Abstract
The life cycle of the widely distributed mayfly, Baetis rhodani, was investigated in Øvre Heimdalen, in the Jotunheimen Mountains of central southern Norway. B. rhodani displayed a shift in life cycle from univoltine below the lake, Øvre Heimdalsvatn (1090 m a.s.l.) to a two-year life cycle in the inflowing stream, Brurskardbekken (1100–1300 m a.s.l.). The day degree sum for the univoltine life cycle was 950 compared to 1500–1550 for the two-year life cycle. Larvae of B. rhodani grew rapidly during the ice-free period (June–October) with growth rates of 2.1–8.1% (mg/day), while growth rates were low during winter (0.3–0.5%). There was a positive correlation between growth (mg/day) and temperature (r 2 = 0.34, p = 0.02). Although larval growth and voltinism were influenced by water temperature, other factors such as food resources are likely to be important in mountain areas. The life cycle plasticity exhibited by B. rhodani has undoubtedly contributed to its success in colonising a wide range of running water habitats throughout Europe.
Introduction
Many aquatic insects demonstrate flexible life cycles allowing them to inhabit a wide range of climates and habitats. Temperature is one of the main factors governing the life history of aquatic insects (Vannote and Sweeney Citation1980; Ward Citation1992), affecting all stages from egg development (Elliott Citation1972; Benech Citation1972a) to fecundity (Ward Citation1992). Studying the same species at different altitudes in the same stream system can provide information on the species' thermal requirements (Nebeker Citation1971; Ward and Berner 1980; Ban and Kawai Citation1986). The mayfly, Baetis rhodani (Pictet) is one of the most widespread ephemeropteran species in Europe, occupying a wide range of different lotic habitats (Ulfstrand Citation1968; Benech Citation1972b; Baekken Citation1981; Rosillon Citation1986). It has the ability to adopt different life cycle strategies ranging from a univoltine cycle in Swedish Lapland (Ulfstrand Citation1968) to two or more generations a year in Austria (Humpesch Citation1979), western Norway (Baekken Citation1981), southern Spain (Alba-Tercedor Citation1986) and Belgium (Rosillon Citation1986). In this investigation B. rhodani has been studied at altitudes ranging from 1090 m a.s.l. in subalpine birch forest to 1300 m a.s.l. in the mid-alpine zone within a catchment in the Jotunheimen Mountains of central southern Norway. The aim of the investigation was to document growth patterns, voltinism and life cycle strategies at different altitudes within the same stream system.
Study area
The investigation was carried out in the streams Brurskardbekken and Hinøgla, in Øvre Heimdalen, on the eastern slopes of the Jotunheimen Mountains in southern Norway (61°25′32′′ N, 8°52′10′′ E). Brurskardbekken flows out of the small lake, Brurskardtjern, at 1309 m a.s.l. From the lake the stream falls gradually until 1250 m a.s.l. and at about 1275 m.a.s.l. it flows through a series of small pools. Below 1250 m a.s.l. the gradient is higher until just before it enters a larger lake, Øvre Heimdalsvatn, at 1090 m a.s.l. The distance between the two lakes is 3.2 km. Brurskardbekken is 2–10 metres wide and the substratum is composed of stones of various sizes with gravel in between. Hinøgla, the outflow of the lake, Øvre Heimdalsvatn, is 8–12 m wide and has mainly stony and rocky substrate, although there are areas of soft sediments. In both lake outlets the stony substrate was generally covered with mosses and algae.
Brurskardbekken lies on the NNE side of the valley and has a high net input of radiant energy during the summer, resulting in higher air temperatures, greater precipitation and higher air humidity than elsewhere in the valley (Johannessen Citation1978). Along the study stream the tree line is at 1180 m a.s.l., one of the highest tree lines in Norway. The catchment and in particular the lake, Øvre Heimdalsvatn, has been extensively studied and is well described in Vik (Citation1978), which also contains information on the outflow stream, Hinøgla. The stream, Brurskardbekken, has been studied earlier by Tangen et al. (Citation1978) and Lillehammer and Brittain (Citation1978).
Four stations at different altitudes were used in the investigation; station 1 at 1090 m a.s.l. about 100 m downstream of Øvre Heimdalsvatn (Hinøgla), Station 2 at 1100 m a.s.l. about 300 m upstream of Øvre Heimdalsvatn, station 3 at 1200 m a.s.l in the steep section and station 4 at 1300 m a.s.l. about 100 m downstream of lake, Brurskardtjern. Stations 1 and 2 are located in subalpine birch forest, while stations 3 and 4 are above the tree line.
