows the same number of claws as the species mentioned above (Behan-Pelletier Citation1989). However, in Z. naias the claws are shaped unusually, the single claw on tarsus I is very robust but weakly curved with two proximoventral spurs, the single claw of tarsus II is similar but shows only one proximoventral spur, the median claws of tarsus III and IV are strong but more curved with one proximoventral spur, and the lateral claws of the latter legs are strongly bent (nearly at a right angle) with a prominent middorsal spur () (Behan-Pelletier Citation1998). Zetomimus cristatus from Chile possesses the same number of claws on the legs as Z. naias and also shows similar hook-shaped lateral claws with middorsal spurs on leg III and IV, but lacks the spurs on the median claws on all legs (Hammer Citation1962). Zetomimus naias is sexually dimorphic and although the claw modifications are not part of the dimorphism (Behan-Pelletier Citation1989), the unique claws still might be somehow involved in a mating ritual. Sexual dimorphism was not reported in Z. cristatus, therefore it could also be that both species inhabit very similar but unique semiaquatic habitats requiring this specific claw structure. Observations in the field are clearly needed to answer that question.
Several oribatid mite species have also adapted to ephemeral rock pools (lithotelmata) where they are mainly active when immersed in water (e.g. Norton and Franklin Citation2018). Chudalupia meridionalis was reported from temporary gravel pools in Western Australia, and in this species all tarsi are heterotridactylous, with a strong median claw and two slender lateral claws that are finely serrate on their upper edge (Wallwork Citation1981). Aquanothrus montanus is known from temporary pools in mountain ranges of South Africa and shows the same claw configuration, but in this species the proral and unguinal tarsal setae are short, thick, and dentate (Engelbrecht Citation1975). The supposedly related Paraquanothrus grahami and P. spooneri both dwell in small, temporary aquatic environments formed by the accumulation of rainwater on rock surfaces in North America, and they have, in contrast to Chudalupia and Aquanothrus, monodactylous pretarsi and the single claw is equipped with a paired dorsolateral row of minute denticles in the proximal half (Norton and Franklin Citation2018). Scapheremaeus rodickae was found in very similar weathering depressions in the U.S.A., often associated with moss and lichen, and shows the exact same claw number and configuration as the Paraquanothrus species (Norton et al. Citation2010). Most of these taxa from ephemeral rock pools burrow into the sediment when the habitat dries out and remain immobile until the water returns (Engelbrecht Citation1975; Norton and Franklin Citation2018), and thus the claws and their structure may also play an important role not only for movement in water but also for digging into the dry ground.
However, claw numbers and shapes strongly vary among all oribatid mites from freshwater habitats and often overlap with characters of terrestrial taxa so that no clear pattern emerges from the comparison. Aquatic and semiaquatic environments are very diverse, showing different microhabitats, substrates, climatic conditions, they can be running or standing, permanent or temporary, etc., consequently, each situation will require different claw morphologies for movement and attachment. Nevertheless, as most taxa are monodactylous or at least show anterior monodactylous legs, there seems to be an evolutionary trend for the reduction of claw number in freshwater mites. The distal tarsal setae are also often modified to support the claws in attaching to the substrate during submergence in water.
Pulvillus/adhesive pads
The membranous region between the claws, also known as empodium, can be modified to form an eversible structure which is usually called the pulvillus (e.g. Dunlop Citation2002). Tarsal suction pads show frictional and adhesive properties, at least in insects (Gorb and Gorb Citation2004), and thus enhance attachment abilities on surfaces where claws alone are not able to interlock sufficiently with the substrate. The occurrence of tarsal pulvilli is a rare phenomenon among Oribatida (Grandjean Citation1970; Behan-Pelletier Citation1988) and in most of the few cases they are interpreted as adaptations to arboreal life (Walter and Behan-Pelletier Citation1993). Well-developed pulvilli are found in adults and immatures of the two known species of the genus Adhaesozetes (Hammer Citation1966; Walter and Behan-Pelletier Citation1993). Adhaesozetes barbarae shows a broad almost lenticular pulvillus at the base of a strong median claw and two tiny curved lateral claws (Hammer Citation1966) () and in A. polyphyllos, the pulvillus is also large, broad, and dorsoventrally flattened, the median claw is strong and accompanied by setae like rudimentary lateral claws (Walter and Behan-Pelletier Citation1993) (). The latter species is a leaf-inhabiting mite from Australian rainforests and grazes on epiphyllic fungi growing on leaves with smooth surfaces or at least closely appressed hairs (Walter and Behan-Pelletier Citation1993). Adhaesozetes barbarae, on the other hand, was yet only found in moss and liverwort in New Zealand (Hammer Citation1966), but based on the presence of conspicuous pulvilli, this species is suggested to be a typical arboreal species (Walter and Behan-Pelletier Citation1993). Members of the genus Phylleremus are found on leaves of woody dicots in arboreal environments of eastern Australia and thus share the same general habitat as A. polyphyllos (Behan-Pelletier and Walter Citation2007). They also possess pulvilli on each tarsus in each stage, but in contrast to Adhaesozetes, they lack the lateral claws completely () and the size of the remaining claw and the pulvillus differs between the anterior and posterior legs, i.e. claws of legs I–II are proportionally larger than those of legs III–IV and the pulvilli of leg I–II are smaller than those of legs III–IV (Behan-Pelletier and Walter Citation2007). These considerable differences may be caused by different microhabitat preferences, the two Phylleremus species basically favour plants with densely tomentous leaves and may also occur on branches and twigs, while A. polyphyllos is often found on plants with smooth leaves and there they are restricted to the leaf surfaces (Behan-Pelletier and Walter Citation2007; Karasawa and Behan-Pelletier Citation2007). Nasozetes sumatrensis was found on the underside of Gardenia leaves associated with galls of gall wasps in Borneo (Grandjean Citation1959a) and shows elongated pretarsi equipped with three equal tarsal claws and a large pulvillus (Grandjean Citation1970) (). Additionally, this species has the ability to reverse the claws completely on the back of the tarsus (Grandjean Citation1970), which may somehow help the mites to attach to the plants under certain circumstances. Wallworkiella nasalis is another species with three equally strong tarsal claws and a distinct pulvillus at their base, which indicates an arboreal lifestyle (). However, this species was collected in a luxurious rainforest in Java, but the exact ecology of this oribatid mite is yet unclear (Hammer Citation1979). Fenichelia porosa (Mahunka, Citation1985b) also shows three equal claws on each leg equipped with a well-developed pulvillus, indicating an arboreal lifestyle, but this species was yet only found in mats of moss in South Africa (Mahunka Citation1985b). Symbioribates aoki is known to occur on the leaves and branches of Castanopsis and Distylium trees and is a monodactylous species with a large empodial claw and a distinct subunguinal pulvillus in adults and immatures (Karasawa and Behan-Pelletier Citation2007). The size of the pulvillus differs between the legs, i.e. it is noticeably smaller on leg I and II (Karasawa and Behan-Pelletier Citation2007) similar to Phylleremus. Brassiella taiwanica was found on leaves of the Golden Trumpet tree and shows three almost equally strong tarsal claws with a large subunguinal pulvillus (Ermilov and Liao Citation2017), if juveniles of this species also exhibit pulvilli is yet unknown.
Figure 8. Different types of tarsal adhesive pads and claws found in oribatid mites. (a) Laterodorsal view of ambulacral structures in A. barbarae (Licneremaeoidea) (after Hammer Citation1966). (b) Ambulacrum of A. polyphyllos (Licneremaeoidea) in lateral view (after Walter and Behan-Pelletier Citation1993). (c) Differently sized tarsal structures shown on legs of P. leei (Licneremaeoidea) (after Behan-Pelletier and Walter Citation2007). (d) Homodactyl claws and pulvillus of N. sumatrensis (Oripodoidea) (after Grandjean Citation1970). (e) Dorsal view of ambulacral structures in W. nasalis (Hammer Citation1979). (f) Juvenile ambulacral structures of C. cymba (Cymbaeremaeoidea) (after Pfingstl & Krisper Citation2011a). (g) Small sized single claw of jumping Z. flabrarius (Zetorchestoidea) (after Grandjean Citation1951). (h) Large claw borne on conspicuously elongated pretarsus in S. auratus (Zetorchestoidea) (after Grandjean Citation1951). mc = main or median claw; lc = lateral claw; pu = pulvillus; ep = elongated pretarsus.
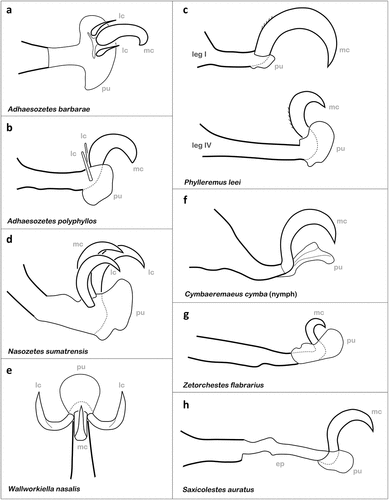
Apart from these taxa, there are other groups of oribatid mites that show tarsal adhesive pads but only in the immature stages. For example, larvae and nymphs of Ametroproctus and Cymbaeremaeus possess a large bilobed subunguinal pulvillus (), which is completely lost in the tridactylous adult (Behan-Pelletier Citation1987, Citation1988). The presence of this pulvillus is considered a unique synapomorphy of these two taxa (Behan-Pelletier Citation1987, Citation1988), but juvenile pulvilli are also supposed to have evolved independently in various other non-soil taxa (Behan-Pelletier Citation1990; Karasawa and Behan-Pelletier Citation2007). Members of the genus Megeremaeus show anvil or rectangular shaped pulvilli in their immature stages, whereas their adults exhibit heterotridactyl claws without a pulvillus (Behan-Pelletier Citation1990). The shape of the pulvillus varies to a certain degree between some species of Megermaeus and can thus even be used to distinguish at least some of the species based on juvenile specimens alone (Behan-Pelletier Citation1990). In Dendroeremaeus krantzi, a species from forest trees in western North America, a continuous reduction of the subunguinal pulvillus is occurring during development. In the larva there is a normal tarsal pulvillus, in the proto- and deutonymph the pulvillus is still present but minute, and in the tritonymph it is completely lost (Behan-Pelletier et al. Citation2005). Cymbaeremaeus and Dendroeremaeus are clearly arboreal taxa but all Ametroproctus and most species of Megeremaeus prefer dry habitats like grasslands and semi-deserts (Behan-Pelletier Citation1988, Citation1990), therefore, tarsal pulvilli seem to be correlated not only with arboreal but also epilithic lifestyles (Karasawa and Behan-Pelletier Citation2007). Why this morphological feature is lost in the adult of the above-mentioned groups is unclear, but for the arboreal species there are at least two possible explanations: (1) three claws on each leg may be sufficient to attach to arboreal microhabitats whereas the single claw of the monodactylous immatures may not be enough for getting a firm grip and therefore an additional adhesive device is needed, or (2) adults and juveniles may show different microhabitat preferences, e.g. adults may be mainly foraging on bark and twigs with rough surfaces, whereas immatures may prefer the leaves with rather smooth surfaces. In a giant stick insect species, the development of adhesive tarsal pads is closely correlated with the ecology of the developmental stages; the nymphs are leaf dwelling and show extensive adhesive structures on their legs while the adults are ground dwelling, possessing strongly reduced pads and significantly larger claws (Gottardo et al. Citation2015). A similar shift in the ecology of the developmental stages of arboreal oribatid mites may be responsible for the observed ontogenetic change in attachment devices, but presently there is no indication for such a scenario.
Apart from an arboreal or epilithic lifestyle, the ability to jump apparently also requires tarsal pads as shown in the monodactylous Zetorchestidae. Most members of this family have modified legs IV and are able to jump considerable heights when disturbed (Grandjean Citation1951; Krisper Citation1990). In the genera Zetorchestes, Belorchestes, and Litholestes, all legs, including the modified fourth leg, have well-developed but very small claws equipped with a distinct pulvillus (Grandjean Citation1951) (). This character is already given in the juvenile stages as indicated in the immatures of Zetorchestes micronychus (Ermilov and Kolesnikov Citation2013). In Diorchestes and Strenzkea the claws of legs II–IV are strongly reduced, either hardly projecting the pulvilli or being completely vestigial (Grandjean Citation1951; Travé Citation1966), a condition known as “adactyly” (Grandjean Citation1951). The larva of Diorchestes still possesses a minute but normal curved claw on leg II which is lost in the subsequent stages (Grandjean Citation1951). In contrast to the adactylous Diorchestes and Strenzkea, members of the genus Saxicolestes show well-developed large claws borne on conspicuously elongated pretarsi equipped with a rather small pulvillus (Grandjean Citation1951) (). Species of this genus are classified as non-jumping (“non sauteur”) but agile fast running mites (Grandjean Citation1951). The same is supposed for Strenzkea depilata, although living specimens have never been observed (Travé Citation1966).
Why the jumping Zetorchestidae possess strongly modified claws and associated pulvilli and how these structures work in action, has not been studied yet in detail. But it is reasonable to assume that a reduction of the claws is necessary to allow immediate detachment during an evasive jump. If attacked, the mite must react instantaneously, and this might not be possible with a large claw hooked firmly into the irregularities of the substrate. The reduction of the claw, on the other hand, results in weaker attachment to the ground when walking, therefore, the presence of an adhesive pad could compensate for this loss of attachment force.
The Zetomotrichidae are not closely related to Zetorchestidae but show the same modifications of leg IV (e.g. Grandjean Citation1954b; Coetzee Citation1993) and are therefore also supposed to be adapted for jumping. Interestingly, they lack tarsal pulvilli and show three tarsal claws on each leg, whereas the median claw is strong, but only half the size of the weak and thin lateral claws, as for example shown in Zetomotrichus lacrimans (Grandjean Citation1954b) or Anoplozetes jamiesoni (Lee and Pajak Citation1987).
In these cases, the weak but larger lateral claws may compensate for the reduced size of the median claw, instead of a pulvillus. However, reducing the claws or claw size seems to be a common adaptation of most oribatid mites being able to perform sudden evasive jumps.
Phoresy
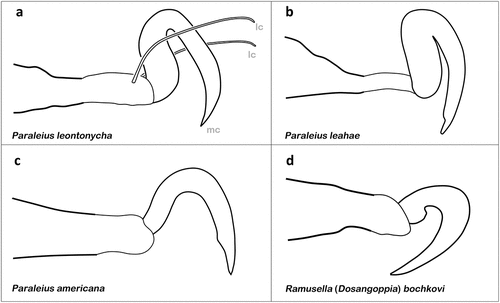
Phoresy is a relatively uncommon phenomenon among oribatid mites and the majority of the few phoretic taxa show no specific morphological adaptations for attaching to the host (Norton Citation1980). They are presumed to hide in depressions and grooves and hold onto irregularities in the host integument with their claws (Norton Citation1980; Ermilov and OConnor Citation2020a). In most cases, the claws of phoretic species are not very different from the claws of non-phoretic taxa, but in certain groups obvious modifications of claw shape as adaptations to phoresy can be observed. The scheloribatid genus Paraleius has evolved phoresy in nearly all of its members and shows the most remarkable claw adaptations for this specific behaviour. Paraleius leontonycha is a broad host generalist, documented from more than 20 bark beetle species (Knee et al. Citation2013; Ahadiyat and Akrami Citation2015) and possesses heterotridactylous legs with sickle shaped strongly hooked median claws on each leg while the lateral claws are weakly developed, looking almost like normal setae () (e.g. Knee Citation2017). The modified median claws allow the mites to hold on to the setae of the xylophagous beetles and to travel with them to new tree galleries (Wunderle et al. Citation1990; Pérez-Iñigo Citation1993; Penttinen et al. Citation2013; Ermilov and Frolov Citation2019). The congeneric Paraleius leahae is a host specialist only found on two bark beetle species and shows nearly the exact same modification of the median claw, but lacks the lateral claws () (Knee Citation2017). Paraleius americana, phoretic on a scarab beetle species, is also monodactylous but the claws are only slightly modified, forming a more or less right angle with an inconspicuous distoventral indentation () (Ermilov and OConnor Citation2020b). Paraleius trinidadensis shows active phoresy on a dipteran insect species and possesses strongly modified monodactylous claws similar to that of P. leontonycha, but only on the first two pairs of legs (Ermilov and OConnor Citation2020b). Apart from the genus Paraleius, obvious modifications of the claws have yet only been observed in the oppiid Ramusella (Dosangoppia) bochkovi (). This species resembles very much P. trinidadensis, it is monodactylous and only the claws of leg I and II are modified (Ermilov and Frolov Citation2019). These mites are supposed to feed on specific fungi on the brood balls of Ceratophyus polyceros and, with their claws curved at a nearly right angle, they are able attach to the setae of the dung beetle and use it as a transport host (Ermilov and Frolov Citation2019).
Figure 9. Examples of claws modified for phoretic behaviour (only tarsi I shown). (a) P. leontonycha (Oripodoidea) (modified after Travé Citation1960). (b) P. leahae (after Knee Citation2017). (c) P. americana (after Ermilov & OConnor Citation2020b). (d) R. (D.) bochkovi (after Ermilov & Frolov Citation2019).
Given these cases, it seems that modified claws are commonly used for the attachment to the host’s setae, but knowledge about where and how phoretic mites cling to the insects’ bodies is yet largely incomplete. There are several species with apparently non-modified claws and although it is assumed that they might hide and hold on to unexposed body places on the ventral side (Ermilov and Frolov Citation2021), observational data is extremely rare. Moreover, the attachment to body structures other than hairs may still require modified claws, but they may be modified to lesser a extent. Perscheloribates gabonensis, for example, is phoretic on passalid beetles and is supposed to have claws without any modification, but looking at the figures of the original description, the claw of leg I appears to be more robust and much stronger curved than the claw of leg IV (Ermilov and Frolov Citation2021; and , p. 778).
Other mites show strongly modified claws but have not been observed to be phoretic yet. Females of Archeremella africana possess a single strongly hooked claw on leg I, the shape is strikingly similar to the claws of P. leontonycha and other phoretic Paraleius species, but phoresy has not been reported in this species (Pérez-Iñigo Citation1981). Further studies on claw shape and phoretic behaviour are clearly needed to uncover the true correlations between claw characteristics and mode and place of attachment to the host.
The last case that is mentioned here, is seen not strictly as a case of phoretic behaviour but may have evolved as a consequence of it. Symbioribates papuaensis is a species that is found in a layer of cryptogamic plants growing on the pronotum and elytra of Papuan weevils. It feeds on these fungi and apparently lives in this “mobile microenvironment” (Aoki Citation1966a), which means it is permanently attached to the beetle and goes wherever the beetle goes. These mites are equipped with short tarsi and very robust and strongly curved claws (Aoki Citation1966a) allowing them to engage in this very unique symbiosis.
