Abstract
This article discusses the use of somatic cell count (SCC) and bacteriological culture (BC) as monitoring tools to assess the udder health situation of dairy goats. Both SCC and BC can be applied for milk samples from individual goats or at bulk milk samples. The causative agent of primary concern in the dairy goat industry is Staphylococcus aureus. This pathogen strongly increases goat milk SCC. The SCC is therefore a useful test to detect S. aureus-infected goats. However, several non-infectious factors, most importantly the stage of lactation, also influence SCC, complicating the interpretation of this test. BC has a low sensitivity for the detection of S. aureus-infected goats, but is a valuable tool to obtain information on which bacterial species are responsible for udder health problems in a herd.
1. Introduction
Monitoring tools can be used to detect changes in udder health status of a goat or a goat herd, in order to be able to intervene at an early stage. When a goat suffers from clinical mastitis, the classical signs of inflammation will be present: pain, swelling, erythema, warmth, and loss of function, i.e., milk yield depression (Smith and Sherman Citation2009). Subclinical mastitis can be defined as an infection of the udder gland, without the visible clinical signs. Subclinical mastitis will thus remain unnoticed, unless additional diagnostic tools are used. Two frequently used tools are somatic cell count (SCC) and bacteriological culture (BC). Whereas clinical mastitis has a low incidence in goats, subclinical mastitis is much more common and SCC is higher in goats than in cows (Bergonier et al. Citation2003; Contreras et al. Citation2007; Paape et al. Citation2007). Evaluation of udder health, especially of the subclinical mastitis situation in a dairy goat herd, requires knowledge of the interpretation of the available diagnostic tools. This review discusses SCC and BC as diagnostic tools for assessment of the udder health situation of an individual dairy goat and of a dairy goat herd. We will first give a brief overview of the agents causing intramammary infections (IMI) in goats, then discuss the SCC and BC, and finalize with some concluding remarks.
2. Pathogens causing IMI
Mastitis in goats can be caused by a number of pathogens, but the most important bacterial genus is Staphylococcus, usually divided into coagulase-negative Staphylococci (CNS) and Staphylococcus aureus.
Staphylococcus aureus can not only cause subclinical IMI, but is also an important cause of clinical mastitis (White and Hinckley Citation1999; Contreras et al. Citation2003). The prevalence of subclinically S. aureus-infected udder halves, as measured by BC from unsymptomatic goats, is usually below 5% (Contreras et al. Citation2003; Schaeren and Maurer Citation2006; Hall and Rycroft Citation2007) and milk yield losses due to subclinical S. aureus-infection are limited (Koop et al. Citation2010b) Therefore, the economical importance of subclinical S. aureus-infection seems to be low. Clinical mastitis in goats has a low incidence of around 2% per year (Bergonier et al. Citation2003; Koop et al. Citation2009), but is often severe. Staphylococcus aureus may cause gangrenous mastitis, which is a peracute form of mastitis, characterized by necrosis of the udder tissue, caused by alpha-toxins (Smith and Sherman Citation2009). The circulation of these toxins also causes general illness and fever and the affected udder gland will eventually fall off (Maisi and Riipinen Citation1991; Contreras et al. Citation2003). This stage is, however, often not reached, because death may occur within 24 h. The severity and painfulness of this disease makes S. aureus-mastitis a serious threat for animal welfare. Because of the high mortality, it is also economically important. Furthermore, S. aureus represents a public health hazard because of the possible shedding of bacteria and toxins in the milk. It is unclear how clinical mastitis cases are related to subclinical S. aureus-infections. A clinical case may not only be the result of a flare-up of a subclinical infection, but may also occur without a preceding subclinical stage. It is also unclear if the strains that are responsible for clinical mastitis cases are also responsible for subclinical infections. It is therefore unknown to what extent subclinical S. aureus infections present a hazard for clinical mastitis.