Methods
Water temperatures were measured every six hours from September 1994 until October 1996 at all stations. The temperature logger at station 2 did not work for 29 days during the summer of 1995, so temperatures for this period were calculated using the daily differences (in %) between 1100 and 1200 m a.s.l. for the same days during the following year.
Samples of B. rhodani were taken from all stations about every second week during the ice free period, from June until October 1995, as well as September 1994 and June 1996. Benthic samples were taken using a kick-net (mesh size 350 μm; net opening 30 × 30 cm) (Frost et al. Citation1971; Brittain Citation1978a). All samples were preserved in 70% ethanol. Three 30 s samples were taken at each station. One additional sample of 3–5 min was also taken at each station in case of low numbers in the 30 s samples. The samples were sorted in the laboratory using a stereo microscope with 6.3 × magnification.
Species identification for B. rhodani was based on Müller-Liebenau (Citation1969). Baetis rhodani was the most abundant ephemeropteran species in the samples, but five other species, Acentrella lapponica (Bengtsson), B. scambus Eaton, B. subalpinus Bengtsson, B. bundyae Lehmkuhl and Siphlonurus lacustris Eaton, were present, but in very low numbers. Larval body length, from the tip of the head to the base of the cerci, was measured in the laboratory to the nearest 0.1 mm, using an ocular micrometer. In the life cycle figures, larvae have been grouped into 0.5 mm size categories. However, 0.1 mm size-classes were used for separating the cohorts.
Growth rates have been calculated for different periods during the year to compare growth rates at different mean temperatures. For the cohort that was present throughout the whole ice-free period, growth has been calculated for the whole period (18 June to 3 October) in addition to three other periods; 18 June to 12 July (a period when temperatures increased), 12 July to 16 August (temperatures were high and stable) and 30 August to 3 October (temperatures decreasing). Growth of larvae hatching from the beginning of August was calculated for the period 16 August to 3 October. Eggs hatched throughout the whole of this period so to avoid taking the newly hatched larvae into account, only the 40 largest larvae of this cohort were used in the calculations. Due to smaller numbers in the samples at station 1 only the 30 largest larvae were used in the growth calculations from 16 August to 3 October 1995. For all samples mean length (in mm) was also converted to biomass (mg dry weight = DW) using the formula DW = aLb , where a and b are constants and L is the larval length in mm. The constants a = 0.0053 and b = 2.875 for the family Baetidae in Benke et al. (Citation1999) have been used.
Emergence traps were installed at each station. At stations 1 and 4, box traps (Davies Citation1950) with sides of 1 m were used, while at stations 2 and 3, smaller traps were used with a length of 1 m and a width and height of 30 cm. All traps were placed in the water with a stone higher than the water level inside the trap. All traps were visited at least every week. Sweep netting was used to collect adult B. rhodani from vegetation and searches were also made under stones along the river bank. Flight periods have been estimated by using the adults found together with the occurrence of mature larvae with dark wing pads.
Results
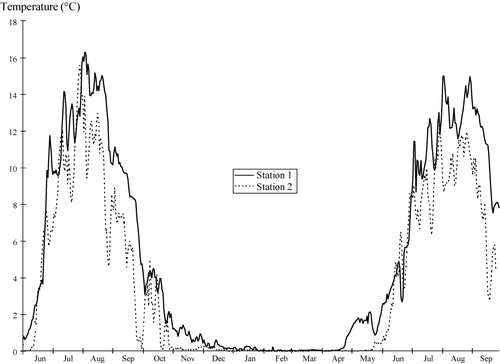
Summer water temperatures were generally between 10 and 15°C, with station 1 usually being 1–2°C warmer (). During winter, temperatures were close to 0°C. The total annual number of degree days differed between stations, ranging from 1007 to 1462 degree days. Stations 1 and 4 situated at lake outlets had the highest number of degree days, 1462 in the outlet of Ø. Heimdalsvatn and 1140 in the outlet of Brurskardtjern. Stations 2 and 3 were similar to each other with 1007 and 1035 degree days, respectively.
Figure 1. Water temperatures at station 1 (outflow of Øvre Heimdalsvatn) and station 2 (inflow of Øvre Heimdalsvatn) from 1 June 1995 to 19 September 1996.