Sexual dimorphism
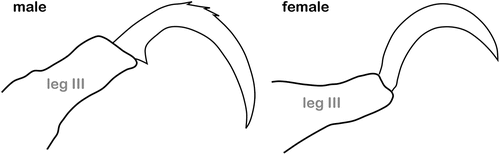
A very small percentage of oribatid mites shows distinct sexual dimorphism and a variety of morphological characters may be affected, e.g. the octotaxic system, number and shape of diverse body setae, cuticular projections, etc. (Behan-Pelletier Citation2015). Claws are also among these characters, but presently there are only four species known to exhibit sexually dimorphic claws. In the intertidal monodactylous Fortuynia atlantica, the females possess relatively smaller and more curved claws on leg I than males (Pfingstl and Kerschbaumer Citation2022b). Although never observed, females are supposed to clasp notogastral protuberances of the males with these modified claws to maintain contact during a courtship or mating sequence (Pfingstl and Kerschbaumer Citation2022b). Females of the monodactylous Archeremella africana also show modified claws on leg I, they are strongly hooked with a widened basal part (Pérez-Iñigo Citation1981) and strongly resemble the claws of phoretic Paraleius species. If these claws are used in a mating ritual or if they are used for being transported to new environments for egg deposition is unknown. The aquatic Hydrozetes ringueleti is monodactylous, except for leg IV, which shows two strongly heteromorphic claws. The males of this species, however, possess a strongly modified claw on leg III sitting on an elongated empodial pretarus (Fernandez Citation1984). The claw itself is curved at a nearly right angle and is equipped with an obvious proximoventral tooth (). In combination with a modified femur II and a spine on femur IV (Fernandez Citation1984), these claws might be used to clasp the female (Pfingstl and Kerschbaumer Citation2022b), but again, observation of any direct contact or mating behaviour is lacking so far. The marine associated Antarctic Podacarus auberti is a heterotridactylous species with a strong but smooth median claw and weaker and dorsally barbed lateral claws equipped with a small distoventral tooth (Grandjean Citation1955). Males of this species show relatively larger claws than females, whereas male claw size increases considerably from leg I to leg IV, resulting in a remarkable size difference between the claw of leg I and leg IV (Grandjean Citation1955). The reason for this unusual and unique sexual claw dimorphism is unclear.
Figure 10. Sexually dimorphic claws borne on tarsus III of Hydrozetes ringueleti (Limnozetoidea) specimens (modified after Fernandez Citation1984).
Extraordinary claw shapes and modifications
Claws of oribatid mites are mostly curved and pointed structures that look very similar among most taxa, at least with the naked eye. There are very few cases in which the shape strikingly deviates from this common pattern and indicates an adaptation to very specific environments. The reasons for these unusual modifications are unknown in most cases and their function can only be inferred from observation of living and moving specimens in the field. In Aribates javensis, such observations were performed allowing researchers to relate behaviour and claw shape. Legs of this species are monodactylous with thin, hardly curved claws that are broadened in the apical half in dorsal view (Aoki et al. Citation1994). This species has a mutualistic relationship with ants. They exclusively inhabit their nests, where they are groomed by the ants and remain remarkably inactive. If they are unattended, they move very slowly or not at all and thus become infested by fungi and die at last (Aoki et al. Citation1994). The weak appearance and the nearly straight shape of their claws is most likely a result of their mostly passive behaviour.
The monotypic Onychobates nidicola, on the other hand, was found in birds’ nests and is supposed to be parasitic (Hammer Citation1967a). All tarsi have a single, long, slightly curved sickle-shaped claw sitting on long thin pretarsal stalk, and only tarsus IV shows an additional long thin outer claw (). The claw can be bent against the pretarsal stalk functioning like a nipper or forceps, allowing the mites to cling to the feathers of the birds (Hammer Citation1967a). Another monotypic species with similar claw modifications is Luisumaoppia molinoensis. This species shows monodactylous legs, with nearly straight, blunt, and backwards directed claws, which are dorsally slightly barbed and borne on elongated and slightly upward curved pretarsi (Ermilov Citation2022) (). Although L. molinoensis was just found in soil and leaf litter from a mountain cloud forest in Peru and not associated with other organisms (Ermilov Citation2022), it is assumed the animals attach themselves to something or someone by using their claws and pretarsi like a forceps, similar to O. nidicola.
Figure 11. Strongly modified and thus unusual claw morphologies shown in oribatid mites. (a) Ambulacrum IV of O. nidicola (Ceratozetoidea) found in bird nests; arrow indicates possible rotation of claw (modified after Hammer Citation1967a). (b) Pretarsus IV of L. molinensis (Oppioidea) (after Ermilov Citation2022). (c) Typical claw shape of M. zealandica (Oppioidea) (after Hammer Citation1968). (d) Ambulacrum II of P. carinatus (Hermannielloidea) bearing a unique ventral spine (after Hammer Citation1961). (e) Typical claw morphology of U. punctatus (Oripodoidea) (after Hammer Citation1961); arrows indicating possible interaction of claw with tarsal cuticular structures. (f) Antiaxial view on tarsi I and IV of A. adjacentis (Protoplophoroidea) highlighting paradox lengths of claws (after Fuangarworn Citation2011).
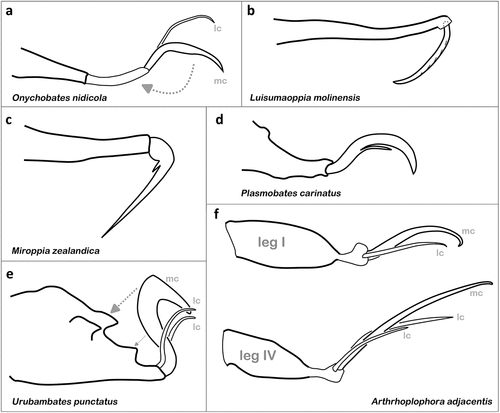
In Miroppia zealandica, the claws of the monodactylous legs also sit on elongated pretarsi, but are sickle-shaped (bent at a nearly right angle) and extremely thin and acute towards the tip (). Additionally, there is a pointed ventral tooth on each claw (Hammer Citation1968). Specimens of this species were collected in wet moss and liverwort on a vertical slope of a small brook in a Southern Beeches Forest in New Zealand (Hammer Citation1968), therefore the claws could be an adaptation to the semiaquatic environment or to the occurrence on vertical areas, but this is pure conjecture and needs to be verified.
A completely different modification can be found in Plasmobates carinatus, discovered in moss and soil in Peru (Hammer Citation1961). Plasmobatidae are usually monodactylous showing a proximoventral tooth on their normally shaped claws, but this species only possesses a ventral tooth on claw II, and this tooth is long and slender (Hammer Citation1961), looking almost like a hair or seta (). Why only leg II is affected and what function this slender tooth fulfils is unknown. Urubambates punctatus, another species from Peru, is heterotridactylous, with two very thin and short lateral claws and a very strong middle claw, which is bent in its distal third at an almost right angle resulting in a sharper outer edge (Hammer Citation1961) (). The same median claw shape can be found in the congeneric Urubambates ueckermanni from soil near termite nests in South Africa, but in this species, the lateral claws are completely lacking (Ermilov et al. Citation2021b). Interestingly, both species show unusual and conspicuous dorsal cuticular teeth on the tarsi (Hammer Citation1961; Ermilov et al. Citation2021b), so if the median claw would be reversed on the back of the tarsus, it would touch these teeth and thus, it could be that these structures somehow interact. Maybe the claws can be temporarily latched to the tarsus, but again observation of living specimens is clearly needed to verify this theory.
In Arthrhoplophora paradoxa, each tarsus is equipped with two slender curved claws that are nearly as long as the whole leg, therefore, the name of this species refers to the “paradoxical” size of these claws (Berlese Citation1910). In the congeneric Arthrhoplophora adjacentis, the claws reach similar sizes (), whereas leg I is bidactylous and legs II–IV are heterotridactylous, with the median claw always being the strongest and longest (Fuangarworn Citation2011). Why these mites need extraordinarily long claws is unclear.
Claw characteristics of systematic taxon groups
Palaeosomata
The Acaronychoidea are supposed to be the most plesiomorphic Oribatida (Norton and Behan-Pelletier Citation2009) and all their members show three claws on their legs (). The lateral claws are typically larger and angularly bent in the pre-terminal part, whereas this part is also laterally widened like a spatula or lamella (e.g. Schubart Citation1968) (). The median claw is very small, hook-like, less than quarter the length of the lateral claws, as for example shown in Stomacarus abresi (Lee Citation1981). In Acaronychus proximus, the median claw on leg I is further strongly reduced (Schubart Citation1968), in Zachvatkinella nipponica, on the other hand, the claws on tarsus I are distinctly larger than those of the other tarsi (Aoki Citation1980). Although they have the typical appearance of acaronychoid claws, the size of the lateral claws varies between the legs of Stomacarus macfarlani. On tarsus I they have the exact same size, on tarsus II the paraxial claw is slightly smaller and less curved, and on tarsi III and IV it is the antiaxial claw that is slightly smaller (Grandjean Citation1957).
Figure 12. Examples of ambulacral claws of Palaeosomata and Enarthronota. (a) Tarsus of S. macfarlani (Acaronychoidea); left in lateral view, right in ventral view (modified after Grandjean Citation1957). (b) Bidactylous ambulacrum of P. hystricinus (Palaeacaroidea) (after Grandjean Citation1954a). (c) C. barbatus (Palaeacaroidea) (after Schubart Citation1968). (d) perfectly bidactylous tarsus of A. artiodactylus (Atopochthonioidea) (after Grandjean Citation1949). (e) M. pulchra (Mesoplophoroidea) (after Grandjean Citation1965a). (f) Claw of C. promecus (Lohmannioidea) with distinct ventrobasal tooth (after Grandjean Citation1950a). (g) Empodial claw of N. thailandica (Hypochthonioidea) showing middorsal spur (after Fuangarworn and Lekprayoon Citation2012). (h) Adhesive pad-like ambulacrum of P. lavoipierrei (Cosmochthonioidea) possessing only an empodial remnant of the claw; left – lateral view, right – dorsal view (after Norton et al. Citation1983).
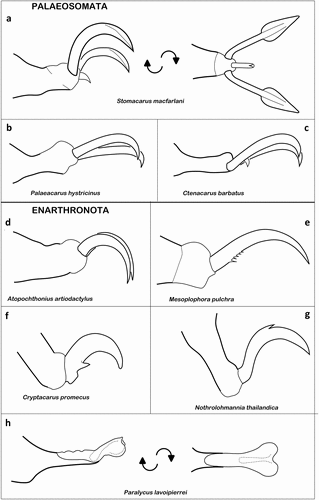
Table 1. Number of tarsal claws (mono, bi- and tridactyly) occurring in all larger systematic groups of Oribatida. Roman numbers in bold indicate that this is the most frequent (>90%) form of dactyly in the respective taxon, other forms are present but rare and exceptional; * indicate that polydactyly (different number of claws among legs of single specimens) may occur, but these are clearly the minority in each group; () is based on an ambiguous report.
The Palaeacaroidea are exclusively bidactylous, as they have completely lost the median claw (). On the first leg, the two remaining lateral claws are weakly curved but of exactly the same size and appearance and on legs II–IV they are more curved and slightly different in size (Grandjean Citation1954a).
The Ctenacaroidea show the largest variation among Palaeosomata in respect to claw number. All legs are tridactylous in Aphelacarus, Adelphacarus, and Ctenacarus, whereas in the two former genera, the median claw is a little bit stronger but shorter than the lateral claws (Grandjean Citation1932a, Citation1952), in Ctenacarus barbatus the median claw is conspicuously smaller than the laterals and reduced to a tiny hook on leg I (Schubart Citation1968) (). In the ctenacaroid genus Beklemishevia, legs II–IV are always tridactylous, with the median claw being shorter than the lateral ones, but leg I can be either bi- or tridactylous (e.g. Akrami and Behmanesh Citation2016). In the case of bidactylous first legs, as for example shown in Beklemishevia galeodula, there are two equally large claws present on the tarsus (Akrami and Behmanesh Citation2016). Species of the genera Neoctenacarus and Gilarovella all show homobidactylous tarsi I and heterotridactylous tarsi II–IV (Moritz Citation1974; Akrami and Behmanesh Citation2016).
Enarthronota
Despite their relatively high diversity, the Brachychthonioidea are all monodactylous taxa (), showing a smooth, strong, and slightly curved claw (e.g. Moritz Citation1976; Schatz Citation2021).
In Atopochthonioidea, Pterochthonius is the only monodactyl genus, showing a robust slightly curved claw on each tarsus (Minor and Ermilov Citation2015). The genera Atopochthonius and Phyllochthonius are exclusively bidactylous, showing a perfect bidactyly (), which means the median claw is completely reduced and the remaining lateral claws are identical in size and shape (Grandjean Citation1949; Travé Citation1967; Fuangarworn Citation2010). The two claws are basically robust, slightly curved (hook-shaped), and strongly pointed, whereas on leg I they can be a little bit smaller and less curved than on the other legs, as for example shown in Atopochthonius artiodactylus (Grandjean Citation1949).
Among Enarthronota, the Hypochthonioidea are known to show extreme body divergence (Norton and Behan-Pelletier Citation2009), but their claws are relatively constant across all taxa. The vast majority of Hypochthonioidea is monodactylous, whereas the single claw may be smooth or equipped with distinct teeth. In Mesoplophora pulchra, there are several tiny hairs instead of the distinct proximoventral tooth (Grandjean Citation1965a) (). Smooth claws can be found in the Eniochthoniidae (e.g. Fujikawa Citation1994; Norton and Behan-Pelletier Citation2007) and in several species of all other hypochthonioid families, as for example in Apoplophora aokii, in Mesoplophora gaveae, in Paulianacarus barlowi, in Torpacarus foveolatus, or in Lohmannia pseudoturcmenica (Schuster Citation1962; Wallwork Citation1962; Mondal et al. Citation1999; Coetzee Citation2001; Ermilov Citation2017a). In several species of Lohmanniidae, there is a distinct basoventral tooth on the tarsal claw (), especially in the genera Annectacarus (e.g. Grandjean Citation1950a; Coetzee Citation2001; Shiji et al. Citation2007), Cryptacarus (e.g. Grandjean Citation1950a; Shiji et al. Citation2007) or Papillacarus (e.g. Ren et al. Citation2018) where this feature is very common. In Mixacarus chapmani, this basoventral tooth is only present on tarsal claw III and IV (Wallwork Citation1962) and in Mixacarus (Phyllolohmannia) tenasserimensis it is only present on tarsus IV (Fuangarworn and Chaisuekul Citation2011). In Mesoplophora ifeana, the number of ventral teeth varies between the legs, tarsal claw I shows a weakly developed midventral tooth, tarsal claw II is bidentate, and tarsal claws III and IV are multidentate with poorly developed teeth looking like tiny spines (Badejo et al. Citation2001). Species of the genus Nothrolohmannia lack ventral teeth but show a dorsal tooth at midlength on each claw () (e.g. Norton Citation2003; Fuangarworn and Lekprayoon Citation2012), and Bedoslohmannia anneae possesses a distinct tooth in a very unusual antiaxial position on each tarsal claw (Fernandez et al. Citation2014a). The monotypic family of Psammochthoniidae is unique among Hypochthonioidea in respect to their claws, because it is the only non-monodactylous family within this group. Psammochthonius kethley shows tridactylous tarsi, with smooth strong claws, whereas the middle claw is slightly larger than the laterals (Fuangarworn and Norton Citation2013). Why this family shows three claws on each leg, in contrast to all other hypochthonioid mites is unknown, but could be somehow related to their occurrence in coastal sandy soils.
The Protoplophoroidea show very diverse claw morphologies, with different claw numbers and shapes. Members of the family of Haplochthoniidae are monodactylous and have mostly a slender and weakly curved claw on each leg (e.g. Grandjean Citation1949; Sanyal et al. Citation2002; Ordouni et al. Citation2021). The Sphaerochthoniidae show three-clawed legs with a strong heterodactyly, the median claw is strong but short, and the lateral claws are longer but considerably weaker, almost setiform (e.g. Mahunka Citation1985a; Schatz Citation2003). The Protoplophoridae show the largest variability when it comes to claw numbers. Species of Protoplophora are mainly heterotridactylous with a thick median claw and weaker lateral claws, e.g. Protoplophora takensis, P. iranica, while only Protoplophora palpalis shows monodactylous tarsi within this genus (Grandjean Citation1932b; Fuangarworn Citation2011; Akrami and Behmanesh Citation2012). The genera Aedelophora, Cryptoplophora, Tauroplophora, Bursoplophora, and Protritia are heterotridactylous with strong median and weaker lateral claws inserting on an elongated pretarus (Grandjean Citation1932b; Gordeeva et al. Citation1998), whereas in single species the length of the claws varies between the legs, for example in Bursoplophora muraiae where all claws gradually elongate from leg I to leg IV and in Protritia palaciosi the claws of leg IV are considerably longer than on all other legs (Mahunka and Mejia-Recamier Citation1998). The few species of the genus Arthrhoplophora may have very unusual claws and either are bidactylous or have a mixture of bidactyly and tridactyly (Subías and Pérez-Iñigo Citation1978; Fuangarworn Citation2011). The very unusual cases of this genus are given in a separate chapter (“Extraordinary claw shapes and modifications”). The family Cosmochthoniidae is characterized by polydactyl legs (Jorrin Citation2014), which means they show different numbers of claws on different legs. Members of the genus Cosmochthonius basically have two claws on tarsus I and three claws on tarsi II–IV, but there are exceptions, as for example Cosmochthonius bengalensis and C. bhutanensis, which possess only two claws on all legs (Ayyildiz and Luxton Citation1990; Seniczak et al. Citation2020). In all cases, the tarsi are heterodactylous, with a strong central claw and sickle-like lateral claws (e.g. Jorrin Citation2014). The same applies to species of the genus Phyllozetes, but these show a different tarsal claw formula with 2-2-2-3, i.e. only tarsus IV is equipped with three claws (Gordeeva Citation1980; Jorrin Citation2014). In the two known species of Krivolutskiella, the tarsal claws are arranged according to the formula 2-3-3-3, similar to most Cosmochthonius species (Gordeeva et al. Citation2007). The family of Pediculochelidae is rather exceptional in terms of tarsal appendages, not only among Protoplophoroidea and Enarthronota, but also among all Oribatida in general. In all species, claws are absent from all tarsi, there is only a minute empodial remnant left providing a thick central support, and there is a lump-like membrane appearing heart-shaped in dorsal view, a sort of a suction disc or adhesive pad (Lavoipierre Citation1946; Norton et al. Citation1983; Xu et al. Citation2020) (). Why the claws are more or less completely reduced is unclear, species of this family were mainly found in very dry habitats, whereas these can be very different, i.e. species were collected in soil, from lily fruits but also associated with other animals, like coccinellid beetles, bark beetles, bees, chicken and rats (Norton et al. Citation1983; Xu et al. Citation2020). The possession of an adhesive disc may point to a phoretic lifestyle, but there is no observation of such behaviour yet and thus it needs to be verified.
Despite being a relatively small group, the Heterochthonioidea are rather variable in terms of claws and show mono-, bi-, and tridactyly. The Heterochthoniidae and Trichthoniidae are monodactylous families (e.g. Martinez and Casanueva Citation1996), the Arborichthoniidae are perfectly homobidactylous (Norton Citation1982; Lotfollahi et al. Citation2016) and the Nanohystricidae are heterotridactylous with the median claw being slighter longer but conspicuously stronger than the lateral claws (Norton and Fuangarworn Citation2015).