In goats, CNS are responsible for the majority of subclinical mastitis cases (Contreras et al. Citation2003), and also clinical mastitis caused by these pathogens has occasionally been described (Deinhofer and Pernthaner Citation1995). CNS comprise a number of different species (Valle et al. Citation1991; Deinhofer and Pernthaner Citation1995; Contreras et al. Citation1997) and the herd level prevalence of CNS is usually between 10% and 30%, and can be as high as 71% (Sheldrake et al. Citation1981). Proper diagnosis of these species is important and should be based on genotypic rather than phenotypic methods (Ruegg Citation2009; Zadoks and Watts Citation2009). In cows, differences in potential to affect udder health between CNS species have been described (Supré et al. Citation2011), but species-specific study on pathogenicity of CNS in goats has been limited and was usually based on phenotypic tests (e.g., Kalogridou-Vassiliadou Citation1991; Deinhofer and Pernthaner Citation1995; Leitner et al. Citation2007). The literature is inconsistent about the question whether CNS in goats are to be seen as major mastitis pathogens. In a review, Bergonier et al. (Citation2003) stated that CNS cannot be considered as minor pathogens. Also, Dulin et al. (Citation1983) write that CNS should be regarded as major pathogens, given their potential to significantly increase SCC and decrease milk yield. Other studies, however, show that although the rise in SCC caused by CNS may be statistically significant, it is not nearly as strong as the effect of S. aureus on SCC (Hunter Citation1984; Hall and Rycroft Citation2007). Also, the negative effect on milk yield could not always be reproduced (Merin et al. Citation2004; Moroni et al. Citation2005; Koop et al. Citation2010b). The importance of this group of species therefore remains to be demonstrated.
Several other pathogens can infect the goat udder, but none of these seems to be as pathogenic as S. aureus or as prevalent as CNS. The most important groups are Streptococci, Gram-negatives, Corynebacteria, Mycoplasmata, and a virus with a tropism for the udder, Caprine arthritis encephalitis virus (CAEV). Streptococci have occasionally been cultured from the goat udder. Environmental streptococci are responsible for the majority of streptococcal IMI, but S. agalactiae is rarely seen (Contreras et al. Citation2003). Gram-negative bacteria have a low prevalence in goats, but can occasionally cause severe clinical mastitis (Hunter Citation1984; Ameh et al. Citation1994). Streptococci as well as Gram-negative bacteria are to be considered as major pathogens because of their strong effect on SCC (Hall and Rycroft Citation2007; Min et al. Citation2007). However, because of their low prevalence, the importance of these bacteria is limited in goats. Corynebacteria have been cultured in several studies, but are considered minor pathogens in goats as they are in cows (Hogan et al. Citation1999; Contreras et al. Citation2003; Schaeren and Maurer Citation2006; Hall and Rycroft Citation2007). Not only in the Mediterranean basin, but also in other places of the world, Mycoplasma spp. can be important causes of mastitis in goats (Bergonier et al. Citation1997; Corrales et al. Citation2004), and are responsible for the contagious agalactia syndrome (Castro-Alonso et al. Citation2009). CAEV is also known to cause udder infections in goats. These infections are characterized by interstitial mastitis and clinical cases are known as “hard udder”. The acute form of this viral mastitis appears at parturition as a very firm udder, almost as a rock, but the overlying skin is loose and free of edema, heat, and erythema and, most importantly, milk flow is almost absent (Smith and Sherman Citation2009). Infection with CAEV does not always result in clinical mastitis, but can also cause subclinical mastitis. Elevated SCC has been reported for CAEV-positive animals (Lerondelle et al. Citation1992; Sánchez et al. Citation2001; Turin et al. Citation2005).
Summarizing, in goats S. aureus is the most important mammary pathogen, because of its capacity of causing clinical disease and thereby impairing welfare and causing economic losses. Diagnostic tools should therefore focus primarily on the detection of S. aureus, rather than on CNS, which are more prevalent, but less harmful.
3. Somatic cell count
SCC is a count of the number of body cells in a quantity of milk, expressed as cells/mL, and can be measured at gland level, at animal level, or at herd level. The herd level SCC (bulk milk SCC) represents a weighted average of the SCC of all animals contributing to the milk in the bulk tank. In the same way, animal level SCC (composite SCC) represents a weighted average of the SCC of both udder glands of a goat. Composite SCC of an infected animal will therefore generally be lower than the gland level SCC of an infected gland, because of dilution with milk from the uninfected udder half.