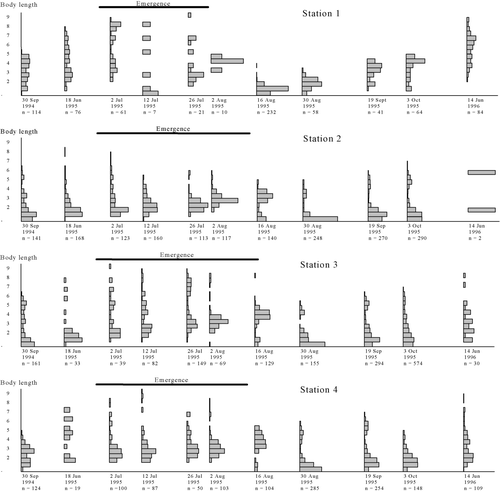
B. rhodani was present in the samples at all stations throughout the year. Two different life cycles for B. rhodani were observed: at station 1 in the outflow of the lake, Øvre Heimdalsvatn, the species was univoltine, while at stations 2–4 above the lake it was clearly semivoltine, taking two years to complete its life cycle ().
Univoltine life cycle
On 16 August most of the eggs in the new generation had hatched. Larvae up to 2 mm in length were present in the samples, indicating that hatching had started shortly after the previous sampling on 2 August (). Hatching may have started even earlier, but the smallest larvae would have been too small to have been collected. Nevertheless, the main hatching period was short, about three weeks.
Egg hatching was followed by a period of rapid growth in length (2.0%/day) until the beginning of October when the growth rate declined (). During winter (3 October–14 June) growth rates were low, 0.2%/day at a mean water temperature of 1.1°C. After ice-break, larvae grew rapidly again (2.3% growth/day) prior to emergence, which started by the end of June and continued through July. Eight larvae with dark wing pads were found at station 1, seven in the 2 July sample and one in the 12 July sample. The number of day degrees needed to complete larval development was estimated to be 950.
Table 1. Growth data for B. rhodani in relation to water temperature at station in the inflow and outflow of Øvre Heimdalsvatn. All dates are in 1995 except for those marked with * which are in 1996. Temperatures are in °C and n is the number of individuals used in the calculations.
Semivoltine life cycle
At stations 2–4 the life cycle of B. rhodani was different from that observed at station 1 (). Most of the larvae collected in the June samples were smaller than 2 mm in length. They grew slowly through the summer at a mean rate of 1.1%/day, reaching about 5 mm at the beginning of October (). During the first half of August larvae smaller than 1 mm in length again appeared in the samples at stations 2–4. The egg incubation time was about one month at a mean temperature of 11°C. The first larvae that hatched grew rapidly until 3 October with a daily growth rate in length of 1.9–2.7% (mean 2.2%). Eggs continued to hatch over a period of at least two months. Slow growth took place during winter (3 October–14 June) with daily growth rates of 0.1–0.2% at mean temperatures of 0.4–0.6°C. In the spring growth rates increased again until the onset of winter. Emergence took place in the following year from the end of June to the middle of August. Hatching and growth over a long period created a wide size distribution. The number of day degrees needed to complete larval development was estimated to be about 1500 at station 2, 1550 at station 3 and 1700 at station 4.
Larval growth
Growth rates of B. rhodani varied considerably between different stages in the life cycle, the small, newly hatched larvae grew at significantly higher rates than the rest of the population. Larvae grew rapidly during the ice-free period (June–October) with growth rates of 0.7–2.67% (mm/day), while growth rates were low during winter (0.12–0.20%). At stations 2–4 daily growth rates varied little through the ice-free period except for the cohort that was present through the whole ice free period at station 3. Linear regression of length against time, using all individual lengths, gave r 2 values in the range 0.66–0.85 (p < 0.001, ) for stations 2–4, demonstrating that growth in length fitted a linear relationship. However, after converting lengths to dry weight, we also calculated growth rates in terms of biomass rather than length. At stations 2–4 growth rates in terms of dry mass varied from 5.2 to 8.1% (mean 6.5%) for the smallest larvae and 2.1 to 2.8% (mean 2.6%) for the largest larvae during the same time period. There was a positive and significant correlation between all growth rates (mg/day) and water temperature (r 2 = 0.34, p = 0.02). However, no significant correlation was found between growth rates at all stations and mean temperature when solely considering the ice free periods (June–October) (r 2 = 0.0004, p > 0.05), despite a temperature range for this period of 5.0–13.2°C.
Discussion
The larvae emerge and the eggs develop during the same summer. At station 1 the main emergence period was from the end of June to the middle of July, with sporadic emergence through August. At stations 2–4 emergence started about one week later. Using experimental data from Elliott (Citation1972) and Benech (Citation1972a), eggs will have started hatching during the first week of August and continued until ice formed at all stations. Small larvae in the spring samples were probably individuals that hatched close to ice formation and due to slow winter growth they were still less than 1 mm long in the spring. Some eggs may also have hatched during the winter and early spring as eggs of B. rhodani exhibit high hatching success even at temperatures as low as 3°C (Elliott Citation1972; Benech Citation1972a). However, the main hatching certainly took place during the second half of August.