Parhyposomata
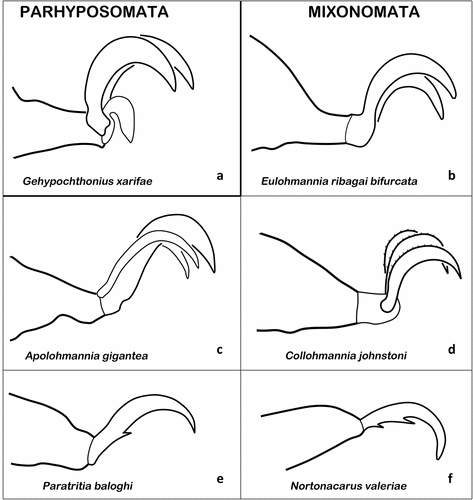
The Parhypochthonioidea show basic conformity in their claw morphology and strongly resemble some Palaeosomata in this respect. The Parhypochthoniidae, Elliptochthoniidae, and Gehypochthoniidae are heterotridactylous with a highly reduced hook-like empodial central claw (Strenzke Citation1963; Norton Citation1975; Mahunka Citation1997a; Martinez and Laborde Citation2000). In Gehypochthonius xarifae, the central claw is barely one third as long as the two equally sized lateral claws (Strenzke Citation1963) () and the regression can even be stronger leading to confusion about the real number of tarsal claws (Martinez and Laborde Citation2000).
Figure 13. Examples of ambulacral claws of Parhyposomata and Mixonomata. (a) Claws of G. xarifae (Parhypochthonioidea) (modified after Strenzke Citation1963). (b) Bidactylous tarsus of E. ribagai bifurcata (Perlohmannioidea) (after Fujikawa Citation2014b). (c) Tarsus of A. gigantea (Perlohmannioidea) (after Aoki Citation1960). (d) Ambulacrum of C. johnstoni (Perlohmannioidea) (after Norton & Sidorchuk Citation2014). (e) Claw of P. baloghi (Euphthiracaroidea) (after Moritz Citation1966). F. N. valeriae (Phthiracaroidea) (after Balogh & Mahunka Citation1992).
Mixonomata
The monogeneric Nehypochthonioidea have tridactylous ambulacra with a reduced central claw (Norton and Metz Citation1980; Aoki Citation2002), very similar to the Parhypochthonioidea or Palaeosomata discussed above. Despite this similarity, a tridactyl ambulacrum with a reduced central claw is supposed to have evolved several times (Norton and Metz Citation1980).
The also monogeneric and species-poor Eulohmannioidea show homobidactylous tarsi with quite prominent claws () (e.g. Fujikawa Citation2014b), whereas sometimes a minute empodial vestige can still be detected (Norton and Behan-Pelletier Citation2009).
In the Perlohmannioidea, with the single family Perlohmanniidae, monodactyly and tridactyly occurs and this difference in claw number gave rise to controversial debates on systematics and classification in this group. The genera Hololohmannia and Perlohmannia are monodactylous, showing a single claw bearing a short spine at the very base of the claw (Grandjean Citation1958b; Kubota and Aoki Citation1998; Ayyildiz et al. Citation2016). For the tridactylous species, a new genus, Apolohmannia, was erected (Aoki Citation1960), but this taxonomic act was questioned and this group is only seen as a subgenus in most recent publications (e.g. Ayyildiz et al. Citation2016). However, these Perlohmannia (Apolohmannia) species possess heterotridactylous tarsi, with a strong median claw, weaker and shorter lateral claws, and all claws may be dorsally serrate (Aoki Citation1960; Fujikawa Citation2008b) ().
The Epilohmannoidea are all monodactylous, whereas slight differences in claw shape and structure may occur. Some species show smooth claws, e.g. Epilohmannia shtanchaeva, while others show dentation or serration on the dorsal side, e.g. E. cylindrica or Epilohmannoides setoensis (Bayartogtokh Citation2000; Fujikawa Citation2008b). In Epilohmannia spathulata, claw thickness varies between legs, with tarsal claws I and II being conspicuously stronger than tarsal claws III and IV (Bayartogtokh Citation2000).
The Collohmannioidea are tridactylous with the empodial claw being equal or very slightly shorter than the laterals (Norton and Behan-Pelletier Citation2009; Norton and Sidorchuk Citation2014). In Collohmannia johnstoni, each claw is additionally equipped with minute inconspicuous barbs along its dorsal curvature () (Norton and Sidorchuk Citation2014).
The Eupthiracaroidea show, in contrast to the Collohmannioidea, considerable variation in claw number. Members of the family Oribotritiidae are mainly heterotridactylous, except for the genera Sobacarus and Paratritia, which possesses monodactylous legs (Moritz Citation1966; Niedbała Citation2000). Among Euphthiracaridae, there are also exclusively monodactylous genera, like Bukitritia, Sumatotritia, and Microtritia, but members of Euphthiracarus can be either mono- or heterotridactylous. Species of Acrotritia can even be mono-, bi-, or heterotridactylous, whereas the latter two traits occur at the same time, i.e. tarsus I is heterobidactylous and tarsi II–IV are heterotridactylous (Niedbała Citation2000). Members of the Synichotritiidae can be either monodactylous by the loss of lateral claws, e.g. Sabahtritia, Temburongia patoi, or they can have heterotridactylous tarsi, as shown in Synichotritia, Apotritia, and one species of Temburongia (Norton and Lions Citation1992; Lions and Norton Citation1998; Niedbała Citation2000; Fuangarworn and Lekprayoon Citation2011). In all Euphthiracaroidea, the heterodactylous condition always includes a strong median claw and a pair of fine lateral claws, and in single cases a small proximoventral tooth can be present on the median claw, as for example in Paratritia baloghi ().
The Phthiracaroidea are among the more species rich oribatid mite superfamilies, but their claws are relatively static and uniform. All members are exclusively monodactylous (Norton and Behan-Pelletier Citation2009), whereas the claw is usually stout but not strongly curved and may possess ventral teeth. In some Phthiracarus, Mantigueracarus, and Neosteganacarus species, there is a single prominent proximoventral tooth present (Ramsay Citation1966; Balogh and Mahunka Citation1992) and in some Atropacarus (Hoplophorella), Nortonacarus, Notophthiracarus, and Steganacarus, there are two ventral teeth with the distal tooth being twice as long and stout as the proximal (Ramsay Citation1966; Balogh and Mahunka Citation1992; Haq and Xavier Citation2005) ().
Desmonomata – Cohort Nothrina
The superfamily of Crotonioidea is rather diverse in terms of claw morphology, whereas single families tend to be consistent in this character while others show strong variation. The Crotoniidae belong to the latter group, they can be either monodactylous or homotridactylous, showing overall smooth claws, but in some cases the lateral claws may be serrate along their dorsal edge (), e.g. Austronothrus rostralis (Colloff and Cameron Citation2009, Citation2014). The family Hermanniidae, on the other hand, is exclusively monodactylous, showing strong claws that may be dorsally slightly barbed, as for example in Phyllhermannia (Woas Citation1981; Colloff Citation2011c; Ermilov and Liao Citation2020). The Nanhermanniidae are also monodactylous, showing a single strong or thick claw that is always smooth () (e.g. Aoki Citation1977; Fujikawa Citation2003a; Nakamura et al. Citation2013). Within the Malaconothridae, species of Tyrphonothrus are consistently homotridactylous with smooth claws (Colloff and Cameron Citation2013), but species of Malaconothrus are either monodactylous or heterotridactylous, and the claws (median as well as laterals) can be smooth or dorsally sparingly barbed (Colloff and Cameron Citation2013; Ermilov and Starý Citation2019). An interesting and unique case is that of Malaconothrus knuellei, where the tarsi I and II are clearly heterodactylous, with the median claw being noticeably larger than the laterals, whereas tarsi III and IV are equipped with three completely equal claws () (Colloff and Cameron Citation2013). The family Nothridae is variable with species being either monodactylous or heterotridactylous and the claws are mostly smooth but sometimes dorsally finely serrate lateral claws may occur (e.g.Fujikawa Citation1999; Colloff Citation2011b). Another interesting case here is that of Nothrus ezoensis from Japan, which is basically monodactylous, but tarsus I may be mono-, bi-, or heterotridactylous (Fujikawa Citation1999) in some instances. The frequency of this variation and the reason for it are unknown. The Trhypochthoniidae also show variation in claw number, where Afronothrus, Allonothrus, Trhypochthoniellus, Trhypochthonius, and Mainothrus have three equal claws on each leg (Choi Citation1996), but there are single exceptions, namely Allonothrus monodactylus and Trhypochthonius monodactylus, both showing only a single claw on all legs instead of three (Mihelčič Citation1957b; Wallwork Citation1960). The genera Archegozetes and Mucronothrus possess only a single claw on each leg (Choi Citation1996).
Figure 14. Examples of ambulacral claws of Desmonomata (Cohort Nothrina). (a) Homotridactylous tarsus of A. curviseta (Crotonoidea) (modified after Colloff and Cameron Citation2009). (b) Empodial claw of N. hiemales (Nanhermannioidea) (after Fujikawa Citation2003b). (c) Claws of M. knuellei (Crotonoidea) showing size variation between anterior and posterior legs (after Colloff and Cameron Citation2013).
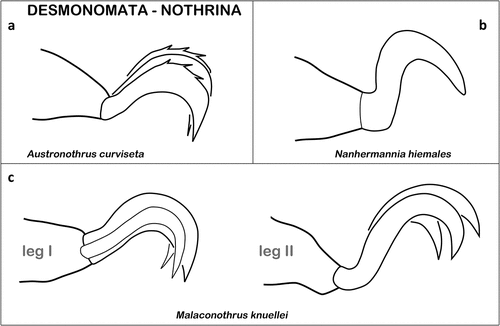
Desmonomata – Cohort Brachypylina
The Hermannielloidea are all monodactylous taxa with a strong tarsal claw that is either smooth or has a single proximoventral tooth and/or is dorsally slightly barbed () (e.g. Grandjean Citation1962; Fujikawa Citation1993; Mizutani et al. Citation2003; Fernandez et al. Citation2011). Hermanniella spiniseta is the only species of this group that was reported to be tridactylous (Mahunka and Mahunka-Papp Citation2007), however, in the same publication it was written that the legs are typical for the genus, therefore the reported tridactylous condition may be a mistake.
Figure 15. Ambulacral claws of Desmonomata (Cohort Brachypylina). (a) Monodactylous tarsus of A. silvanus (Hermannielloidea) (modified after Fujikawa Citation1993). (b) Tarsus of K. arthurjacoti (Damaeoidea) (after Norton et al. Citation2022); tarsus given in full length to highlight small relative size of claw. (c) Ambulacrum of S. roynortoni (Cepheoidea) (afer Ermilov Citation2020c). (d) Reduced claw on tarsus I of S. schusteri (Ameroidea) (after Grandjean Citation1966). (e) Ambulacrum of L. daamsae (Oppioidea) showing two rows of dorsal barbs (after Ermilov et al. Citation2014). (f) Tarsal claws of P. auberti (Ameronothroidea); left – all claws overview, right – lateral claw in detail (after Grandjean Citation1955).
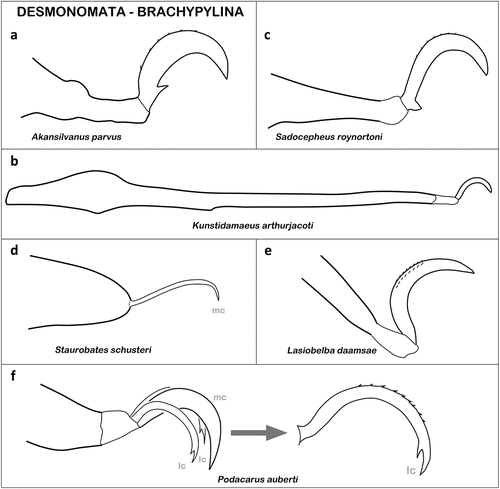
Members of the Neoliodoidea are all homotridactylous, with three strong claws, that can be dorsally slightly serrate in some cases (e.g. Fujikawa Citation2001; Nakamura et al. Citation2010).
The Plateremaeoidea are overall a tridactylous group, only in the genera Nacunansella and Licnodamaeolus are there are single monodactylous species among other non-monodactylous species (e.g. Ermilov and Hugo-Coetzee Citation2012). Apart from these exceptions, plateremaeoid members possess three heterodactylous claws, with the median being the strongest, whereas in a few cases the heterodactyly may be only weakly developed, as for example in Licnobelba (Ruiz et al. Citation1990). The claws are mostly small in relation to the leg length and always sit on a tarsal pedicel, whereas the length of this pedicel or stalk is highly variable in some groups – like Pedrocortesella, where it ranges from very short to long (Hunt Citation1996). In this genus, the distal part of the tarsus often shows a recess to receive the empodial claw (e.g. Hunt Citation1996), however, the function of this recess is unknown. In several species of the genus Aleurodamaeus, the size of the claws may also become progressively larger from leg I to IV (Hugo-Coetzee Citation2013).
Despite being a genera- and species-rich group, the Damaeoidea show hardly any variation concerning their claws, with all species being monodactylous with a single smooth claw that is relatively small in relation to their slender long legs () (e.g. Miko Citation2008; Norton et al. Citation2022).
The majority of taxa of Cepheoidea are clearly monodactylous, having a single normal claw, e.g. Cercocepheidae, Eutegaeidae, Microtegeidae, and Nosybeidae. In the family Anderemaeidae, the genera Cristeremaeus and Epieremulus are monodactylous while members of Anderemaeus are heterotridactylous, showing minute inconspicuous spines on the dorsal edge of the claws (Norton and Ermilov Citation2019). Most genera within the family Cepheidae also have just a single claw on each leg, the exceptions are Conoppia, Ommatocepheus, Pilocepheus, Sphodrocepheus, and Tritegeus, which show three claws on each tarsus (Bayartogtokh and Ermilov Citation2021). Only the genus Eupterotegaeus includes both monodactylous and tridactylous species (Bayartoghtokh and Ermilov Citation2021). The tridactylous species are usually slightly heterodactylous and only Ommatocepheus is known to show clear homodactyly (Woolley and Higgins Citation1964). The monodactylous species show a normal smooth claw, but sometimes a distinct ventrobasal tubercle may be present, as for example in Sadocepheus nortonroyi (Ermilov Citation2020c) ().
Polypterozetoidea are all monodactylous, whereas a basal tooth and a slight dorsal serration may be present, as for example shown in Polypterozetes cherubin or in Podopterotegaeus altimonticola (Grandjean Citation1959b; Piffl Citation1972). In Podopterotegaeus miyamaensis, the basal tooth and the serration are lacking but instead there are one or two tiny dents at the mid-portion of the dorsal claw aspect (Fujikawa Citation2008a).
The Microzetoidea comprise nearly 200 species, all these species show monodactylous tarsi, with a smooth mostly weakly curved and therefore rather long claw (e.g. Grandjean Citation1936; Engelbrecht Citation1972).
In Ameroidea, the situation is similar, despite a relatively high family and species diversity, all taxa are monodactylous. The single pretarsal claw is mostly slender, smooth, and not strongly curved. Exceptions from this common configuration are Montgaillardia callitoca, in which there is a small spine at the base of the claw (Grandjean Citation1961), and the Staurobatidae, in which the claw of tarsus I may be either strongly reduced to a nearly hair-like structure () or it is completely lost including the whole apotele (Grandjean Citation1966). These reductions are supposed to be an evolutionary consequence of leg I being only used for sensorial purposes and not for walking anymore (Grandjean Citation1966). The genus Heterobelba also respresents an exception among Ameroidea, because legs I–III of all species are typically monodactylous, whereas leg IV is tridactylous with a strong median claw and slightly less strong lateral claws (e.g. Beck Citation1962).
The Zetorchestoidea (also referred to as Eremaeoidea by some authors, e.g. Norton and Behan-Pelletier Citation2009) possess two monodactylous families, namely the Arceremaeidae showing normal single pretarsal claws and the Zetorchestidae having strongly reduced claws. The considerable reduction of the claw size in the latter family is supposed to be an adaptation to the jumping ability shown in members of this family (Grandjean Citation1951). The claw and its associated structures in Zetorchestidae are given in detail in an earlier chapter (see ‘Pulvillus/adhesive pads). All other zetorchestoid families are tridactylous with slight variation between some taxa. The Niphocepheidae are homodactylous showing three equally strong claws that may be dorsally barbed (Travé Citation1959; Behan-Pelletier Citation1982). The Megeremaeidae are heterotridactylous (Behan-Pelletier Citation1990), whereas the Eremaeidae possess hetero-, e.g. Eremaeus, as well as homotridactylous members, e.g. Asperemaeus (Behan-Pelletier Citation1982).
The Gustavioidea represent a mostly tridactylous group, but monodactyly and also bidactyly may occur in single families. Members of the family Astegisidae show either three heterodactylous tarsal claws, as for example the genera Astegistes, Furcoppia, and Maorizetes (e.g. Hammer Citation1966; Choi Citation1998; Grobler Citation2003) or they are monodactyl, showing a single normal claw, i.e. the genera Cultroribula, Lamellozetes, and Monofurcoppia, as well as the species Sulcoribula laticuspidata (Covarrubias Citation1967; Hammer Citation1971; Pérez- Iñigo and Sarasola Citation1995; Ermilov and Minor Citation2015a). The small family Multioribulidae is tridactylous, with a single exception, namely the monotypic Peloptoribula spinulosa, which shows two strongly heterodactylous claws on each tarsus (Mahunka Citation1984). The monogeneric Gustaviidae show all three nearly equal tarsal claws, the median claw is only slighter thicker than the laterals (e.g. Hammer Citation1977). The monogeneric Kodiakellidae also possess three tarsal claws but here, the median claw is by far strongest (Hammer Citation1967b). The Tenuialidae and Peloppidae show overall heterotridactylous legs (e.g. Norton Citation1983; Lindo Citation2011, Citation2015; Zhang and Jin Citation2016), only members of the peloppiid genus Parapyroppia show monodactyly (e.g. Hirauchi Citation1998). The diverse Liacaridae are basically tridactylous, whereas claws can be either nearly homodactylous, as for example in Xenilloides aenigmaticus and Xenillus amazonensis (Pérez-Iñigo and Baggio Citation1989, Citation1997), or they are heterodactylous with the claws showing a slight dorsal serration, as in many Liacarus and Xenillus species (e.g. Ermilov and Starý Citation2017, Citation2021). The genera Birsteinius and Planoristes are the only monodactylous groups within the Liacaridae (Iturrondobeitia and Subías Citation1978; Wang et al. Citation2001).
Carabodoidea are all monodactylous taxa, with relatively few variations in claw morphology. Most of the taxa show simple strong smooth tarsal claws, but claws with a deep ventral indentation or tooth are quite common in the family Carabodidae, e.g. Afticarabodes, Antongilibodes, Austrocarabodes, etc. (Hugo-Coetzee Citation2011; Fernandez et al. Citation2013, Citation2014b), and claws with a dorsal serration or several tiny dorsal teeth can be found in Nippobodidae, e.g. Leobodes (Ermilov and Martens Citation2021), in Dampfiellidae, e.g. Dampfiella (Mahunka Citation2000), in Otocepheidae, e.g. Eurostocepheus (Zheng and Chen Citation2021) and in all Tokunocepheidae (Aoki Citation1966b; Fujikawa Citation2014a).