In Europe, the standard to be met for the collection of raw cow's milk from the production holding or for acceptance at treatment or processing establishments is 400 × 103 cells/mL (EEC Council directive 92/46, 1992). For goat milk, no such standard exists in the EU. However, in several other countries, goat milk SCC limits are comparable to cow milk SCC limits (Pirisi et al. Citation2007), which are often impossible to meet for dairy goat farmers. Some researchers feel that the use of the same SCC limit for cows and goats is unfair because of differences in physiology between the species (Wilson et al. Citation1995; Manlongat et al. Citation1998; White and Hinckley Citation1999). These authors point out that in many cases, the high SCC of goats is not accompanied by positive BC. This is supported by the fact that several studies have shown non-infectious factors to be related to SCC in goats, such as stage of lactation (Perrin and Baudry Citation1993; Gomes et al. Citation2006; Olechnowicz and Sobek Citation2008; Koop et al. Citation2010b), oestrus (McDougall and Voermans Citation2002; Moroni et al. Citation2007; Talafha et al. Citation2008), parity (Wilson et al. Citation1995; Luengo et al. Citation2004), breed (Park Citation1991; Boscos et al. Citation1996), and various causes of stress which have their effect probably via milk yield depression (Lerondelle et al. Citation1992).
A number of different cell types contribute to the SCC. Polymorphonuclear neutrophilic leukocytes (PMN) are the most abundant cell type in goat milk and constitute 45–74% of the SCC in uninfected glands and 71–86% in infected glands (Dulin et al. Citation1983). Macrophages and lymphocytes are less abundant and the fraction of these cells decreases with infection. About 6% of the cells in uninfected goat milk are epithelial in origin (Paape et al. Citation2001). The function of PMN is to ingest and kill invading mastitis pathogens (Paape and Capuco Citation1997). It is therefore surprising that these cells are present in high numbers in milk from apparently uninfected animals. Rota et al. (Citation1993) showed that PMN increase during lactation and Manlongat et al. (Citation1998) explained this late-lactation rise with the presence of higher chemotactic activity in non-mastitic, late-lactation milk, and concluded that this phenomenon was non-pathological and could play a physiologic regulatory role in mammary gland involution.
Summarizing, large numbers of inflammatory cells are not only seen in milk from infected but also from culture-negative goats, and SCC seems to be affected by infectious as well as non-infectious factors. When SCC is used as a diagnostic tool to measure the udder health status of a goat or a goat herd, these non-infectious factors should be taken into account.
3.1. SCC at goat level
To monitor udder health of an individual goat, SCC is probably the most relevant tool. In a recent article using latent class models, we showed that composite SCC has acceptable test characteristics for use as a screening test. At a cut-off value of 1500 × 103 cells/mL, sensitivity was estimated at 0.86 and specificity at 0.95, for detecting S. aureus-infected goats in early lactation (Koop et al. Citation2011). Furthermore, SCC is much cheaper than BC of individual animals, and less time consuming than the California Mastitis Test (CMT). Especially in the case of a herd that already participates in a Dairy Herd Improvement (DHI) program with routine sampling for milk components measurement, the additional costs of measuring SCC are low. When using DHI samples, SCC measurement is at animal level, and thus gives an indication of the probability that at least one of the two udder glands is infected, which is more efficient than testing a sample for each individual gland. Still, with large herd sizes, the logistic aspects of DHI testing are substantial, and a farmer should choose wisely at what stage of lactation and at what frequency the SCC measurement should be performed.