Several authors have found temperature to be the major factor affecting growth rates in Ephemeroptera (e.g. Brittain Citation1976, Citation1978b, Citation1982; Elliott et al. Citation1988; Huryn Citation1996; Briers et al. Citation2004). More specifically, Humpesch (Citation1979) documented that temperature accounted for 82% of the variation in growth rate of B. rhodani in two Austrian streams. In our study of B. rhodani only 34% of the variation in growth rates was explained by temperature, so clearly other factors that influence growth rates are playing an important role. There was a substantial difference in the number of degree days needed to complete larval development in the one and two year life cycles, despite that fact that the size of individuals at emergence was similar at all stations. The higher number of degree days obviously did not change the size of the final instar as is frequently the case with summer and winter generations of B. rhodani (Wise Citation1980). Growth rates during the life cycle at stations 2–4 may be adapted to the two year life cycle. High growth rate for the small larvae and slower growth for the larger larvae may result in a higher fitness for B. rhodani in this environment. Although larval growth and voltinism was influenced by water temperature, other factors are clearly important in this mountain area where limiting factors such as food resources (Söderström Citation1988) may come into play to a greater extent.
The present data show the great flexibility in life cycle displayed by B. rhodani, ranging from two or more generation per year in much of Europe including lowland southern Norway (Bækkken 1981; Brittain, unpublished data), to univoltine and seminvoltine life cycles in mountain areas, such as those in the present investigation. This life cycle plasticity, also shown in its congener Baetis alpinus Pictet (López-Rodríguez et al. Citation2008 and references therein), has undoubtedly contributed to its success, frequently occurring in high densities in streams and rivers throughout Europe. It has been suggested that B. rhodani is a complex of several forms of unknown taxonomic status (Engblom Citation1996), possibly cryptic species. This may explain some of its apparently widespread distribution and its ability to adapt to different regimes of temperature and food resources and this may also explain some of the differences observed in the relationship between water temperature and growth rates documented in field studies.
Acknowledgements
Thanks are due to Morten Due, Norwegian Water Resources and Energy Directorate (NVE), who kindly arranged loan of temperature loggers and provided the subsequent temperature data.
References
- Alba-Tercedor , J. 1986 . ‘Ecología Distribución y Ciclos de Desarrollo de Efemerópteros de Sierra Nevada (Granada, España). II: Baetidae (Insecta: Ephemeroptera)’ . Limnetica , 1 : 234 – 246 .
- Baekken , T. 1981 . ‘Growth patterns and food habits of Baetis rhodani, Capnia pygmaea and Diura nanseni in a west Norwegian river’ . Holarctic Ecology , 4 : 139 – 144 .
- Ban , R. and Kawai , T. 1986 . ‘Comparison of the life cycles of two mayfly species between upper and lower part of the same stream’ . Aquatic Insects , 8 : 207 – 205 .
- Benech , V. 1972a . ‘Etude experimentale de l'incubation des oeufs de Baetis rhodani Pictet’ . Freshwater Biology , 2 : 243 – 252 .
- Benech , V. 1972b . ‘Le polyvoltinisme chez Baetis rhodani Pictet (Insecta, Ephemeroptera) dans un ruisseau a truites des Pyrenees-Atlantiques, le Lissuraga’ . Annales de Hydrobiologie , 3 : 141 – 171 .
- Benke , A. , Huryn , A. D. , Smock , L. A. and Wallace , J. B. 1999 . ‘Length-mass relationships for freshwater macroinvertebrates in North America with particular reference to the southeastern United States’ . Journal of the North American Benthological Society , 18 : 308 – 343 .
- Briers , R. A. , Gee , J. H.R. and Geoghegan , R. 2004 . ‘Effects of the North Atlantic Oscillation on growth and phenology of stream insects’ . Ecography , 27 : 811 – 817 .
- Brittain , J. E. 1976 . ‘Experimental studies on nymphal growth in Leptophlebia vespertina (L.) (Ephemeroptera)’ . Freshwater Biology , 6 : 445 – 449 .
- Brittain , J. E. 1978a . ‘Sparkemetoden - fordeler, ulemper og anvendelser’ . Fauna , 31 : 56 – 58 .
- Brittain , J. E. 1978b . ‘The Ephemeroptera of Øvre Heimdalsvatn’ . Holarctic Ecology , 1 : 239 – 254 .
- Brittain , J. E. 1982 . ‘Biology of mayflies’ . Annual Review of Entomology , 27 : 119 – 147 .