The Oppioidea are the largest superfamily in the Oribatida and comprise altogether more than 1000 species (Norton and Behan-Pelletier Citation2009). In view of this diversity, it is surprising that all members of this superfamily show monodactyly and only the monogeneric Tuparezetidae with two known species are tridactylous (Spain Citation1969). These two species possess dorsally finely barbed claws with the median being longer and twice as thick as the laterals (Spain Citation1969). All the other Oppioidea show a single tarsal claw, which is in most cases relatively slender, smooth, and weakly curved. In some cases, the claw may be dorsally slightly barbed or serrated, as for example in several Lasiobelba (Ermilov et al. Citation2014) and Geminoppia amatholensis (Ermilov et al. Citation2021a). In Lasiobelba daamsae, there are even two rows of small teeth on the dorsal edge of each claw (Ermilov et al. Citation2014) (), and in Machadobelba ugandensis and Gittella kontschani there is an additional tubercle at the ventral base of the claw (Ermilov Citation2020a; Ermilov et al. Citation2021c). For the oppioid families Enantioppiidae and Platyameridae, there is no information about their claws to be found in the literature yet.
The Trizetoidea are all monodactylous, without exception, their tarsal claws are mostly smooth and normally curved. In single cases, claws can be dorsally serrate as for example in Allosuctobelba alexanderkhaustovi (Ermilov and Stary Citation2018), or they can be weakly curved and strongly blunt, i.e. Niosuctobelba sphagnicola (Ermilov Citation2017b).
The mostly parthenogenetic Tectocepheoidea are also characterized by monodactylous legs; the singly claw is either smooth or can be dorsally sparsely roughened, e.g. Tectocepheus elegans and T. satsumaensis (Fujikawa Citation2017), or show a single or several weak dorsal teeth as in Tectocepheus velatus (Nübel-Reidelbach Citation1994). The size of the claw is equal on all legs of a species (Nübel-Reidelbach Citation1994), but the angle of the claw shows strong variation between different Tectocepheus species and ranges from 40–180° (Fujikawa Citation2017). Tegeozetes miyajima is the only species of Tectocepheoidea with three claws on each tarsus, whereas the median claw is strong and the laterals are very thin (Fujikawa Citation2006).
The freshwater aquatic and semi-aquatic Limnozetoidea comprise two families, the Hydrozetidae and Limnozetidae. Hydrozetidae are basically monodactylous but show interesting variations on the fourth leg (e.g. Fernandez and Travé Citation1984). Tarsus IV may also be bidactylous, with the lateral claw being longer and considerably thinner than the strong median claw, e.g. Hydrozetes behanpelletierae (Bayartogtokh and Ermilov Citation2019). There also may be a third claw on leg IV in H. tridactylous (Abd-el-Hamid Citation1964) and specific sexual dimorphic modifications of the claws in H. ringueleti (Fernandez Citation1984), details about these were already given in the section “Freshwater environments.” The Limnozetidae show all heterotridactyl legs without any specific modifications (Behan-Pelletier Citation1989).
Most members of the superfamily Ameronothroidea are marine associated taxa and in an earlier section (“The littoral environment”), the specific correlation of claw length and shape with this specific environment was already discussed in detail. Apart from size and shape, Ameronothroidea show relatively few variations in claw characteristics. The littoral Fortuyniidae and the limnic Tegeocranellidae are exclusively monodactylous taxa, showing a single large and smooth tarsal claw (e.g. Behan-Pelletier Citation1997b; Pfingstl Citation2017). The littoral Selenoribatidae are also purely monodactylous species, but here additional teeth-like structures can be often found on the single claws, for example Selenoribates arotroventer possesses two proximoventral teeth on the claw and Selenoribates satanicus shows in addition to a proximoventral tooth even a proximodorsal tooth (e.g. Pfingstl Citation2017) (). The southern hemispheric Podacaridae are all heterotridactylous taxa, with a strong median and conspicuously thinner lateral claws, that may either be smooth or dorsally finely serrate. Podacarus auberti is exceptional because it is the only member in this group showing a subsidiary tooth on the thinner lateral claws (Grandjean Citation1955) (). The Ameronothridae are the only family of Ameronothroidea having mono- and heterotridactylous species. In Ameronothrus, most species are heterotridactylous, with a strong median and two weaker lateral claws, that may be slightly serrated, with only Ameronothrus bilineatus, A. nigrofemoratus, and A. schneideri being monodactylous, having a single smooth claw (Schubart Citation1975). As these three species usually occur on soft substrates (e.g. marsh, silt) while all the other species prefer hard surfaces (rocks, concrete, trees), it was suggested that claw number possibly correlates with habitat characteristics (Schubart Citation1975). In the subfamily of Aquanothrinae, all Aquanothrus species are heterotridactylous and all Paraquanothrus species are monodactylous (e.g. Norton and Franklin Citation2018). Bidactylous members are completely lacking in the superfamily of Ameronothroidea. Only a single specimen of a single population of the usually monodactylous A. schneideri from a saltmarsh near Cuxhaven in Germany was reported to show bidactylous ambulacra on at least five of its legs (Schubart Citation1975).
Figure 16. Examples of ambulacral claws in Desmonomata (Cohort Brachypylina). (a) Bidentate claw of the littoral S. satanicus (Ameronothroidea) (modified after Pfingstl Citation2017). (b) Tarsal claws of E. malleensis (Eremaeozetoidea) (after Colloff Citation2012). (c) Homodactylous tarsus of M. brevipes (Licneremaeoidea) (after Pfingstl & Krisper Citation2011b). (d) Bidactylous ambulacrum of B. pilosus (Licneremaeoidea) antiaxial view (after Bayartogtokh & Smelyansky Citation2003). (e) Variation of claw number between legs of A. phongnhae (Achipterioidea) antiaxial view (after Ermilov & Vu Citation2012). (f) Ambulacrum of M. penetrabilis (Oripodoidea) (after Grandjean Citation1959c). (g) Claws of G. robisoni (Oripodoidea) (after Behan-Pelletier & Knee Citation2019).
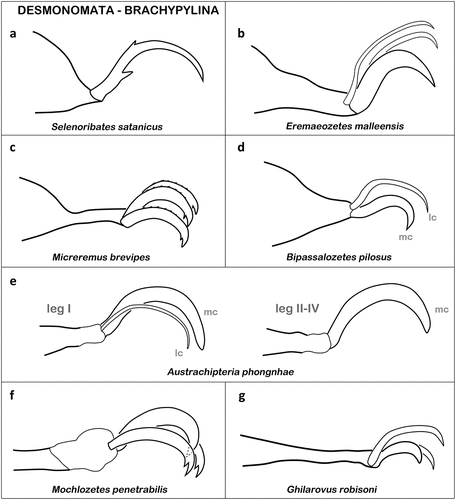
In Cymbaeremaoidea, monodactylous and tridactylous members can be found whereas the former are clearly the minority. The monotypic Seteremaeus and Spineremaeus, as well as several species of Scapheremaeus, show only a single strong tarsal claw (Hammer Citation1971; Rios and Palacios-Vargas Citation1998; Colloff Citation2011a). Legs with three equally strong tarsal claws are present in Bulleremaeus (Hammer Citation1966), Glanderemaeus (Balogh and Csiszár Citation1963) and Cymbaeremaeus (Fujikawa Citation2002). In Cymbaeremaeus silva, these equally strong claws are additionally equipped with two dorsal rows of dents and several basal spines (Fujikawa Citation2002). The genera Sculpteremaeus, Ametroproctus, Scapuleremaeus, and many species of Scapheremaeus are heterotridactylous, with a strong median claw and slender lateral claws (Behan-Pelletier Citation1987; Colloff Citation2010, Citation2011a; Behan-Pelletier and Ermilov Citation2020).
The relatively small superfamily of Eremaeozetoidea, comprises the Eremaeozetidae and Idiozetidae, and both families show monodactylous as well heterotridactylous members (e.g. Schatz Citation2001; Colloff Citation2012), the latter with a strong median and two thin and hyaline lateral claws ().
Licneremeoidea represent a quite diverse group and the same applies to their claw morphologies. The Charassobatidae and Lamellareidae are exclusively monodactylous families. In the former, the single tarsal claw may bear a basal ventral spine, as in Charassobates cavernosus (Grandjean Citation1958a), and in the latter, the claw may either be smooth or dorsally slightly roughened (e.g. Coetzee Citation1987; Ermilov Citation2020b). The Licneremaeidae are all heterotridactylous, with a strong median and very fine lateral claws (e.g. Fernandez et al. Citation1997; Bonfim et al. Citation2017) that are usually smooth, but in Licneremaeus braziliensis and Licneremaeus indicus there are small spines arranged in dorsal and lateral lines on these claws (Bonfim et al. Citation2017; Arun and Ramani Citation2020). Almost all Micreremaeidae show tridactylous legs, except for species of the genus Phylleremus, which are monodactylous, showing an additional tarsal pulvillus (Behan-Pelletier and Walter Citation2007; further details on this exceptional group are given herein in section “Pulvillus, adhesive pads”). The three tarsal claws of all other members of this family are homodactylous or at least nearly homodactylous showing a fine dorsal dentation (e.g. Martinez and Palacios-Vargas Citation1998; Pfingstl and Krisper Citation2011b). In Micreremus brevipes, all claws are born on an elongated ambulacral stalk and each show a small incision close to the forefront (Pfingstl and Krisper Citation2011b) (). Almost all Scutoverticidae show three tarsal claws on each leg, the median claw is usually strong and robust, the laterals are rather thin, and all claws may be dorsally slightly dentate (e.g. Balogh Citation1970; Mahunka Citation1982; Pfingstl et al. Citation2010a, Citation2010b). The monogeneric Lamellovertex and the small group of Scutoverticosus represent the only monodactylous exceptions within the family of Scutoverticidae (Kok Citation1968b; Bernini Citation1976). The Passalozetidae show the largest variation of claw numbers in Licneremaeoidea. Passalozetes demeteri is the only mondactylous taxon (Mahunka Citation1987), besides, there are numerous species showing two tarsal claws, with a strong and a weaker but longer paraxial claw (), and there are several taxa showing typical heterotridactylous legs (Grandjean Citation1932c; Engelbrecht Citation1974b; Bayartogtokh and Aoki Citation1997). The possession of bidactylous legs in certain Passalozetes species led to the erection of the genus Bipassalozetes to accommodate this unusual group (Mihelčič Citation1957a).
Phenopelopoidea show basically heterotridactylous tarsi, with a strong median and conspicuously weaker lateral claws. The dorsal edges of either all (e.g. Bayartogtokh and Aoki Citation1999; Behan-Pelletier and Walter Citation2009) or just of the lateral claws may be finely serrated (Nübel-Reidelbach and Woas Citation1992; Pfingstl and Krisper Citation2010). In Phenopelopidae, the genus Nesopelops is exceptional in being exclusively monodactylous, with a single smooth tarsal claw that may possess a proximoventral tooth (Hammer Citation1973).
The Achipterioidea are a quite diverse and variable group in terms of tarsal claw numbers. Within Achipteriidae, the genera Cerachipteria and Cubachipteria are exclusively monodactylous, with a single smooth tarsal claw (e.g. Grandjean Citation1935; Ren et al. Citation2019). The genera Achipteria and Dentachipteria, on the other hand, comprise only heterotridactyl members, showing a median strong claw and weak lateral claws (e.g. Nevin Citation1974; Hirauchi and Aoki Citation1997; Seniczak and Seniczak Citation2016). In Anachipteria, most members show typical heterotridactylous legs, but there are monodactylous exceptions, as for example A. svetlanae (Ermilov and Anichkin Citation2014). In Campachipteria, there are monodactylous and heterotridactylous species in almost equal numbers (e.g. Aoki Citation1995; Ren et al. Citation2019), whereas the tridactylous taxa were subsumed under the subgenus Campachipteria (Triachipteria) (Subías Citation2022). In the genus Austrachipteria, there are monodactylous and heterotridactylous species, but there is also a single species, namely Austrachipteria phongnhae, where the number of claws varies among specimens and even among legs (Ermilov and Vu Citation2012). The most common case is that leg I is bidactylous, with a strong and a very weak lateral claw, and legs II–IV show a single strong claw (). Epactozetidae are basically tridactylous, showing very weak and thin lateral claws (e.g. Grandjean Citation1930; Mahunka Citation1998), but in the genus Truncozetes, there is one monodactylous species, T. monodactylus and two species, T. ecuadoriensis and T. paraecuadoriensis, that show a single claw on tarsus I and three claws on tarsi II–IV (Ermilov et al. Citation2013, Citation2015). In Tegoribatidae, monodactylous and heterotridactylous species are equally common. The genera Lemurobates, Paraphysobates, and Protectoribates show only a single tarsal claw on each leg (e.g. Mahunka Citation1985b, Citation1997b; Behan-Pelletier Citation2017) whereas Plakoribates, Physobates, Pseudotectoribates, Scutozetes, and Tectoribates all possess a strong median and two weak lateral claws on each leg (e.g. Hammer Citation1952; Popp Citation1960, Citation1962; Ruiz et al. Citation1991; Behan-Pelletier and Walter Citation2013). Members of the genera Ceratobates, Hypozetes, and Tegoribates are either mono- or heterotridactylous (e.g. Behan-Pelletier Citation2017; Ermilov et al. Citation2017) and the usually monodactylous Neophysobates shows a single exception, namely N. incrassatus with heterobidactylous legs (Ermilov and Minor Citation2015b).
The Oribatelloidea comprise a single family that shows varying tarsal claw numbers. The genera Ophidiotrichus, Joelia, Lamellobates, Novoribatella, Fenestrobates, Palmitalia, and Siciliotrichus are exclusively monodactylous taxa (Bernini Citation1983; Engelbrecht Citation1986; Nübel-Reidelbach and Woas Citation1992; Pérez-Iñigo and Peña Citation1997; Behan-Pelletier Citation2013; Ermilov and Behan-Pelletier Citation2014), whereas Adoribatella, Cuspidozetes, and Ferolocella are solely heterotridactylous (Hammer Citation1962; Behan-Pelletier Citation2013). The genus Oribatella is more complex, having mono-, bi-, and tridactylous species. Subías (Citation2022) even divides the species according to their claw numbers in different subgenera: Oribatella (Monoribatella) species show a single smooth tarsal claw, Oribatella (Bioribatella) species possess a strong inner and one thin outer claw, and Oribatella (Oribatella) species are characterized by a strong median and two thin smooth lateral claws. Prionoribatella impar is also heterobidactylous but represents an unusual case, because the inner claw is weak and thin, and the outer claw is strong and not vice versa (Aoki Citation1976a).
The Oripodoidea represent a very large superfamily and its members show a huge variety of tarsal claw morphologies. Tarsal attachment devices range from mono- to tridactylous, they can comprise smooth claws, dorsally serrate claws, claws with basal spines, claws with distal teeth, elongated pretarsi, and pulvilli. Bidactylous members represent the minority among Oripodoidea, mono- and tridactylous species are almost equally frequent. A specific claw feature that is characteristic for this superfamily is the small ventrodistal or subsidiary tooth or notch that can be found only on the lateral claws of tridactylous taxa (). This feature occurs in nearly each of the numerous oripodoid members, but is especially common in Mochlozetidae (Grandjean Citation1959c, Citation1960) and was suggested to be an adaptation to an arboreal lifestyle (Grandjean Citation1959c). Outside Oripodoidea, distally notched lateral claws have only yet been reported in the marine associated Podacarus auberti living on sub-Antarctic coasts and islands (Grandjean Citation1955). The Mochlozetidae are all heterotridactylous taxa, with a strong median and two weaker lateral claws (Grandjean Citation1960). The same applies to Caloppiidae, Drymobatidae, Neotrichozetidae, Sellnickiidae, Stelchobatidae, Tubulozetidae, and Zetomotrichidae (e.g. Oudemans Citation1927; Grandjean Citation1930; Travé Citation1961, Citation1965b; Behan-Pelletier and Knee Citation2019), whereas in Caloppiidae and Stelechobatidae, the lateral claws may be almost the same size and shape as the median claw. In Zetomotrichidae all claws are borne on an elongated pretarsus and the median claw is stronger but considerably smaller than the laterals (). The Campbellobatidae, Nesozetidae, and Symbioribatidae are exclusively monodactylous taxa, showing a single smooth tarsal claw (Wallwork Citation1964; Hammer Citation1971, Citation1979). The Parakalummidae are mostly heterotridactylous and show only very few monodactylous taxa, as for example Parakalumma piton (Mahunka Citation1998). Oribatulidae are similarly mainly heterotridactylous, and only species of Gerloubia and the monotypic genera Haloribatula, Neoluccoppia, and Pontiobates show monodactylous tarsi (Schuster Citation1957; Hammer Citation1977; Tseng Citation1984; Luxton Citation1989). The Oripodidae are also dominated by heterotridactylous species, whereas in some taxa, the heterodactylous condition may be weakly expressed, e.g. Oripoda (Corpuz-Raros Citation2010). Monodactyly is nevertheless quite common in some oripodid genera, e.g. Birobates, Brachyoripoda, Cryptoribatula, and Parapirnodus (e.g. Hammer Citation1973; Ohkubo Citation1980; Martinez et al. Citation1996; Bayartogtokh et al. Citation2020). The Haplozetidae and Scheloribatidae are the largest families among Oripodoidea and they are also the most diverse groups in terms tarsal claw characteristics. There is even strong variation within certain genera. In Haplozetidae, there are, besides the numerous monodactylous and heterotridactylous species, also bidactylous representatives that show a strong and a weak claw on each tarsus. Haplozetes bayartogtokhi and Haplozetes biheterodactylus possess a thick median claw and a weak terminally notched antiaxial claw (Ermilov and Tolstikov Citation2015; Ermilov et al. Citation2019) while other Haplozetes members are either mono- or heterotridactylous. The genus Magyaria is mostly monodactylous but there are also bidactylous species and interestingly, one of these species, namely Magyaria javensis, bears a thin medial and a strong lateral claw on tarsi I–II and a strong medial and a weak lateral claw on tarsi III–IV (Hammer Citation1979). In the genus Protoribates, there are also bidactylous species that are subsumed under the subgenus Biunguis (Subías Citation2022). The monogeneric Aokibates possesses monodactylous legs I–III, but a heterobidactylous leg IV (Mahunka Citation1988). In Scheloribatidae mono- and tridactylous taxa also represent the majority, and heterobidactylous members can only be found in the genera Hemileius (e.g. Lee Citation1989) and Scheloribates (Hammer Citation1961). In the latter, these are subsumed under the subgenus Bischeloribates (Subías Citation2022).