3.1.1. Timing of SCC measurement
For three reasons, we think the optimal stage of lactation for SCC measurement in goats is at peak lactation (between week 6 and week 18 of lactation): (1) Stage of lactation is an important factor influencing SCC, increasing SCC with increasing days in milk (Koop et al. Citation2010b). Sensitivity of SCC therefore increases in late lactation, but specificity is highest at peak lactation (Koop et al. Citation2011). The choice for high sensitivity or high specificity depends on the prevalence of the disease in the population and the costs associated with false positive and false negative results. Because of the relatively low prevalence of major pathogens, the high specificity is more important than the high sensitivity in order to keep an acceptable positive and negative predictive value. The weight given to false negative or false positive findings should be driven by the consequences of these findings, such as costs. The costs associated with false positive or false negative test results are unknown, but we do know that the number of false positive test results will be much higher than the number of false negative results and will thus have a bigger impact. Imagine a herd of 1000 goats with a constant prevalence of subclinical major infections of 5%. With the sensitivity and specificity estimated by Koop et al. (Citation2011) for peak and late lactation, the number of false positives is 38 at peak lactation and 86 at late lactation, whereas the number of false negatives is 10 at peak lactation and 5 at late lactation. This shows that the costs of a false negative finding must be at least 10 times as high as the costs of a false positive finding, to prefer the late lactation test over the peak lactation test. Peak lactation therefore seems to be the optimal timing of SCC measurement. (2) As stated before, estrus is known to increase SCC in goats (McDougall and Voermans Citation2002; Moroni et al. Citation2007). Therefore, SCC measurement later in lactation will give false positive results when breeding has commenced. (3) Earlier detection enables to intervene earlier, and thus may save more losses caused by reduced milk yield and transmission to other goats.
3.1.2. Frequency of SCC measurement
In cows, DHI testing is usually done every 3–6 weeks. In dairy goats, the optimal frequency of testing may be lower. Frequent testing can have two goals: (1) to detect new subclinical infections earlier and (2) to be able to make a stricter rule for positive cases, by requiring multiple high SCC to mark the animal as truly infected. Because of the low incidence of S. aureus during lactation (Leitner et al. Citation2007), it may not be cost-efficient to repeat a sampling to detect new subclinical infections earlier. However, data supporting economic calculations are lacking. Requiring multiple high SCC to differentiate truly persistently infected animals from accidental high cell counts caused by other reasons, may be worthwhile, because many factors can cause a brief elevation of SCC, such as various causes of stress or diseases other than mastitis (Lerondelle et al. Citation1992; Haenlein Citation2002). Repeated sampling for SCC may help differentiate between such false positive high SCC and true positive high SCC caused by infection with a major pathogen. Bergonier et al. (Citation2003) did describe an increased specificity when the criterion for infection involved multiple high SCC measurements rather than one. However, the increased specificity came at the cost of decreased sensitivity, so the use of repeated SCC measurements may be preferable if high specificity is valued higher than high sensitivity, which was discussed before. Measuring SCC twice or thrice around the peak of lactation may be ideal, but more detailed study of the dynamics of infection with S. aureus and of SCC are needed to determine the optimal frequency.
3.1.3. SCC threshold
One can differentiate between fixed and dynamic thresholds. A threshold that is fixed at a certain number of cells/mL is most commonly used, because of the ease of implementation. There may, however, be some benefits to a dynamic threshold that can vary over the course of lactation and possibly also with parity. Haenlein et al. (Citation2002) used linear regression to calculate the average SCC at various days in milk and translated this into a series of threshold values varying from 556 × 103 cells/mL at 90 days in milk to 1206 × 103 cells/mL at 305 days in milk. This method did, however, not take into account that SCC is log-normally distributed and it assumed that the mean SCC would be the optimal threshold value to distinguish the infected from non-infected which was not validated. Nevertheless, the principle of using various thresholds to correct for stage of lactation may be valuable. Another approach may be to correct for milk yield, since the effect of stage of lactation was hypothesized to be caused by a dilution effect (Koop et al. Citation2010b).
When SCC is measured around the peak of lactation, a threshold of 1500 × 103 cells/mL gives reasonable test characteristics, according to latent class analysis (Koop et al. Citation2011). A higher threshold will quickly reduce sensitivity. However, if the same static threshold is also used for later stages of lactation, a threshold of 2000 × 103 cells/mL or even higher may be needed to keep the number of false positives within acceptable limits. In an optimal situation, the threshold should be adapted to the specific situation on a farm and should be guided by the herd prevalence of major pathogens and by the goals of the farmer. If a farmer is willing to invest more in udder health, a lower threshold can be used to find more infected animals and thus make more progress. At the same time, if the prevalence is high, a too low threshold will result in many alerts. It will generally be economically unfeasible to cull or separate all of these animals. Furthermore, high numbers of test positive animals may be discouraging. A better approach may be to set achievable goals each year and set the goals higher (and the SCC threshold lower) with improving udder health.