- Davies , D. M. 1950 . ‘A study of the blackfly population of a stream in Algomin Park, Ontario’ . Transactions of the Royal Canadian Institute , 59 : 121 – 160 .
- Elliott , J. M. 1972 . ‘Effect of temperature on the time of hatching in Baetis rhodani (Ephemeroptera: Baetidae)’ . Oecologia , 9 : 47 – 51 .
- Elliott , J. M. , Humpesch , U. H. and Macan , T. T. 1988 . ‘Larvae of the British Ephemeroptera: a key with ecological notes’ . Scientific Publications of the Freshwater Biological Association , 49 : 145
- Engblom , E. 1996 . “ ‘Ephemeroptera, Mayflies’ ” . In The Aquatic Insects of North Europe , Edited by: Nilsson , A. 13 – 53 . Stenstrup , , Denmark : Apollo Books .
- Frost , S. , Huni , A. and Kershaw , W. E. 1971 . ‘Evaluation of a kicking technique for sampling stream bottom fauna’ . Canadian Journal of Zoology , 49 : 167 – 173 .
- Humpesch , U. H. 1979 . ‘Life cycles and growth rates of Baetis spp. (Ephemeroptera: Baetidae) in the laboratory and in two stony streams in Austria’ . Freshwater Biology , 9 : 467 – 479 .
- Huryn , A. D. 1996 . ‘Temperature-dependent growth and life cycle of Deleatidium (Ephemeroptera: Leptophlebiidae) in two high-country streams in New Zealand’ . Freshwater Biology , 36 : 351 – 361 .
- Johannessen , T. W. 1978 . ‘The local climate of the Øvre Heimdalen valley’ . Holarctic Ecology , 1 : 93 – 102 .
- Lillehammer , A. and Brittain , J. E. 1978 . ‘The invertebrate fauna of the streams in Øvre Heimdalen’ . Holarctic Ecology , 1 : 271 – 276 .
- López-Rodríguez , M. J. , Tierno de Figueroa , J. M. and Alba-Tercedor , J. 2008 . ‘Life history and larval feeding of some species of Ephemeroptera and Plecoptera (Insecta) in the Sierra Nevada (Southern Iberian Peninsula)’ . Hydrobiologia , 610 : 277 – 295 .
- Müller-Liebenau , I. 1969 . ‘Revision der europäischen arten der gattung Baetis Leach, 1815 (Insecta, Ephemeroptera)’ . Gewässer und Abwässer , 48/49 : 1 – 214 .
- Nebeker , A. V. 1971 . ‘Effect of temperature at different altitudes on the emergence of aquatic insects from a single stream’ . Journal of the Kansas Entomological Society , 44 : 26 – 35 .
- Rosillon , D. 1986 . ‘Life cycles of four ephemeropteran species in a chalky stream’ . Polskie Archiwum Hydrobiologii , 33 : 21 – 31 .
- Söderström , O. 1988 . ‘Effects of temperature and food quality on life-history parameters in Parameletus chelifer and P. minor (Ephemeroptera): a laboratory study’ . Freshwater Biology , 20 : 295 – 303 .
- Tangen , K. , Müller , K. and Brettum , P. 1978 . ‘Periphytic and drifting microalgae in a tributary stream of Øvre Heimdalen’ . Holarctic Ecology , 1 : 148 – 154 .
- Ulfstrand , S. 1968 . ‘Life cycles of benthic insects in Lapland streams (Ephemeroptera, Plecoptera, Trichoptera, Diptera Simuliidae)’ . Oikos , 19 : 167 – 190 .
- Vannote , R. L. and Sweeney , B. W. 1980 . ‘Geographic analysis of thermal equilibria: a conceptual model for evaluation the effect of natural and modified thermal regimes on aquatic insect communities’ . The American Naturalist , 115 : 667 – 695 .
- Vik , R. (ed.) . 1978 . ‘The lake, Øvre Heimdalsvatn, a subalpine freshwater ecosystem . Holarctic Ecology , 1 : 81 – 320 .
- Ward , J. V. 1992 . Aquatic Insect Ecology 1. Biology and habitat , New York : Wiley .
- Ward , J. V. and Berner , L. 1980 . “ ‘Abundance and altitudinal distribution of Ephemeroptera in a Rocky Mountain stream’ ” . In Advances in Ephemeroptera Biology , Edited by: Flannagan , J. F. and Marshall , K. E. 169 – 177 . New York : Plenum Press .
- Wise , E. J. 1980 . ‘Seasonal distribution and life histories of Ephemeroptera in a Northumbrian river’ . Freshwater Biology , 10 : 101 – 111 .