Ceratozetoidea are not as diverse as Oripodoidea in terms of claws and most members are either monodactylous with a single smooth tarsal claw or heterotridactylous with a strong median and two weaker lateral claws that may be all smooth or dorsally slightly serrated – in few cases, the median claw may be equipped with minute proximal cusp, e.g. Zetomimus naias (Behan-Pelletier Citation1998). The families Euzetidae, Humerobatidae, Maudheimiidae, and Ramsayellidae possess exclusively heterotridactylous members (e.g. Behan-Pelletier and Mahunka Citation1993; Coetzee Citation1997; Weigmann Citation2006) but there is no family that is exclusively monodactylous. The families Mycobatidae and Ceratozetidae possess several purely monodactylous taxa, for example the genera Allomycobates, Ellipsozetes, Minunthozetes, Mycozetes, Allozetes, Guatemalozetes, and Punctizetes (e.g.Hammer Citation1967a; Spain Citation1968, Citation1971; Aoki Citation1976b; Bernini Citation1980; Tseng Citation1984; Weigmann Citation2006; Behan-Pelletier and Eamer Citation2008). Heterotridactylous and monodactylous members together occur only in the mycobatid Cryptobothria, Mycobates, Ceratozetes, Macrogena, and Umbellozetes (Wallwork Citation1963; Behan-Pelletier et al. Citation2001; Fujikawa Citation2003b; Behan-Pelletier and Eamer Citation2009; Ermilov and Minor Citation2015c). All other genera of these two families are strictly heterotridactylous (e.g. Weigmann Citation2006). In Chamobatidae, most members show heterotridactylous tarsi and only the genus Pedunculozetes is characterized by monodactyly (Hammer Citation1962). However, in this family, tarsal claws, no matter if one or three, are often borne on an elongated pretarsus that is almost as long as the claws themselves (e.g. Sellnick Citation1928; Hammer Citation1962). The Ceratokalummidae basically also show mono- and heterotridactylous members, but the genus Cultrobates is very special because it shows strong variation between species and even between the legs of a single species. Cultrobates heterodactylus shows a single claw on leg I but three heterodactylous claws on legs II–IV, Cultrobates quadricuspidatus possesses two claws on tarsus I and three claws on tarsi II–IV, whereas Cultrobates nipponicus exhibits two claws, a strong median and a weak, on all legs (Willmann Citation1930; Aoki Citation1982). The Zetomimidae, except for the heterotridactylous Naiazetes reevesi (Behan-Pelletier Citation1986), show altogether a similar heterodactyly. Species of Zetomimus possess monodactylous legs I–II but heterotridactylous legs III–IV, while species of Heterozetes show only a monodactylous leg I and heterotridactylous legs II–IV (e.g. Behan-Pelletier Citation1998). The monotypic Onychobatidae also exhibit heterodactyly with one claw on leg I–III and two claws on leg IV, their claws are additionally specially modified, which is supposed to facilitate parasitic behaviour (Hammer Citation1967a) (for details see section “Extraordinary claw shapes and modifications”).
Finally, the Galumnoidea usually have three claws, with the median claw mostly considerably thicker than the laterals, all claws either smooth or dorsally barbed, in rare cases mono- or bidactylous tarsi may occur (e.g. Ermilov and Klimov Citation2017). In Galumnidae, claw numbers are mostly stable within genera, exceptions are Allogalumna, Leptogalumna, Mirogalumna, and Neoctenogalumna, which have mono- as well as tridactylous members (Ermilov and Klimov Citation2017). The genera Pergalumna and Trichogalumna comprise subgenera with different claw numbers, i.e. species of Pergalumna (Pergalumna) and Trichogalumna (Trichogalumna) with the typical three tarsal claws, and Pergalumna (Bigalumna) and Trichogalumna (Tanzanycha) with two unequal claws (Ermilov and Klimov Citation2017). The genus Heterogalumna is the only galumnoid taxon showing heterodactylous legs, their tarsi I–III bear a single smooth claw and tarsus IV is equipped with three small smooth claws (Ermilov and Klimov Citation2017). In the family Galumnellidae, claw number strongly varies within genera. In Galumnella, there are mono-, bi-, and tridactylous species, and in Porogalumnella there are mono- and bidactylous members (Balakrishnan and Haq Citation1982; Mahunka Citation1995). Based on this intrageneric variation, the genera Monogalumnella (with one tarsal claw), Bigalumnella (with two tarsal claws), and Trichogalumnella (with three tarsal claws) were suggested to be junior subjective synonyms of Galumnella (Ermilov and Klimov Citation2017). Galumnopsis and Iberogalumnella show stable claw numbers including only heterotridactylous species (Ermilov and Klimov Citation2017) and Trypogalumnella with only monodactylous members (Mahunka Citation1995).
Conclusions
There is a huge diversity of ambulacral claw morphologies present in oribatid mites and this diversity largely does not follow a strict phylogenetic or systematic pattern. Most taxon groups show large variations and only few groups have uniform tarsal claw structures. Lifestyle and ecology apparently play an important role in shaping claw morphologies, such a correlation is best seen in phoretic species possessing specifically modified claws to attach to their hosts or in intertidal mites having large claws with specific shapes for different substrates to withstand tidal flooding and wave action. Nevertheless, in most cases, the correlation between claw morphology and lifestyle remains unclear and no common rules can be postulated with the present data. The microhabitats the mites live in are too cryptic and complex for us to understand yet, and evolution surely found more than one way to solve specific problems. Apart from that, our knowledge about the ecology of most species is absolutely insufficient and observation of living specimens is no common practice, at least in oribatid mites. To really understand why there is such a diversity of tarsal claw morphologies and what function each claw type fulfils and how they operate in detail, we need much more ecological and observational data, as well as experimental studies using specific performance tests. In view of the more than 11,000 known oribatid mite species, there is a lot of work left to do but also a lot of hidden potential for highly interesting studies and for applications in other fields of research.
Acknowledgments
I thank two anonymous reviewers for their valuable comments improving this review article. Thanks also to Michaela Kerschbaumer for sharing my fascination about claws and for working together with me in a very ambitious project about claws of oribatid mites. Finally, I would like to thank Roy Norton for allowing so many of us to make use of his comprehensive pdf-library comprising nearly all existing literature about oribatid mites. This priceless resource was an enormous help when writing this review.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Abd-El-Hamid ME. 1964. Hydrozetes tridactylus n. sp., eine neue Art der Gattung Hydrozetes Berlese, 1902 von Ägypten (Acari, Oribatei). Sitzungsberichte der österreichischen Akademie der Wissenschaften mathematisch natur. Klasse Abt. I. 173:369–382.
- Ahadiyat A, Akrami MA. 2015. Oribatid mites (Acari:Oribatida) associated with bark beetles (Coleoptera: Curculionidae: Scolytinae) in Iran, with a review on Paraleius leontonychus (Berlese) and a list of bark beetles in association with this species. Persian Journal of Acarology. 4:355–371.
- Akrami MA, Behmanesh M. 2012. A new oribatid mite of the genus Protoplophora Berlese, 1910 (Acari: Oribatida: Protoplophoridae) from Iran. International Journal of Acarology. 38:168–176.
- Akrami MA, Behmanesh M. 2016. Palaeosomatid mites (Acari: Oribatida) of Iran, with description of a new species of Beklemishevia and supplementary description of Gilarovella demetrii Lange, 1974. Systematic & Applied Acarology. 21:195–208.
- Aoki J-I. 1960. Eine dreikrallige Gattung der Familie Perlohmanniidae (Acarina: Oribatei). Japanese Journal of Zoology. 12:507–511.
- Aoki J-I. 1966a. Epizoic symbiosis: an oribatid mite, Symboribates papuensis, representing a new family, from cryptogamic plants growing on backs of Papuan weevils (Acari: Cryptostigmata). Pacific Insects. 8:281–289.
- Aoki J-I. 1966b. A remarkable new oribatid mites from South Japan (Cryptostigmata: Tokunocepheidae, fam. nov.). Acarologia. 8:258–364.
- Aoki J-I. 1976a. The oribatid mites of Pseudosasa—grass zone at the highest point of the Island of Yaku-shima, South Japan. Memoires of the National Science Museum, Tokyo. 9:145–150.
- Aoki J-I. 1976b. Zonation of oribatid mite communities around Sesshogawara of Kusatsu, a spouting area of sulfurous acid gas, with description of two new species. Bulletin of the Institute of Environmental Science and Technology, Yokohama National University. 2:177–186.
- Aoki J-I. 1977. Studies on Oribatei (Acarina) from the South Pacific III. Cosmohermannia monstruosa, n. sp. from New Guinea. Pacific Insects. 17:229–232.
- Aoki J-I. 1980. A revision of the oribatid mites of Japan IV. The families Archeonothridae, Palaeacaridae and Ctenacaridae. Annotationes Zoologicae Japonenses. 53:124–136.
- Aoki J-I. 1982. New species of oribatid mites from the southern islands of Japan. Bulletin of the Institute of Environmental Science and Technology, Yokohama National University. 8:173–188.
- Aoki J-I. 1995. Oribatid mites of high-altitude forests of Taiwan II. Mt. Nan-hu-ta Shan. Special Bulletin of the Japanese Society for Coleopterology, Tokyo. 4:123–130.
- Aoki J-I. 2002. The second representative of the family Nehypochthoniidae found in Mishima City of Central Japan. Bulletin of the Kanagawa Prefecture Museum (Natural Science). 31:23–25.
- Aoki J-I, Takaku G, Ito F. 1994. Aribatidae, a new myrmeciphilous oribatid mite from Java. International Journal of Acarology. 20:3–10.
- Arun A, Ramani N. 2020. Two remarkable new species of oribatid mites (Acari: Oribatida) from Acacia litter of Kerala, India. Zootaxa. 4877:539–558.
- Atyeo WT. 1979. The pretarsi of astigmatid mites. Acarologia. 20:244–269.
- Ayyildiz N, Luxton M. 1990. The genus Cosmochthonius Berlese, 1910, (Oribatida: Cosmochthoniidae). Acarologia. 31:279–284.
- Ayyildiz N, Subías LS, Baran Ş. 2016. Review of the family Perlohmanniidae (Acari: Oribatida) with description of a new species from Turkey. Biologia. 71:323–327.
- Badejo MA, Woas S, Beck L. 2001. Mesoplophora ifeana, a new species of ptychoid mite (Acari, Oribatida) from Nigeria. Andrias. 15:65–73.
- Balakrishnan MM, Haq MA. 1982. A new species of Porogalumnella (Acari: Oribatei: Galumnellidae) from Kerala, India. Indian Journal of Acarology. 7:20–25.
- Balogh J. 1970. New oribatids (Acari) from New Guinea. II. Acta Zoologica Academiae Scientiarum Hungaricae. 16:291–344.
- Balogh J, Csiszár J. 1963. The zoological results of Gy. Topál’s collectings in South Argentina. Annales Historico-Naturales Musei Nationalis Hungarici, Pars Zoologica. 55:463–485.
- Balogh J, Mahunka S. 1992. New phthiracarid taxa from Brazilian soils (Acari, Oribatida). Acta Zoologica Hungaricae. 38:159–174.
- Bayartogtokh B. 2000. Oribatid mites of the Genus Epilohmannia (Acari: Oribatida: Epilohmanniidae) from Japan and Mongolia. Systematic & Applied Acarology. 5:187–206.
- Bayartogtokh B, Aoki J-I. 1997. Oribatid Mites of the Genus Bipassalozetes Mihelčič, 1957 (Acari: Oribatei; Passalozetidae) from Mongolia. Acta Arachnologica. 46:87–99.
- Bayartogtokh B, Aoki J-I. 1999. Oribatid mites of the Family Phenopelopidae (Acari: Oribatida) from Mongolia. Journal of the Acarological Society Japan. 8:117–134.
- Bayartogtokh B, Ermilov SG. 2019. A new species of Hydrozetes (Acari: Oribatida, Hydrozetidae) from Ecuador and a key to Neotropical species. International Journal of Acarology. 45:328–334.
- Bayartogtokh B, Ermilov SG. 2021. A new genus of Cepheidae and reassessment of the family (Acari: Oribatida). Systematic & Applied Acarology. 26:1198–1212.
- Bayartogtokh B, Itioka T, Kitora H, Meleng P, Shimano S. 2020. New findings of poronotic oribatid mites (Acari: Oribatida) from the high canopy of a Bornean tropical rain forest. International Journal of Acarology. 46:73–82.
- Bayartogtokh B, Smelyansky IE. 2003. New and little known species of Bipassalozetes and some other related genera (Acari: Oribatida) from Russia and Kazakhstan. Mongolian Journal of Biological Sciences. 1:31–48.
- Beck L. 1962. Beiträge zur Kenntnis der neotropischen Oribatidenfauna. 2. Nothridae, Camisiidae, Heterobelbidae (Arach., Acari). Senckenbergiana Biologica. 43:385–407.
- Behan-Pelletier VM. 1982. Descriptions of new species and a new genus of Oribatei (Acari) from the Soviet Subarctic. The Canadian Entomologist. 114:855–871.
- Behan-Pelletier VM. 1986. Ceratozetidae (Acari: Oribatei) of the western North American Subarctic. The Canadian Entomologist. 118:991–1057.
- Behan-Pelletier VM. 1987. Redefinition Of Ametroproctus (Acari: Oribatida) with descriptions of new species. The Canadian Entomologist. 119:505–536.
- Behan-Pelletier VM. 1988. Systematic relationships of Ametroproctus, with modified definition of Cymbaeremaeidae (Acari: Oribatida). In: Channabasavanna GP, Viraktamath CA, editors. Progress in acarology. Vol. 1. New Delhi: Oxford and IBH Publishing Co., Pvt. Ltd; p. 301–307.
- Behan-Pelletier VM. 1989. Limnozetes (Acari: Oribatida: Limnozetidae) of northeastern North America. The Canadian Entomologist. 121:453–506.
- Behan-Pelletier VM. 1990. Redefinition of Megermaeus (Acari: Megeremaeidae) with description of new species, and nymphs of M. montanus Higgins and Woolley. Canadian Entomologist. 122:875–900.
- Behan-Pelletier VM. 1997a. Naiazetes reevesi n. g., n. sp. (Acari: Oribatida: Zetomimidae) from semi-aquatic habitats of eastern North America. Acarologia. 37:345–355.
- Behan-Pelletier VM. 1997b. The semiaquatic genus Tegeocranellus (Acari: Oribatida: Ameronothroidea) of North and Central America. The Canadian Entomologist. 129:537–577.
- Behan-Pelletier VM. 1998. Ceratozetoidea (Acari: Oribatida) of lowland tropical rainforest, La Selva, Costa Rica. Acarologia. 39:349–381.
- Behan-Pelletier VM. 2013. Adoribatella, Ferolocella, Joelia and Ophidiotrichus (Acari, Oribatida, Oribatellidae) of North America. Zootaxa. 3637:254–284.
- Behan-Pelletier VM. 2015. Review of sexual dimorphism in brachypyline oribatid mites. Acarologia. 55:127–146.
- Behan-Pelletier VM. 2017. Tegoribatidae of North America, with proposal of Protectoribates gen. nov., and new species (Acari, Oribatida, Tegoribatidae). Zootaxa. 4337:151–197.
- Behan-Pelletier VM, Eamer B 2007. Aquatic Oribatida: adaptations, Constraints, Distribution and Ecology. In: Morales-Malacara JB, Behan-Pelletier VM, Ueckermann E, Pérez TM, Estrada-Venegas EG, Baddi M, editors. Acarology XI: Proceedings of the International Congress. Sociedad Latinoamericana de Acarología (México): Instituto de Biología and Facultad de Ciencias, Universidad Nacional Autonóma de México; p. 71–82.
- Behan-Pelletier VM, Eamer B. 2008. Mycobatidae (Acari: Oribatida) of North America. The Canadian Entomologist. 140:73–110.
- Behan-Pelletier VM, Eamer B. 2009. Ceratozetes and Ceratozetoides (Acari: Oribatida: Ceratozetidae) of North America. The Canadian Entomologist. 141:246–308.
- Behan-Pelletier VM, Eamer B, Clayton M. 2001. Mycobatidae (Acari: Oribatida) of Pacific Northwest canopy habitats. The Canadian Entomologist. 133:755–775.
- Behan-Pelletier VM, Eamer B, Clayton M. 2005. Dendroeremaeidae n. fam., from forest trees in western North America (Acari: Oribatida: Licneremaeoidea). Acarologia. 46:321–339.
- Behan-Pelletier VM, Ermilov SG. 2020. Sculpteremaeus olszanowskii gen. nov., sp. nov. (Acari, Oribatida, Cymbaeremaeidae) from chaparral in California, USA, with a reassessment of Cymbaeremaeidae. Zootaxa. 4790:341–357.
- Behan-Pelletier VM, Knee W. 2019. Ghilarovus robisoni n. sp., first record of Zetomotrichidae (Acari, Oribatida) from North America. Acarologia. 59:226–241.
- Behan-Pelletier VM, Lindo Z. 2023. Oribatid mites. Biodiversity, Taxonomy and Ecology. Boca Raton (London, NY): CRC Press.
- Behan-Pelletier VM, Mahunka S. 1993. Description of Humerobates setosus sp. n. (Acari: Humerobatidae) from South Africa. Folia Entomologica Hungarica. 54:9–16.
- Behan-Pelletier VM, Walter DE. 2007. Phylleremus n. gen., from leaves of deciduous trees in eastern Australia (Oribatida: Licneremaeoidea). Zootaxa. 1386:1–17.
- Behan-Pelletier VM, Walter DE. 2009. Unduloribates from North America (Acari, Oribatida, Unduloribatidae). Zootaxa. 2294:47–61.
- Behan-Pelletier VM, Walter DE. 2013. Phylogenetic relationships of Tectoribates: nymphal characters of new North American species place the genus in Tegoribatidae (Acari, Oribatida). Zootaxa. 3741:459–489.
- Berlese A. 1910. Lista di nuove specie e generi di Acari. Redia. 6:242–271.
- Bernini F. 1976. Notulae Oribatologicae XV. Lamellovertex, un nuovo genere per Scutovertex caelatus Berlese 1895 (Acarida, Oribatei). Redia. 59:311–321.
- Bernini F. 1980. Notulae Oribatologicae XXIV. Gli Acari Oribatei di alcune piccole grotte del Senese. Redia. 63:359–405.
- Bernini F. 1983. Notulae Oribatologicae XXX. Siciliotrichus siculus n. gen., n. sp. di Sicilia (Acarida, Oribatida). Animalia. 10:141–147.
- Bonfim GS, Silva JG, Oliveira AR. 2017. A new species of Licneremaeus (Oribatida: Licneremaeidae) from Brazil, with an identification key to the known species of the genus. Systematic & Applied Acarology. 22:1872–1883.
- Chatterjee T, Pfingstl T, Pešić V. 2018. A checklist of marine littoral mites (Acari) associated with mangroves. Zootaxa. 4442:221–240.
- Choi S-S. 1996. A new genus and a new species of aquatic oribatid mite (Acari: Oribatida) from Korea. Korean Journal of Soil Zoology. 1:1–4.
- Choi S-S. 1998. Two new species of oribatid mites from Baekrockdam of Mt. Halla in Cheju-do, Korea. Journal of Asia-Pacific Entomology. 1:211–216.
- Coetzee L. 1987. The South African Lamellareidae Balogh, 1972 (Acari: Oribatei). Navorsinge van die Nasionale Museum Bloemfontein. 5:325–353.
- Coetzee L. 1993. New genera and species of the family Zetomotrichidae Grandjean, 1954 (Acari, Oribatida, Oripodoidea) from South Africa. Navorsinge van die Nasionale Museum Bloemfontein. 9:133–179.
- Coetzee L. 1997. The Antarctic mite genus Maudheimia (Acari, Oribatida). Navorsinge van die Nasionale Museum Bloemfontein. 13:101–135.
- Coetzee L. 2001. New species of the family Lohmanniidae (Acari, Oribatida) from South Africa. Navorsinge van die Nasionale Museum Bloemfontein. 17:53–67.
- Colloff MJ. 2010. The hyperdiverse oribatid mite genus Scapheremaeus (Acari: Oribatida: Cymbaeremaeidae) in Australia, with descriptions of new species and consideration of biogeographical affinities. Zootaxa. 2475:1–38.
- Colloff MJ. 2011a. A new genus of oribatid mite, Spineremaeus gen. nov. and three new species of Scapheremaeus (Acari: Oribatida: Cymbaeremaeidae) from Norfolk Island, South-west Pacific, and their biogeographical affinities. Zootaxa. 2828:19–37.