3.2. SCC at herd level
Bulk milk SCC represents a weighted average of the SCC of all lactating animals and thus gives an indication of the subclinical mastitis situation of the entire herd. The interpretation of bulk milk SCC in goat farms differs from bulk milk SCC in a dairy cattle farm, because goats are seasonal breeders, which causes a yearly fluctuation in bulk milk SCC. This will have an effect on the optimal threshold and timing of bulk milk SCC measurement.
3.2.1. Bulk milk SCC threshold
When setting bulk milk SCC goals, the fact that goats are seasonal breeders should be taken into account, and thresholds should be specific for each month or season of the year. Whereas cows calve more or less constantly throughout the year, parturition in a herd of goats usually occurs in a limited period of time. Still, there is some variation to when this period occurs. In the Netherlands, for example, most goats kid during February, but the actual peak may occur anywhere between December and April and some herds even have a peak in October. This makes it more difficult to compare SCC between herds, because if, for instance, the lowest 10% would be taken as the target for a particular month of the year, these 10% would probably consist part of herds that are closer to peak lactation than the average Dutch herd. Therefore, a farmer may decide to compare the SCC with the SCC that was measured in his own herd in the previous year. If the SCC results of the population are used as a benchmark, the farmer should be aware of the moment of kidding in the own herd relative to the average moment of kidding in the population. If, for instance, parturition occurs in fall for a substantial part of the herd, the bulk milk SCC is expected to be lower during winter, but higher during the summer months.
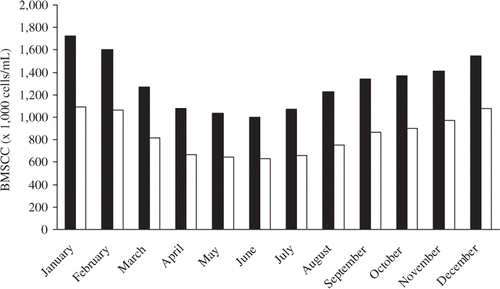
Another factor that will have an effect is the fraction of animals with an extended lactation. An extended lactation is a lactation of two or more consecutive years without kidding (Salama et al. Citation2005; Schuiling Citation2007). If many animals have an extended lactation, SCC is expected to be somewhat higher than average in summer, but lower than average around the moment kidding would normally happen. Based on the dataset described by Koop et al. (Citation2009), the effect of moment of kidding and extended lactation was explored. The effect of extended lactation turned out to be more pronounced than the effect of kidding moment. SCCs of herds with <10% extended lactations was 300–400 × 103 cells/mL higher in January/February, and 300–400 × 103 cells/mL lower than the other herds in September/October. With these influencing factors in mind, some goal setting can be done. Based on Dutch goat bulk milk SCC records of 2008, the median and lower 10th percentile were calculated for each month of the year. shows that half of all herds stay below 1700 × 103 cells/mL throughout the year and stay even below 1000 × 103 cells/mL during the summer months. These values can be used as benchmarks, but obviously have no biological meaning in the sense that they discriminate between good and bad udder health. Further study is needed to know what bulk milk SCC levels reflect a low prevalence of S. aureus in the herd.
3.2.2. Timing of bulk milk SCC measurement
The sensitivity of bulk milk SCC to detect changes in udder health will be highest during January–June, when SCC decreases gradually. If in this period, SCC stabilizes or even increases, something is going wrong. An increase in SCC in the second half of the year can be seen as a normal phenomenon, and it is difficult to assess whether the increase is steeper than normal. This is even more so, because of the increase in SCC due to the breeding in fall. Therefore, monitoring of bulk milk SCC should be confined to the first 6 months after the start of the kidding season, which in the Dutch example coincides approximately with the first 6 months of the year.