- Colloff MJ. 2011b. A review of the oribatid mite family Nothridae in Australia, with new species of Novonothrus and Trichonothrus from rain forest and their Gondwanan biogeographical affinities (Acari: Oribatida). Zootaxa. 3005:1–44.
- Colloff MJ. 2011c. New species of the oribatid mite genus Phyllhermannia Berlese, 1916 (Acari, Oribatida, Hermanniidae) from wet forests in south-eastern Australia show a high diversity of morphologically-similar, short-range endemics. Zootaxa. 2770:1–60.
- Colloff MJ. 2012. New eremaeozetid mites (Acari: Oribatida: Eremaeozetoidea) from the south-western Pacific region and the taxonomic status of the Eremaeozetidae and Idiozetidae. Zootaxa. 3435:1–39.
- Colloff MJ. 2013. Species-groups and biogeography of the oribatid mite family Malaconothridae (Oribatida: Malaconothroidea), with new species from the south-western Pacific region. Zootaxa. 3722:401–438.
- Colloff MJ, Cameron SL. 2009. Revision of the oribatid mite genus Austronothrus Hammer (Acari:Oribatida):sexual dimorphism and a re-evaluation of the phylogenetic relationships of the family Crotoniidae. Invertebrate Systematics. 23:87–110.
- Colloff MJ, Cameron SL. 2013. A phylogenetic analysis and taxonomic revision of the oribatid mite family Malaconothridae (Acari: Oribatida), with new species of Tyrphonothrus and Malaconothrus from Australia. Zootaxa. 3681:301–346.
- Colloff MJ, Cameron SL. 2014. Beyond Moa’s Ark and Wallace’s Line: extralimital distribution of new species of Austronothrus (Acari, Oribatida, Crotoniidae) and the endemicity of the New Zealand oribatid mite fauna. Zootaxa. 3780:263–281.
- Corpuz-Raros LA. 2010. Some mites of the family Oripodidae (Acari: Oribatida) from the Philippines. Philippine Entomologist. 24:1–17.
- Covarrubias R. 1967. New Oribatids (Acarina) from Chile. Opuscula Zoologica Budapest. 7:89–116.
- Dunlop JA. 2002. Character states and evolution of the chelicerate claws. In: Toft S, Scharff N, editors. European arachnology. Aarhus: Aarhus University Press; p. 345–354.
- Engelbrecht CM. 1972. A new South African microzetid genus, Afrozetes (Microzetidae: Oribatei). Acarologia. 14:484–496.
- Engelbrecht CM. 1974a. The genus, Hydrozetes Berlese, 1902 (Acari: Oribatei) in South Africa. Navorsinge van die Nasionale Museum Bloemfontein. 3:41–49.
- Engelbrecht CM. 1974b. The genus, Passalozetes Grandjean, 1932 (Acari: Oribatei) in South Africa. Navorsinge van die Nasionale Museum Bloemfontein. 3:29–38.
- Engelbrecht CM. 1975. New ameronothroid (Oribatei, Acari) taxa from the Republic of South Africa and the Islands Gough and Marion. Navorsinge van die Nasionale Museum Bloemfontein. 3:53–88.
- Engelbrecht CM. 1986. New oribatid taxa and distribution records predominantly from Southern Africa (Acari: Oribatei: Oribatelloidea). Navorsinge van Die Nasionale Museum Bloemfontein. 5:169–250.
- Ermilov SG. 2017a. A new species of Lohmannia (Lohmannia) (Acari, Oribatida, Lohmanniidae) from Vietnam, with supplementary description of L. (Lohmannia) turcmenica (Bulanova-Zachvatkina, 1960). Systematic & Applied Acarology. 22:193–207.
- Ermilov SG. 2017b. Two new species of the family Suctobelbidae (Acari, Oribatida) from Chile. Entomological Review. 97:677–683.
- Ermilov SG. 2020a. Contribution to the knowledge of oribatid mites (Acari, Oribatida) of Uganda, with description of a new species of the genus Machadobelba (Machadobelbidae). Systematic & Applied Acarology. 25:1021–1031.
- Ermilov SG. 2020b. First record of the genus Lamellarea (Acari, Oribatida, Lamellareidae) from the Neotropical region. Ecologica Montenegrina. 27:74–79.
- Ermilov SG. 2020c. Sadocepheus nortonroyi sp. nov. (Acari, Oribatida, Cepheidae) from Chile, with identification key to known species of the genus. Systematic & Applied Acarology. 25:869–880.
- Ermilov SG. 2021. Contribution to knowledge of Mainothrus (Acari, Oribatida, Trhypochthoniidae), with description of two new species from Russia and Brunei. Systematic & Applied Acarology. 26:2059–2069.
- Ermilov SG. 2022. Luisumaoppia molinoensis gen. nov., sp. nov. (Acari, Oribatida, Oppiidae) from Peru. Zootaxa. 5105:131–138.
- Ermilov SG, Alvarado-Rodríguez O, Retana-Salazar AP. 2015. Two new species of oribatid mites (Acari, Oribatida) with auriculate pteromorphs from Costa Rica, including a key to all species of Galumna (Galumna) of the Neotropical region. Systematic & Applied Acarology. 20:273–285.
- Ermilov SG, Anichkin AE. 2014. Taxonomic study of oribatid mites (Acari, Oribatida) of Bi Dup—Nui Ba National Park (southern Vietnam). Zootaxa. 3834:1–86.
- Ermilov SG, Behan-Pelletier VM. 2014. Revision of Fenestrobates (Acari, Oribatellidae) with description of F. marauni sp. nov., from South America, and new diagnosis for Oribatellidae. Zootaxa. 3827:258–272.
- Ermilov SG, Frolov AV. 2019. Ramusella (Dosangoppia) bochkovi (Acari, Oribatida, Oppiidae), a new subgenus and species of oribatid mites phoretic on Ceratophyus polyceros (Pallas, 1771) (Coleoptera, Geotrupidae) from Russia. Systematic & Applied Acarology. 24:209–221.
- Ermilov SG, Frolov AV. 2021. New data on oribatid mites (Acari, Oribatida) phoretic on passalid beetles (Coleoptera, Passalidae) from the Afrotropical and Oriental regions, with descriptions of three new species from Congo, Gabon and Ghana. Systematic & Applied Acarology. 26:769–787.
- Ermilov SG, Hugo-Coetzee E. 2012. Two new species from South Africa, with remarks on generic diagnosis of Licnodamaeolus Covarrubias, 1998 and taxonomic status of Nacunansella Fernandez & Cleva, 1998 (Acari: Oribatida: Licnodamaeidae). Zootaxa. 3167:32–44.
- Ermilov SG, Hugo-Coetzee E, Khaustov AA. 2017. Oribatid mites (Acari, Oribatida) inhabiting nests of the termite Trinervitermes trinervoides (Sjöstedt) in the Franklin Game Reserve (Bloemfontein, South Africa), with description of a new species of the genus Ceratobates (Tegoribatidae). Systematic & Applied Acarology. 22:1715–1732.
- Ermilov SG, Hugo-Coetzee E, Khaustov AA. 2021a. Contribution to the knowledge of Geminoppia (Acari, Oribatida, Oppiidae), with description of a new species from South Africa. Acta Zoologica Academiae Scientiarum Hungaricae. 67:211–222.
- Ermilov SG, Hugo-Coetzee E, Khaustov AA, Khaustov VA. 2021b. New faunistic and taxonomic data on oribatid mites (Acari, Oribatida) inhabiting soil and termite nests in the Golden Gate Highlands National Park (eastern South Africa). International Journal of Acarology. 47:152–158.
- Ermilov SG, Khaustov AA. 2016. Juvenile instars of Siculobata (Paraleius) leontonycha (Acari, Oribatida, Scheloribatidae). Systematic & Applied Acarology. 21:1379–1391.
- Ermilov SG, Klimov PB. 2017. Generic revision of the large-winged mite superfamily Galumnoidea (Acari, Oribatida) of the world. Zootaxa. 4357:1–72.
- Ermilov SG, Kolesnikov VB. 2013. Morphology of juvenile stages of Zetorchestes micronychus (Acari, Oribatida, Zetorchestidae). Entomological Review. 93:646–658.
- Ermilov SG, Liao J-R. 2017. Contribution to the knowledge of the oribatid mite genus Brassiella (Acari: Oribatida: Caloppiidae). Biologia. 72:1041–1048.
- Ermilov SG, Liao J-R. 2020. Contribution to the knowledge of oribatid mites of the genus Phyllhermannia (Acari, Oribatida, Hermanniidae) in Taiwan. Systematic & Applied Acarology. 25:1924–1934.
- Ermilov SG, Martens J. 2021. Contribution to the knowledge of the oribatid mite genus Leobodes (Acari, Oribatida, Nippobodidae). Acarologia. 61:995–1014.
- Ermilov SG, Minor M. 2015a. New species of oribatid mites (Acari: Oribatida) of the genera Austrachipteria (Achipteriidae), Cultroribula (Astegistidae) and Microlamellarea (Lamellareidae) from New Zealand. Biologia. 70:1501–1519.
- Ermilov SG, Minor M. 2015b. Two new species of Neophysobates (Acari, Oribatida, Tegoribatidae) from New Zealand. Acarologia. 55:285–295.
- Ermilov SG, Minor M. 2015c. The oribatid mite genus Macrogena (Acari, Oribatida, Ceratozetidae), with description of two new species from New Zealand. ZooKeys. 506:13–26.
- Ermilov SG, OConnor BM. 2020a. New Perscheloribates species (Acari, Oribatida, Scheloribatidae) phoretic on beetles (Insecta, Coleoptera). Acarologia. 60:289–300.
- Ermilov SG, OConnor BM. 2020b. Two new species of insect phoretic Siculobata (Paraleius) (Acari, Oribatida, Scheloribatidae) from U.S.A. and Trinidad. Acta Zoologica Academiae Scientiarum Hungaricae. 66:329–343.
- Ermilov SG, Sandmann D, Marian F, Maraun M. 2013. Two new species of oribatid mites of the genus Truncozetes (Acari, Oribatida, Epactozetidae) from Ecuador. ZooKeys. 303:23–31.
- Ermilov SG, Sandmann D, Scheu S. 2019. New and interesting species of sacculonotic Haplozetidae (Acari, Oribatida, Haplozetidae) from Indonesia. Zootaxa. 4656:459–474.
- Ermilov SG, Shtanchaeva UY, Subías LS, Martens J. 2014. Two new species of oribatid mites of Lasiobelba (Acari, Oribatida, Oppiidae) from Nepal, including a key to all species of the genus. Zookeys. 424:1–17.
- Ermilov SG, Starý J. 2017. First record of the family Liacaridae (Acari, Oribatida) from Vietnam, with description of two new species. Systematic & Applied Acarology. 22:456–466.
- Ermilov SG, Starý J. 2018. New and interesting oribatid mites (Acari, Oribatida) from Hanoi (Northern Vietnam). Systematic & Applied Acarology. 23:61–77.
- Ermilov SG, Starý J. 2019. New taxa of oribatid mites (Acari, Oribatida) from the Korup National Park, Cameroon. The genus Malaconothrus Berlese, 1904 (Malaconothridae). Journal of Zoology. 98:504–512.
- Ermilov SG, Starý J. 2021. Two new species of oribatid mites of the genus Xenillus (Acari, Oribatida, Liacaridae) from Bolivia. Acta Zoologica Academiae Scientiarum Hungaricae. 67:301–311.
- Ermilov SG, Subías LS, Shtanchaeva UY, Friedrich S. 2021c. Contribution to the knowledge of the oribatid mite genus Gittella (Acari, Oribatida, Oppiidae), with description of a new species from Peru. Acarologia. 61:1015–1022.
- Ermilov SG, Tolstikov AV. 2015. New species and records of mites of the superfamily Oripodoidea (Acari, Oribatida) from Brazil. Systematic & Applied Acarology. 20:629–640.
- Ermilov SG, Vu QM. 2012. Two new species of oribatid mites (Acari: Oribatida) from Phong Nha-Ke Bang National Park of central Vietnam. International Journal of Acarology. 38:160–167.
- Ermilov SG, Weigmann G. 2015. A new species of Trhypochthoniellus (Acari: Oribatida: Trhypochthoniidae) from Chile, with remarks on diagnosis of the genus. Biologia. 70:1495–1500.
- Evans GO. 1992. Principles of Acarology. Wallingford: C.A.B. International.
- Feduccia A. 1993. Evidence from Claw Geometry Indicating Arboreal Habits of Archaeopteryx. Science. 259:790–793.
- Fernandez NA. 1984. Contribution à la connaissance de la famille Hydrozetidae. I. Hydrozetes (Argentinobates) ringueleti nov. sub-gen., nov. sp. Acarologia. 25:307–317.
- Fernandez N, Cleva R, Theron P. 2011. Malgachebates peyrierasi n. gen., n. sp. (Acari: Oribatida: Plasmobatidae) from Madagascar. International Journal of Acarology. 37:61–74.
- Fernandez N, Marcangeli J, Eguaras M. 1997. Les acariens (Oribates) des zones arides d’Argentine. II. Huilicheremaus michaii, n. gen., n. sp. Acarologia. 38:79–85.
- Fernandez N, Theron P, Leiva S, Rollard C, Tiedt L. 2014b. Revision of the family Carabodidae (Acari: Oribatida) VI. Mangabebodes kymatismosi gen. nov., sp. nov. and Antongilibodes paulae gen. nov., sp. nov. from Madagascar. International Journal of Acarology. 40:296–319.
- Fernandez N, Theron P, Rollard C. 2013. Revision of the family Carabodidae (Acari: Oribatida) IV. Afticarabodes anjavidilavai gen. nov., sp. nov., Rugocepheus joffrevillei sp. nov. and redefinition of the genus Rugocepheus Mahunka, 2009. International Journal of Acarology. 39:462–480.
- Fernandez N, Theron P, Rollard C, Castillo ER. 2014a. Oribatid mites from deep soils of Hòn Chông limestone hills, Vietnam: the family Lohmanniidae (Acari: Oribatida), with the descriptions of Bedoslohmannia anneae n. gen., n. sp., and Paulianacarus Vietnamese n. sp. Zoosystema. 36:771–787.
- Fernandez N, Travé J. 1984. La variabilité chaetotaxique et la néotrichie gastronotique des Hydrozetidae (Oribates). Acarologia. 25:407–417.
- Fuangarworn M. 2010. Two new species of the oribatid mite genus Phyllochthonius Travé, 1967 (Acari: Oribatida: Phyllochthoniidae) from Thailand. Zootaxa. 2521:26–36.
- Fuangarworn M. 2011. Two new species of protoplophorid mites (Acari: Oribatida: Protoplophoridae) from Thailand. Zootaxa. 2732:59–67.
- Fuangarworn M, Chaisuekul C. 2011. Two new species of the oribatid mite subgenus Phyllolohmannia (Oribatida: Lohmanniidae: Mixacarus) from Thailand. International Journal of Acarology. 37:114–128.
- Fuangarworn M, Lekprayoon C. 2011. New species of oribatid mites in the families Synichotritiidae and Phthiracaridae from Thailand, with a checklist of Thai Euptyctima (Acari: Oribatida: Euphthiracaroidea, Phthiracaroidea). Zootaxa. 3106:24–41.
- Fuangarworn M, Lekprayoon C. 2012. Description of two new species of Nothrolohmannia Balogh, 1968 (Acari: Oribatida: Hypochthoniidae) from Thailand, with key to known species. Zootaxa. 3170:45–54.
- Fuangarworn M, Norton RA. 2013. Psammochthoniidae n. fam., a paedomorphic family of oribatid mites (Oribatida: Enarthronota) from sandy soil in Thailand, Brazil and the USA. Zootaxa. 3691:473–499.
- Fujikawa T. 1993. Oribatid Mites from Picea glehnii Forest at Mo-Ashoro, Hokkaido (9) A New Species Belonging to a New Genus of the Family Hermanniellidae. Edaphologia. 50:15–22.
- Fujikawa T. 1994. Oribatid mites from Picea glehnii forest at Mo-Ashoro, Hokkaido (10) A new species of the family Eniochthoniidae. Edaphologia. 52:19–27.
- Fujikawa T. 1999. Eight new species of the Genus Nothrus. Edaphologia. 63:5–54.
- Fujikawa T. 2001. A new Oribatid species of Liodidae from Mt. Hayachine in Northern Nippon. Edaphologia. 68:17–22.
- Fujikawa T. 2002. Three species of Cepheidae and Cymbaeremaeidae (Acari: Oribatida) from Nippon. Edaphologia. 69:13–23.
- Fujikawa T. 2003a. Five Species оf Nanhermanniidae (Acari: Oribatida) from Nippon. Edaphologia. 71:1–8.
- Fujikawa T. 2003b. Thirteen new species from the Shirakami-Sanchi World Heritage area, Nippon (Acari: Oribatida). Acarologia. 63:369–392.
- Fujikawa T. 2006. Oribatid mites (Acari, Oribatida) from World Cultural Heritage Area in Miyajima, Japan. Edaphologia. 80:1–24.
- Fujikawa T. 2008a. A new species of Podopterotegaeus (Acari: Oribatida). Acarologia. 48:105–109.
- Fujikawa T. 2008b. Eleven new species from Shikoku Island in Nippon (Acari, Oribatida). Acarologia. 48:69–103.
- Fujikawa T. 2014a. The second species of Tokunocepheidae (Acari: Oribatida) from Chiran-cho, South Japan. Edaphologia. 95:1–5.
- Fujikawa T. 2014b. The second representative of the family Eulohmanniidae Grandjean, 1931 (Acari: Oribatida) from Japan. Edaphologia. 93:1–10.
- Fujikawa T. 2017. A review of Tectocepheus Berlese, 1896 (Acari: Oribatida: Tectocepheidae) from Japan, with a new species from southern Kyushu. Edaphologia. 101:7–26.
- Gorb SN, Gorb EV. 2004. Ontogenesis of the attachment ability in the bug Coreus marginatus (Heteroptera, Insecta). The Journal of Experimental Biology. 207:2917–2924.
- Gordeeva E. 1980. Oribatid mites of the family Cosmochthoniidae (Oribatei). [in Russian]. Zoologicheskii Zhurnal. 59:838–850.
- Gordeeva E, Niemi R, Petrova-Nikitina AD. 1998. A new genus and species, Tauroplophora aureonata, from the Crimea, Ukraine, and a ne species, Cryptoplophora asiatica, from Turkmenistan (Acari, Oribatida, Protoplophoridae). Acarologia. 39:185–190.
- Gordeeva E, Penttinen R, Subías L, Petrova A. 2007. A new species, Krivolutskiella pennata sp. n., from the eastern Mediterranean and new data for K. pubescens Gordeeva, 1980 (Cosmochthoniidae, Acarina, Oribatida). Acarologia. 47:165–171.
- Gottardo M, Vallotto D, Beutel RG. 2015. Giant stick insects reveal unique ontogenetic changes in biological attachment devices. Arthropod Structure & Development. 44:195e199.
- Grandjean F. 1930. Oribates nouveaux de la région Caraïbe. Bulletin de la Société zoologique de France. 55:262–284.
- Grandjean F. 1932a. Au sujet des Palaeacariformes Trägardh. Bulletin du Muséum National d’Histoire Naturelle (2e série). 4:411–426.
- Grandjean F. 1932b. La famille des Protoplophoridae (Acariens). Bulletin de la Société zoologique de France. 57:10–36.
- Grandjean F. 1932c. Observations sur les Oribates (3e série). Bulletin du Muséum National d’Histoire Naturelle (2e série). 4:292–306.
- Grandjean F. 1935. Observations sur les Oribates (9e série). Bulletin du Muséum National d’Histoire Naturelle (2e série). 7:280–287.
- Grandjean F. 1936. Les Microzetidae n. fam. (Oribates). Bulletin de la Société zoologique de France. 61:60–93.
- Grandjean F. 1941. L’ambulacre des Acariens (1re série). Bulletin du Muséum National d’Histoire Naturelle. 13:422–429.
- Grandjean F. 1943. L’ambulacre des Acariens (2e série). Bulletin du Muséum National d’Histoire Naturelle. 15:303–310.
- Grandjean F. 1948. Sur les Hydrozetes (Acariens) de l’Europe occidentale. Bulletin du Muséum National d’Histoire Naturelle (2e série). 20:328–335.
- Grandjean F. 1949. Les Enarthronota (Acariens) (2e série). Annales de la Science Naturelle (II). 10:29–58.
- Grandjean F. 1950a. Études sur les Lohmanniidae (Oribates, Acariens). Archives de Zoologie expérimentale et générale. 87:95–162.
- Grandjean F. 1950b. Sur deux escpèces du genre Dometorina n. g. et les mœurs de D. plantivaga (Berl.) (Acariens, Oribates). Bulletin de la Société Zoologique de France. 75:224–242.
- Grandjean F. 1951. Etude sur les Zetorchestidae (Acariens, Oribates). Mémoires du Muséum National d’Histoire Naturelle. 4:1–50.
- Grandjean F. 1952. Observations sur les Palaeacaroides (2e serié). Bulletin du Muséum National d’Histoire Naturelle (2e série). 24:460–467.
- Grandjean F. 1954a. Etude sur les Palaeacaroides. Mémoires du Muséum National d’Histoire Naturelle. 7:179–274.
- Grandjean F. 1954b. Zetomotrichus lacrimans acarien sauteur (Acari, Zetomotrichidae). Annales de la Société entomologique de France. 123:1–16.
- Grandjean F. 1955. Sur un Acarien des iles Kerguélen. Podacarus auberti (Oribate Mémoires du Muséum National d’Histoire Naturelle. 8:109–150.
- Grandjean F. 1957. Observations sur les Palaeacaroides (4e série). Bulletin du Muséum National d’Histoire Naturelle (2e série). 29:213–220.
- Grandjean F. 1958a. Charassobates cavernosus Grandj. 1929 (Acarien, Oribate). Mémoires du Muséum National d’Histoire Naturelle. 16:121–140.
- Grandjean F. 1958b. Perlohmannia dissimilis (Hewitt) (Acarien, Oribate). Mémoires du Muséum National d’Histoire Naturelle. 16:57–120.
- Grandjean F. 1959a. Observations sur les Oribates (40e serie). Bulletin du Muséum National d’Histoire Naturelle (2e Serié). 31:359–366.
- Grandjean F. 1959b. Polypterozetes cherubin Berl. 1916 (Oribate). Acarologia. 1:147–180.
- Grandjean F. 1959c. Sur le genre Mochlozetes Grandj. 1930 (Oribate). Acarologia. 1:453–474.
- Grandjean F. 1960. Les Mochlozetidae n. fam. (Oribates). Acarologia. 2:102–148.
- Grandjean F. 1961. Les Amerobelbidae (Oribates). Première partie. Acarologia. 3:303–343.
- Grandjean F. 1962. Au sujet des Hermanniellidae (Oribates). Deuxieme partie. Acarologia. 4:632–670.
- Grandjean F. 1965a. Nouvelles observations sur les Oribates (4e série). Acarologia. 7:91–112.
- Grandjean F. 1965b. Oribates mexicains (2e série) Stelechobates megalotrichus n.g., n. sp. Acarologia. 7:532–563.
- Grandjean F. 1966. Les Staurobatidae n. fam. (Oribates). Acarologia. 8:696–727.
- Grandjean F. 1970. Nouvelles observations sur les Oribates. Acarologia. 12:849–876.
- Grobler L. 2003. The genus Furcoppia (Acari, Oribatida) from South Africa. Navorsinge van die Nasionale Museum Bloemfontein. 19:161–179.
- Hammer M. 1952. Investigations on the microfauna of Northern Canada, Part I. Acta Arctica. 4:1–108.
- Hammer M. 1961. Investigations on the Oribatid fauna of the Andes Mountains. II. Peru. Biologiske Skrifter Det Kongelige Dankse Videnskabernes Selskab. 13:202.
- Hammer M. 1962. Investigations on the Oribatid fauna of the Andes Mountains. III. Chile. — Biologiske Skrifter Det Kongelige Dankse Videnskabernes Selskab. 13:127.
- Hammer M. 1966. Investigations on the oribatid fauna of New Zealand. Part I. Biologiske Skrifter Det Kongelige Dankse Videnskabernes Selskab. 15:157.
- Hammer M. 1967a. Investigations on the oribatid fauna of New Zealand. Part II. Biologiske Skrifter Det Kongelige Dankse Videnskabernes Selskab. 15:103.
- Hammer M. 1967b. Some oribatids from Kodiak Island near Alaska. Acta Arctica. 14:1–25.
- Hammer M. 1968. Investigations on the oribatid fauna of New Zealand. Part III. Biologiske Skrifter Det Kongelige Dankse Videnskabernes Selskab. 16:128.
- Hammer M. 1971. On some Oribatids from Viti Levu, the Fiji Islands. Det Kongelige Danske Videnskabernes Selskab Biologiske Skrifter. 16:96.
- Hammer M. 1973. Oribatids from Tongatapu and Eua, the Tonga Islands, and from Upolu, Western Samoa. Det Kongelige Danske Videnskabernes Selskab Biologiske Skrifter. 20:98.
- Hammer M. 1977. Investigations on the Oribatid fauna of North-West Pakistan. Det Kongelige Danske Videnskabernes Selskab Biologiske Skrifter. 21:106.
- Hammer M. 1979. Investigations on the Oribatid fauna of Java. Det Kongelige Danske Videnskabernes Selskab Biologiske Skrifter. 22:126.
- Haq MA, Xavier A. 2005. Four new species of phthiracarid mites (Acari: Oribatei) from Malabar, Kerala, India. Zoos’ Print Journal. 20:2062–2071.
- Heethoff M, Koerner L. 2007. Small but powerful: the oribatid mite Archegozetes longisetosus Aoki (Acari, Oribatida) produces disproportionately high forces. The Journal of Experimental Biology. 210:3036–3042.
- Hirauchi Y. 1998. Two New Species of the Family Metrioppiidae from Central Japan (Acari: Oribatida). Edaphologia. 61:7–13.
- Hirauchi Y, Aoki J-I. 1997. A new species of the genus Achipteria from Mt. Tateyama, Central Japan (Acari: Oribatida). Edaphologia. 59:5–9.
- Hugo-Coetzee EA. 2011. Three new species of Austrocarabodes (Oribatida: Carabodidae) and notes on Austrocarabodes pinnatus Mahunka, 1986, from South Africa. Zootaxa. 3011:1–15.
- Hugo-Coetzee EA. 2013. New species of Aleurodamaeus Grandjean, 1954 (Oribatida: Aleurodamaeidae) from South Africa. Zootaxa. 3670:531–556.
- Hunt GS. 1996. A review of the genus Pedocortesella Hammer in Australia (Acarina: Cryptostigmata: Pedrocortsellidae). Records of the Australian Museum. 48:223–286.
- Iturrondobeitia JC, Subías LS. 1978. Contribución al conocimiento de los Oribátidos (Acarida Oribatida) del País Vasco, II. Boletín de la Asociación española de Entomología. 2:87–90.
- Jorrin J. 2014. Two new arthronotic mites from the South of Spain (Oribatida, Cosmochthoniidae), with a new subgenus and species of Cosmochthonius and one new species of Phyllozetes. Acarologia. 54:183–191.
- Karasawa S, Behan-Pelletier VM. 2007. Description of a Sexually Dimorphic Oribatid Mite (Arachnida: Acari: Oribatida) from Canopy Habitats of the Ryukyu Archipelago, Southwestern Japan. Zoological Science. 24:1051–1058.
- Karasawa S, Hijii N. 2004. Morphological modifications among oribatid mites (Acari: Oribatida) in relation to habitat differentiation in mangrove forests. Pedobiologia. 48:383–394.
- Kerschbaumer M, Pfingstl T. 2021. Testing for phylogenetic signal in claws suggests great influence of ecology on Caribbean intertidal arthropods (Acari, Oribatida). Scientific Reports. 11:4398.
- Knee W. 2017. A new Paraleius species (Acari, Oribatida, Scheloribatidae) associated with bark beetles (Curculionidae, Scolytinae) in Canada. Zookeys. 667:51–65.
- Knee W, Forbes MR, Beaulieu F. 2013. Diversity and host use of mites (Acari: Mesostigmata,Oribatida) phoretic on bark beetles (Coleoptera:Scolytinae): global generalists, local specialists? Annals of the Entomological Society America. 106:339–350.
- Kok OB. 1968a. Studies on the Taxonomy of the South African Tectocepheidae Grandjean 1953 (Oribatei, Acari). Zoologica Africana. 3:155–183.
- Kok OB. 1968b. Two new oribatid mites from South Africa (Oribatei, Acari). Acarologia. 10:696–710.
- Krantz GW, Baker GT. 1982. Observations on the plastron mechanism of Hydrozetes sp. (Acari: Oribatida: Hydrozetidae). Acarologia. 23:273–277.
- Krisper G. 1990. Das Sprungvermögen der Milbengattung Zetorchestes (Acari, Oribatida). Zoologische Jahrbücher Anatomie. 120:289–312.
- Kubota T, Aoki J-I. 1998. Hololohmannia alaskensis from Alaska, Representing a New Genus and Species of the Family Perlohmanniidae (Acari: Oribatida). Edaphologia. 60:17–21.
- Lavoipierre M. 1946. A new acarine parasite of bees. Nature. 158:130.
- Lee DC. 1981. Sarcoptiformes (Acari) of South Australian soils. 1. Notation. 2. Bifemorata and Ptyctima (Cryptostigmata). Records of the South Australian Museum. 18:199–222.
- Lee DC. 1989. Hemileius (Acarida: Cryptostigmata: Scheloribatidae) from South Australian soils. Records of the South Australian Museum. 23:97–111.
- Lee DC, Pajak GA. 1987. Anoplozetes, a new genus of Zetomotrichidae (Acarida: Cryptostigmata) from South Australia. Transactions of the Royal Society of South Australia. 111:99–103.
- Lindo Z. 2011. Five new species of Ceratoppia (Acari: Oribatida: Peloppiidae) from western North America. Zootaxa. 3036:1–25.
- Lindo Z. 2015. A rare new species of Metrioppia (Acari: Oribatida: Peloppiidae) from a Pacific Northwest temperate rainforest. The Canadian Entomologist. 147:553–563.
- Lindquist EE. 1985. External Anatomy. In: Helle W, Sabelis MW, editors. Spider Mites, their Biology, Natural Enemies and Control. Vol. 1A. Amsterdam: Elsevier; p. 3–28.
- Lions J-C, Norton RA. 1998. North American Synichotritiidae (Acari: Oribatida) 2. Synichotritia spinulosa and S. caroli. Acarologia. 39:265–284.
- Lotfollahi P, Movahedzade E, Abbasi A, Shimano S, Norton RA. 2016. Second species of the family Arborichthoniidae (Acari: Oribatida), from agricultural soil in Iran. International Journal of Acarology. 42:229–234.
- Luxton M. 1989. New taxa of intertidal mites (Acari). Journal of Natural History. 23:407–428.
- Mahunka S. 1982. Oribatids from the eastern part of the Ethiopian Region (Acari). I. Acta Zoologica Academiae Scientiarum Hungaricae. 28:293–336.
- Mahunka S. 1984. Oribatids of the eastern part of the Ethiopian Region (Acari). VI. Acta Zoologica Hungarica. 30:393–444.
- Mahunka S. 1985a. Oribatids from Africa (Acari: Oribatida) I. Acta Zoologica Hungarica. 31:295–339.
- Mahunka S. 1985b. Oribatids from Africa (Acari: Oribatida) II. Folia Entomologica Hungarica. 46:73–113.
- Mahunka S. 1987. Oribatids from Africa (Acari: Oribatida), V. Folia Entomologica Hungarica. 48:105–128.
- Mahunka S. 1988. New and interesting mites from the Geneva Museum LXI. Oribatids from Sabah (East Malaysia) III (Acari: Oribatida). Revue Suisse de Zoologie. 95:817–888.
- Mahunka S. 1995. Oribatids from Sabah, East Malaysia (Acari Oribatida, Parakalummoidea n. stat. and Galumnoidea). Tropical Zoology. 8:269–308.
- Mahunka S. 1997a. Oribatids from Brunei II. (Acari: Oribatida). (Acarologica Genavensia LXXXII). Revue suisse de zoologie 104:661–700.
- Mahunka S. 1997b. Oribatids from Madagascar III. (Acari: Oribatida). (Acarologica Genavensia LXXXIII). Revue suisse de zoologie 104:115–170.
- Mahunka S. 1998. New data on Oribatids (Acari: Oribatida) from St. Lucia (Antilles). (Acarologica Genavensia LXXXIX). Revue Suisse de Zoologie. 105:839–877.
- Mahunka S. 2000. Oribatids from Sabah (East Malaysia) VIII (Acari: Oribatida: Dampfiellidae and Otocepheidae). (Acarologica Genavensia LXXXVI). Revue Suisse de Zoologie. 107:675–720.
- Mahunka S, Mahunka-Papp L. 2007. Taxonomical and faunistical studies on oribatids collected in Kenya (Acari: Oribatida) I. Acta Zoologica Academiae Scientiarum Hungaricae. 53:51–74.
- Mahunka S, Mejia-Recamier BE. 1998. Two new protoplophorid oribatids from Mexico (Acari: Oribatida). Miscellanea Zoologica Hungarica. 12:61–66.
- Manning PL, Payne D, Pennicott J, Barret PM, Ennos RA. 2006. Dinosaur killer claws or climbing crampons? Biology Letters. 2:110–112.
- Martinez RI, Casanueva ME. 1996. Un nuevo acaro Cosmochthoniidae de Chile: gozmanyina pehuen n. sp. (Acari: Oribatida). Gayana Zoologica. 60:63–67.
- Martinez PA, Fernandez NA, Monetti LN. 1996. La famille Oripodidae dans la République d’Argentine. I. Parapirnodus proposis n. sp. Acarologia. 37:357–362.
- Martinez PA, Laborde VB. 2000. Gehypochthonius marianoi n. sp. (Acari: Oribatida), from sand dunes in coastal Argentina. Acarologia. 41:381–389.
- Martinez PA, Palacios-Vargas JG. 1998. A new species of Phylloribatula Balogh & Mahunka (Acari: Oribatei: Fenichellidae) from Argentina. Acarologia. 39:165–171.
- Mihelčič F. 1957a. Zur Systematik und Ökologie der Gattung Passalozetes Grandjean. Zoologischer Anzeiger. 158:24–26.
- Mihelčič F. 1957b. Oribatiden Südeuropas VII. Zoologischer Anzeiger. 159:44–68.
- Miko L. 2008. Taxonomy of European Damaeidae (Acari: Oribatida) II. Porobelba weigmanni n. sp. (Oribatida, Damaeidae), from East Slovakia, with comments on other known species of the genus. Zootaxa. 1844:55–62.
- Minor MA, Ermilov SG. 2015. First record of Atopochthonioidea from New Zealand, with description of Pterochthonius roynortoni sp. n. (Acari, Oribatida, Pterochthoniidae). Acarina. 23:132–138.
- Mizutani Y, Shimano S, Aoki J-I. 2003. A New Species of Hermanniella (Acari: Oribatida: Hermanniellidae) from Forest Soil in Tokyo. Journal of the Acarological Society Japan. 12:87–91.
- Mondal BK, Kundu BG, Roy S. 1999. A new cryptostigmatid mite (Acari: Oribatei, Apoplophoridae) from Darjeeling, India. Records of the Zoological Survey India. 97:73–78.
- Moritz M. 1966. Paratritia baloghi n. g., n. sp., eine neue Gattung und Art der Familie Euphthiracaridae (Acari: Oribatei) aus Deutschland. Acarologia. 8:374–381.
- Moritz M. 1974. Beiträge zur Kenntnis der Oribatiden (Acari) Europas. VI. Neoctenacarus hastilis nov. gen., nov. spec., eine neue Ctenacaridae aus der DDR (Palaeosomata, Ctenacaroidea). Abhandlungen und Berichte des Naturkundemuseums Görlitz. 48:1–14.
- Moritz M. 1976. Revision der europäischen Gattungen und Arten der Familie Brachychthoniidae (Acari, Oribatei). Teil J. Allgemeiner Teil: brachychthoniidae Thor, 1934. Spezieller Teil: liochthonius v. d. Rammen, 1959, Verachthonius nov. gen. und Paraliochthonius nov. gen. Mitteilungen aus dem zoologischen Museum in Berlin. 52:1–136.
- Nakamura K, Nakamura Y-N, Fujikawa T. 2013. Oribatid mites (Acari, Oribatida) from Tohoku (Northeast Japan), collected after tidal wave in 2011. Acarologia. 53:41–76.
- Nakamura Y-N, Fukumori S, Fujikawa T. 2010. Oribatid fauna (Acari, Oribatida) from the Kumaya cave of Iheya village in Central Ryukyu arc, south Japan, with a description of several new species. Acarologia. 50:439–477.
- Nevin FR. 1974. A new genus and two new species of Achipteriidae from New York State (Acari: Cryptostigmata: Oribatei). Journal of the New York Entomological Society. 82:177–182.
- Niedbała W. 2000. The ptyctimous mites fauna of the Oriental and Australian regions and their centres of origin (Acari: Oribatida). Genus. supplement:1–493.
- Norton RA. 1975. Elliptochthoniidae, a new mite family (Acarina: Orihatei) from mineral soil in California. New York Entomological Society. 83:209–216.
- Norton RA. 1977. A review of F. Grandjean’s system of leg chaetotaxy in the Oribatei (Acari) and its application to the family Damaeidae. In: Dindal DL, editor. Biology of Oribatid Mites. Syracuse: SUNY College of Environmental Science and Forestry; p. 33–61.
- Norton RA. 1980. Observations on phoresy by oribatid mites (Acari: Oribatei). International Journal of Acarology. 6:121–130.
- Norton RA 1982. Arborichthonius n. gen., an unusual enarthronote soil mite (Acarina: Oribatei) from Ontario. Proceedings of the Entomological Society Washington 84:85–96.
- Norton RA. 1983. The Tenuialidae (Acari: Oribatei): new diagnoses for supraspecific taxa. Acarologia. 24:203–217.
- Norton RA. 2003. Nothrolohmannia baloghi sp. n. (Acari: Oribatida), from rainforest in Papua New Guinea, and re-evaluation of Nothrolohmanniidae. Acta Zoologica Academiae Scientiarum Hungaricae. 49:25–42.
- Norton RA, Behan-Pelletier VM. 2007. Eniochthonius mahunkai sp. n. (Acari: Oribatida: Eniochthoniidae), from North American Peatlands, with a redescription of Eniochthonius and a key to North American species. Acta Zoologica Academiae Scientiarum Hungaricae. 53:295–333.