4. Bacteriological culture
4.1. BC at goat level
In many diagnostic studies, authors used bacterial culture of a milk sample to determine the true infection status of the udder gland. Various criteria have been used to differentiate infected from uninfected udder halves, usually something like the growth of 1 or more or 5 or more identical colonies from a 10 µL inoculum (Contreras et al. Citation1996). However, the validity of bacterial culture as a diagnostic test can be questioned. Sánchez et al. (Citation2004) described an effect of sampling time (pre- or postmilking) on the probability of positive BC, and he also described the effect of storage of goat milk samples on the recovery of pathogens (Sánchez et al. Citation2003). Also in cows, some authors have pointed out that the use of routine BC methods does not always result in the detection of all pathogens present in the udder gland (Buelow et al. Citation1996; Sol et al. Citation2002). Therefore, BC seems to be a useful, but imperfect test to diagnose IMI (Erskine and Eberhart Citation1988; Sanford et al. Citation2006). Still, it is often seen as the best available method (Torres et al. Citation2009) and is therefore used as the reference test in many evaluations of other tests, such as SCC, and is also frequently used in practice. Latent class analysis was used to estimate the sensitivity of BC. For the detection of S. aureus-infected goats, sensitivity was low (Koop et al. Citation2011), which strongly reduces its value as a confirmation test of animals with high SCC suspect of S. aureus infection, because many false negative results will be obtained. The value of BC lies primarily in the fact that it can give an indication of which pathogens are present in a herd. As discussed under Section 2, in goat herds, most intramammary pathogens are Staphylococci, but other organisms may also sometimes cause problems and may be detected easier with culture. Knowledge of which organisms are responsible for udder health problems in a herd, should guide interventions, based on transmission characteristics of the pathogen, for example.
4.1.1. BC as the reference test for IMI
Recently, attempts to give a definition of IMI have been published, which were all based upon various combinations of several BC results over a certain period of time and were to be used as a “pseudo-gold standard” in mastitis research. Using conjoint analysis, Andersen et al. (Citation2010) evaluated expert opinions on the definition of IMI and came with two criteria for a positive diagnosis: (1) the organism of interest is isolated on the test day with at least 10 colonies (1000 cfu/mL), or (2) the organism of interest was isolated at least twice in 3 weekly samplings. This definition was used by Dohoo et al. (Citation2011) to estimate the test characteristics of culture of a single milk sample. Three weekly milk samples were tested with bacteriology and the status of the middle sample was tested against the gold standard status, based on the three samples together. These single milk samples were categorized according to the number of colonies cultured per 0.01 mL (≥1, ≥2 or ≥10), if the organism was grown in pure culture or not, and if the accompanying SCC was higher than 200 × 103 cells/mL or not. These characteristics resulted in 12 possible definitions of IMI. With all 12 definitions, the specificity of a single sample for the detection of S. aureus was found to be higher than 0.99, but sensitivity decreased from 0.90 to 0.44 upon inclusion of stricter criteria. For comparison of different definitions of IMI based on BC, the papers of Andersen et al. (Citation2010) and Dohoo et al. (Citation2011) are very useful and the use of culture of multiple repeated milk samples is probably the closest to a gold standard one can get. However, to determine the true sensitivity and specificity of BC per se, tests other than BC are needed. Polymerase chain reaction (PCR) for the detection of mastitis pathogens may be such a test, and is a promising tool in mastitis research. Several authors have shown that real-time PCR often gives many more positive samples than BC (Taponen et al. Citation2009; Koskinen et al. Citation2010; Bexiga et al. Citation2011). Although the analytical sensitivity and specificity of this PCR was shown to be good (Koskinen et al. Citation2009), no information is available about the diagnostic sensitivity and specificity. The benefit of PCR over bacterial culture lies mainly in the fact that it can detect a lower concentration of bacteria in a sample, and it also detects bacteria that would not easily grow on conventional culture media, for instance, because of the fact that bacteria may reside in the defense cells. Still, if bacteria are present at too low concentrations or are even completely absent from a sample, e.g., because of intermittent shedding, the test will give a false negative result. Real-time PCR is thus also no true gold standard test, but will probably have a higher sensitivity than BC for certain bacterial species. Further validation of the use of PCR for the diagnosis of IMI in goats is therefore needed.