- Norton RA, Behan-Pelletier VM. 2009. Suborder Oribatida. In: Krantz GW, Walter DE, editors. A manual of Acarology – third edition. Texas: Texas Tech University Press; p. 430–564.
- Norton RA, Behan-Pelletier VM, Wang H. 1996. The aquatic oribatid mite genus Mucronothrus in Canada and the western U.S.A. (Acari: Trhypochthoniidae). Canadian Journal of Zoology. 74:926–949.
- Norton RA, Ermilov SG. 2019. Anderemaeus (Acari, Oribatida)—overview, three new species from South America and reassessment of Anderemaeidae supported by ontogeny. Zootaxa. 4647:241–289.
- Norton RA, Ermilov SG, Miko L. 2022. Kunstidamaeus arthurjacoti sp. nov. (Oribatida, Damaeidae), first report of the genus in North America. Systematic & Applied Acarology. 27:482–496.
- Norton RA, Franklin E. 2018. Paraquanothrus n. gen. from freshwater rock pools in the USA, with new diagnoses of Aquanothrus, Aquanothrinae, and Ameronothridae (Acari, Oribatida). Acarologia. 58:557–627.
- Norton RA, Franklin E, Crossley DA Jr. 2010. Scapheremaeus rodickae n. sp. (Acari: Oribatida: Cymbaeremaeidae) associated with temporary rock pools in Georgia, with key to Scapheremaeus species in eastern USA and Canada. Zootaxa. 2393:1–16.
- Norton RA, Fuangarworn M. 2015. Nanohystricidae n. fam., an unusual, plesiomorphic enarthronote mite family endemic to New Zealand (Acari, Oribatida). Zootaxa. 4027:151–204.
- Norton RA, Lions J-C. 1992. North American Synichotritiidae (Acari: Oribatida) 1. Apotritia walker n. g., n. sp., from California. Acarologia. 33:285–301.
- Norton RA, Metz LJ. 1980. Nehypochthoniidae (Acari: Oribatei), a new mite family from the southeastern United States. Annals of the Entomological Society of America. 73:54–62.
- Norton RA, OConnor BM, Johnston DE 1983. Systematic relationships of the Pediculochelidae (Acari: Acariformes). Proceedings of the Entomological Society Washington 85:493–512.
- Norton RA, Sidorchuk E. 2014. Collohmannia johnstoni n. sp. (Acari, Oribatida) from West Virginia (U.S.A.), including description of ontogeny, setal variation, notes on biology and systematics of Collohmanniidae. Acarologia. 54:271–344.
- Norton RA, Williams DD, Hogg ID, Palmer SC. 1988. Biology of oribatid mite Mucronothrus nasalis (Acari: Oribatida: Trhypochthoniidae) from a small coldwater springbrook in eastern Canada. Canadian Journal of Zoology. 66:622–629.
- Nübel-Reidelbach E. 1994. Taxonomie und Systematik der Gattung Tectocepheus BERLESE, 1895 (Acari, Oribatei). Andrias. 12:3–94.
- Nübel-Reidelbach E, Woas S. 1992. Einige basale Arten der cepheiden und der pterogasterinen Entwicklungslinie der Höheren Oribatiden (Acari, Oribatei). Andrias. 9:75–119.
- Ohkubo N. 1980. A New Species of the Genus Brachyoripoda (Acari, Oribatida) from Japan. Annotationes Zoologicae Japonenses. 53:137–139.
- Ohkubo N. 1987. A new species of Zetomimus (Acari: Oribatei) from Japan. Zoological Science. 4:897–902.
- Ojeda M, Velez P, Espinosa-Asuar L, Eguiarte LE, Souza V. 2020. A new species of Trhypochthoniellus (Acari: Oribatida: Trhypochthoniidae) from Cuatro Ciénegas, Coahuila, Mexico, and a key to the world species. Systematic & Applied Acarology. 25:974–985.
- Ordouni F, Akrami MA, Ramroodi S. 2021. New species of primitive oribatid mite of the family Haplochthoniidae (Acari, Oribatida) from southeastern Iran. Systematic & Applied Acarology. 26:2109–2117.
- Oudemans AC. 1927. Acarologische Aanteekeningen, LXXXVIII. Entomologische Berichten. 7:257–268.
- Penttinen R, Viiri H, Moser JC. 2013. The mites (Acari) associated with bark beetles in the Koli National Park in Finland. Acarologia. 53:3–15.
- Pérez-Iñigo C. 1981. Resultados de la expedición Peris-Alvarez a la isla de Annobón (13) Oribatid mites (2nd series). Eos. 57:201–212.
- Pérez-Iñigo C. 1993. Acari, Oribatei, Poronota. In: Ramos MA, Alba J, Belĺs X, Gosálbez J, Guerra A, Macpherson E, Martin F, Serrano J, Templado J, editors. Fauna Ibérica. Vol. 3. Madrid: Museo Nacional de Ciencias Naturales, CSIC; p. 266.
- Pérez-Iñigo C, Baggio D. 1989. Oribates édaphiques du Brésil (V) Oribates de l’état de São Paulo (Deuxième Partie). Acarologia. 30:261–274.
- Pérez-Iñigo C, Baggio D. 1997. Oribates édaphiques du Brésil (X) Quelques Oribates de l’état de Para. Acarologia. 38:403–413.
- Pérez-Iñigo C, Peña MA. 1997. Ácaros oribátidos (Acari, Oribatei) de Gran Canaria (III). Boletín de la Asociación española de Entomología. 21:165–183.
- Pérez- Iñigo C, Sarasola M. 1995. Soil oribatid mites from Uruguay (I) (Acari, Oribatei). Three new species from the Department of Cerro Largo. Acarologia. 36:65–73.
- Pfingstl T. 2017. The marine-associated lifestyle of ameronothroid mites (Acari, Oribatida) and its evolutionary origin: a review. Acarologia. 57:693–721.
- Pfingstl T, Kerschbaumer M. 2022a. Like parent, like child – Ontogenetic development of claws of intertidal arthropods (Acari, Oribatida) from different ecological niches. Arthropod Structure & Development. 67:101143.
- Pfingstl T, Kerschbaumer M. 2022b. Sexually dimorphic claws predict courtship and mating sequence in the intertidal oribatid mite Fortuynia atlantica (Acari, Oribatida). Acarologia. 62:666–671.
- Pfingstl T, Kerschbaumer M, Shimano S. 2020. Get a grip–evolution of claw shape in relation to microhabitat use in intertidal arthropods (Acari, Oribatida). PeerJ. 8:e8488.
- Pfingstl T, Krisper G. 2010. Development and morphology of Unduloribates undulatus (Berlese, 1914) (Acari: Oribatida) and some remarks on the Unduloribatidae. Acta Zoologica Academiae Scientiarum Hungaricae. 56:119–138.
- Pfingstl T, Krisper G. 2011a. Juvenile stages of the arboricolous mite Cymbaeremaeus cymba (Nicolet, 1855) (Acari: Oribatida: Cymbaeremaeidae). International Journal of Acarology. 37:175–189.
- Pfingstl T, Krisper G. 2011b. The nymphs of Micreremus brevipes (Acari: Oribatida) and complementary remarks on the adult. Acta Zoologica Academiae Scientiarum Hungaricae. 57:351–367.
- Pfingstl T, Schäffer S, Ebermann E, Krisper G. 2010b. The discovery of Scutovertex ianus sp. nov. (Acari, Oribatida) – a combined approach of comparative morphology, morphometry and molecular data. Contributions to Zoology. 79:39–55.
- Pfingstl T, Schäffer S, Krisper G. 2010a. Re-evaluation of the synonymy of Latovertex Mahunka, 1987 and Exochocepheus Woolley and Higgins, 1968 (Acari: Oribatida: Scutoverticidae). International Journal of Acarology. 36:327–342.
- Piffl E. 1972. Zur Systematik der Oribatiden (Acari). (Neue Oribatiden aus Nepal, Costa Rica und Brasilien ergeben eine neue Familie der Unduloribatidae und erweitern die Polypterozetidae um die Gattungen Podopterotegaeus, Nodocepheus, Eremaeozetes und Tumerozetes). Khumbu Himal. 4:269–314.
- Popp E. 1960. Neue Oribatiden aus Ägypten (Acarina). Bulletin de la Société entomologique d’Égypte. 44:203–221.
- Pugh PJA, King PE, Fordy MR. 1987. Ambulacral structure in the terrestrial moiety of the intertidal Acari, and its relationship with the lifestyle of the Acari. Acarologia. 28:3–13.
- Ramsay GW. 1966. Three new box-mites (Acari: Oribatei: Phthiracaroidea) from the Brothers, Cook Strait, New Zealand. New Zealand Journal of Science. 9:901–912.
- Ren G, Yang M, Liang W, Xie L. 2018. Two new species of the subgenus Papillacarus (Oribatida, Lohmanniidae, Papillacarus) from China. Zootaxa. 4462:100–114.
- Ren G, Yang M, Liang W, Zheng Q. 2019. Two new species of Achipteriidae (Acari, Oribatida) from China. Zootaxa. 4647:336–347.
- Ribas SC, Velloso A, Teixeira-Filho P, Rocha-Barbosa O, Evangelista H, Dos Santos E. 2004. Structure of claws and toes of two tropidurid lizard species of Restinga from Southeastern Brazil: adaptations to the vertical use of the habitat. Revista Chilena de Historia Natural. 77:599–606.
- Rios G, Palacios-Vargas JG. 1998. Especias nuevas de Scapheremaeus (Acari: Oribatei: Cymbaeremaeidae) de México. Anales Instituto de Biología Universidad Nacional Autónoma de México. Serie Zoología. 69:181–215.
- Ruiz E, Kahwash MAM, Subías LS. 1990. Cuatro nuevos Gymnodamaeoideos del Sur de España (Acari, Oribatida, Gymnodamaeoidea). Boletín de la Real Sociedad Española de Historia Natural (Sección Biologica). 85:39–49.
- Ruiz E, Subías LS, Kahwash MAM. 1991. Nuevos Oribatellidae (Acari, Oribatida) del sur de España. Boletín de la Real Sociedad Española de Historia Natural (Sec. Biol.). 87:143–149.
- Sanyal AK, Basak S, Barman RP. 2002. Three new species of oribatid mites (Acarina, Oribatida: Haplochthoniidae) from the Antarctic continent. Acarina. 10:57–63.
- Schatz H. 2001. The genus Eremaeozetes (Acari:Oribatida) on the Galápagos Islands. Acarologia. 41:475–493.
- Schatz H. 2003. New Sphaerochthonius species from the Neotropical region (Acari: Oribatida). Revue Suisse de Zoologie 110:111–124.
- Schatz H. 2021. A new species of Brachychthoniidae (Acari: Oribatida) from the Eastern Central Alps (Austria, Tyrol), with the proposal of a new genus. Acarologia. 61:365–379.
- Schatz H, Behan-Pelletier V. 2008. Global diversity of oribatids (Oribatida: Acari: Arachnida). Hydrobiologia. 595:323–328.
- Schatz H, Behan-Pelletier VM, OConnor BM, Norton R. 2011. Suborder Oribatida van der Hammen, 1968. In: Zhang Z-Q, editor. Animal biodiversity: an outline of higher-level classification and survey of taxonomic richness. Zootaxa 3148. Auckland (New Zealand): Magnolia Press; p. 237.
- Schubart H. 1968. Neue Palaeacaroidea (Oribatei) aus Amazonien. Amazoniana. 1:251–256.
- Schubart H. 1975. Morphologische Grundlagen für die Klärung der Verwandtschaftsbeziehungen innerhalb der Milbenfamilie Ameronothridae (Acari, Oribatei). Zoologica. 123:23–91.
- Schuster R. 1957. Haloribatula tenareae nov. gen., nov. spec., eine neue Oribatide aus dem mediterranen Eulitoral (Acari). Zoologischer Anzeiger. 159:121–127.
- Schuster R. 1962. Neue Mesoplophora- Vorkommen in der Neotropis (Arach., Acari, Oribatei). Senckenbergiana Biologica. 43:489–495.
- Sellnick M. 1928. Formenkreis Hornmilben, Oribatei. In: Brohmer P, Ehrmann P, Ulmer G, editors. Die Tierwelt Mitteleuropas. Vol. 3(9). Leipzig: Quelle und Meyer; p. 1–42.
- Seniczak A, Seniczak S. 2016. Morphological ontogeny of Achipteria gigantea sp. nov. (Acari: Oribatida: Achipteriidae) from northern Spain, with comments on Achipteria. Systematic & Applied Acarology. 21:1571–1590.
- Seniczak A, Seniczak S. 2020. Morphological ontogeny of Limnozetes solhoyorum sp. nov. (Acari: Oribatida: Limnozetidae) from Norway, with comments on Limnozetes Hull. Systematic & Applied Acarology. 25:327–348.
- Seniczak A, Seniczak S. 2021. Morphological ontogeny of Limnozetes schatzi sp. nov. (Acari: Oribatida: Limnozetidae) from Norway. Systematic & Applied Acarology. 26:1974–1991.
- Seniczak S, Seniczak A, Kaczmarek S, Marquardt T, Jangazieva B. 2020. Morphological ontogeny of Cosmochthonius oralensis sp. nov. (Acari: Oribatida: Cosmochthonidae) from Kazakhstan, and comments on Cosmochthonius Berlese. Systematic & Applied Acarology. 25:31–50.
- Shiji MT, Haq MA, Ramani N. 2007. Two new species of lohmanniid mites (Acari: Oribatei) from Kerala, India. Systematic & Applied Acarology. 12:229–236.
- Spain AV. 1968. a new genus of arboreal Mycobatidae from New Zealand (Acari: Cryptostigmata). Acarologia. 10:516–521.
- Spain AV. 1969. A new genus and two new species or arboreal Oppiidae (Acari: Cryptostigmata) from New Zealand. Pacific Insects. 11:155–163.
- Strenzke K. 1963. Entwicklung und Verwandtschaftsbeziehungen der Oribatidengattung Gehypochthonius (Arach., Acari). Senckenbergiana Biologica. 44:231–255.
- Subías LS. 2022. Listado sistemático, sinonímico y biogeográfico de los ácaros oribátidos (Acariformes, Oribatida) del mundo (excepto fósiles). Monografías electrónicas. 12:1–538.
- Subías LS, Pérez-Iñigo C. 1978. Descripción de Bursoplophora ibérica nov. g. y nov. sp. y algunas consideraciones sobre la familia Protoplophoridae (Acari, Oribatei). Eos. 52:387–398.
- Tinius A, Russell AP. 2017. Points on the curve: an analysis of methods for assessing the shape of vertebrate claws. Journal of Morphology. 278:150–169.
- Travé J. 1959. Sur le genre Niphocepheus Balogh 1943, les Niphocepheidae, famille nouvelle. (Acarien, Oribates). Acarologia. 1:475–498.
- Travé J. 1960. Contribution a l´etude de la fauna de la Massane (3e note) Oribates (Acariens). Vie et Milieu. 11:209–232.
- Travé J. 1961. Sur un nouveau genre d’Oribates: neotrichozetes. Acarologia. 3:364–375.
- Travé J. 1966. Oribates (Acariens) des Pyrénées orientales (4e série) Zetorchestidae (2e série. Strenzkea depilata n. g., n. sp. Vie et Milieu, Ser. C. 17:809–828.
- Travé J. 1967. Phyllochthonius aoutii nov. gen. spec., un Enarthronota (Acarien, Oribate) nouveau de Côte d’Ivoire, avec la création d’une superfamille nouvelle, Phyllochthonoidea. Zoologische Mededelingen. 42:83–105.
- Tseng Y-H. 1984. Taxonomical study of oribatid mites from Taiwan. Chinese Journal of Entomology. 4:27–74.
- Wallwork JA. 1960. Some Oribatei from Ghana III. Two new species of the genus Allonothrus (Van der Hammen). Acarologia. 2:568–574.
- Wallwork JA. 1962. Some Oribatei from Ghana. X. The family Lohmanniidae. Acarologia. 4:457–487.
- Wallwork JA. 1963. The Oribatei (Acari) of Maquarie Island. Pacific Insects. 5:721–769.
- Wallwork JA. 1964. Insects of Campbell Island. Appendix. Campellobates acanthus n. gen., n. sp. (Acari: Cryptostigmata). Pacific Insects Monograph. 7:601–606.
- Wallwork JA. 1981. A new aquatic oribatid mite from Western Australia (Acari: Cryptostigmata: Ameronothridae). Acarologia. 22:333–339.
- Walter DE, Behan-Pelletier VM. 1993. Systematics and ecology of Adhaesozetes polyphyllos sp. nov. (Acari: Oribatida: Licneremaeoidea), a leaf-inhabiting mite from Australian rainforests. Canadian Journal of Zoology. 71:1024–1040.
- Walter DE, Proctor H. 1999. Mites: ecology, evolution and behaviour. Wallingford (Oxon, UK): CAB International.
- Wang H-F, Solhoy T, Shen J, Xu R-M. 2001. New species and new records of oribatid mites from Tibet, China. Acta Zootaxonomica Sinica. 26:401–413.
- Weigmann G. 2006. Die Tierwelt Deutschlands begründet 1925 von Friedrich Dahl 76. Teil. Hornmilben (Oribatida). Keltern: Verlag Goecke & Evers; p. 520.
- Willmann C. 1930. Neue Oribatiden aus Guatemala. Zoologischer Anzeiger. 88:239–246.
- Willmann C. 1931. Moosmilben oder Oribatiden (Cryptostigmata). Die Tierwelt Deutschlands I. 22:79–200.
- Woas S. 1981. Zur Taxonomie und Phylogenie der Hermanniidae Sellnick 1928 (Acari, Oribatei). Andrias. 1:7–88.
- Wolff JA, Huber SJ, Gorb SN. 2015. How to stay on mummy’s back: morphological and functional changes of the pretarsus in arachnid postembryonic stages. Arthropod Structure & Development. 44:301–312.
- Woolley TA, Higgins HG. 1964. A new species of Ommatocepheus Berlese, 1913, from North Carolina. (Acarina: Oribatei, Carabodidae). Transactions of the American Microscopical Society. 83:26–28.
- Wunderle I, Beck L, Woas S. 1990. Ein Beitrag zur Taxonomie und Ökologie der Oribatulidae und Scheloribatidae (Acari, Oribatei) in Südwestdeutschland. Andrias. 7:15–60.
- Xu Y, Zhu Y-Z, Wu J-Q, Zhang F-P. 2020. A new species of the genus Paralycus from Fujian, China. Acarologia. 60:481–487.
- Yamamoto Y, Aoki J-I. 1998. Two new oribatid mites of the genus Trimalaconothrus from Yunnan Province in China (Acari: Oribatida: Malaconothridae). Edaphologia. 61:15–21.
- Zani P. 2000. The comparative evolution of lizard claw and toe morphology and clinging performance. Journal of Evolutionary Biology. 13:316–325.
- Zhang Y, Jin D. 2016. Two new species and two newly recorded species of Tenuialidae in China, with an updated key to the family (Acari: Oribatida: Gustavioidea). Zoological Systematics. 41:243–252.
- Zheng L, Chen J. 2021. Two new species and new records of Otocepheidae (Acari, Oribatida) from Yunnan, Southwest China. Zookeys. 1073:177–199.