4.2. BC at herd level
BC of bulk milk can be used as a tool to monitor the subclinical mastitis situation in a herd and gives information on which pathogens are present in the herd. The bacterial flora of bulk milk can originate from (at least) three different sources: (1) bacteria residing in or on the mammary gland of the goat contribute to the bulk milk bacterial flora, (2) bacteria can enter the milk system from the environment (the milking barn) during milking, and (3) bacteria may grow in the interior of the milking machine because of insufficient cleaning of the system, and pieces of this bacterial plaque may enter the bulk milk during milking. BC of bulk milk will thus detect bacteria that originate from any of these three sources. We showed that CNS make an important contribution to the total bacterial count and that the number of Staphylococci is related to the bulk milk SCC (Koop et al. Citation2010a). In dairy cattle, the presence of (especially contagious) mastitis pathogens was related to bulk milk SCC (Olde Riekerink et al. Citation2006; Rysanek et al. Citation2009), but this was not found for S. aureus in goat milk (Koop et al. Citation2010a). Still, the detection of mastitis pathogens may be a useful tool to identify and monitor udder health problems in a herd of goats. Isolation of contagious mastitis pathogens from bulk milk indicates the presence of one or more infected udder glands in a herd of cows (Olde Riekerink et al. Citation2010). In goats, the culture procedure should primarily focus on the detection of S. aureus. Bulk milk measurements in goat herds have a different interpretation than in cow milk, because the relative contribution of one infected goat to the bulk milk is smaller than the contribution of one infected cow. Therefore, the sensitivity of bulk milk culture for the detection of S. aureus carriers is probably lower in goats than in cows. The sensitivity is even further reduced because of the generally higher total bacterial counts in goat milk. The abundance of other bacteria makes it more difficult to discern specific bacterial species. Research is needed to estimate the sensitivity of BC of bulk milk to detect S. aureus-infected goats. It is important to know the detection limit and the relationship between the number of infected animals and the bulk milk S. aureus count. This requires culture or SCC measurement of a large number of goats in each herd, and culture of bulk milk samples of these herds.
5. Conclusions
Udder health monitoring in goats should primarily aim at identifying goats infected with S. aureus. SCC and BC are valuable diagnostic tools, but have several limitations. For SCC, the effects of non-infectious factors complicate the interpretations of test results. The main drawback of BC is its low sensitivity for the diagnosis of S. aureus. Knowledge of the epidemiological background is necessary for a correct application and interpretation of these tests.
References
- Ameh , JA , Addo , PB , Adekeye , JO , Gyang , EO , Teddek , LB and Abubakar , Y . 1994 . Gangrenous caprine coliform mastitis . Small Rumin Res , 13 : 307 – 309 .
- Andersen , S , Dohoo , IR , Olde Riekerink , R and Stryhn , H . 2010 . Diagnosing intramammary infections: evaluating expert opinions on the definition of intramammary infection using conjoint analysis . J Dairy Sci , 93 : 2966 – 2975 .
- Bergonier , D , Berthelot , X and Poumarat , F . 1997 . Contagious agalactia of small ruminants: current knowledge concerning epidemiology, diagnosis and control . OIE Rev Sci Technol , 16 : 848 – 873 .
- Bergonier , D , de Cremoux , R , Rupp , R , Lagriffoul , G and Berthelot , X . 2003 . Mastitis of dairy small ruminants . Vet Res , 34 : 689 – 716 .
- Bexiga , R , Koskinen , MT , Holopainen , J , Carneiro , C , Pereira , H , Ellis , KA and Vilela , CL . 2011 . Diagnosis of intramammary infection in samples yielding negative results or minor pathogens in conventional bacterial culturing . J Dairy Res , 78 : 49 – 55 .
- Boscos , C , Stefanakis , A , Alexopoulos , C and Samartzi , F . 1996 . Prevalence of subclinical mastitis and influence of breed, parity, stage of lactation and mammary bacteriological status on Coulter Counter Counts and California Mastitis Test in the milk of Saanen and autochthonous Greek goats . Small Rumin Res , 21 : 139 – 147 .
- Buelow , KL , Thomas , CB , Goodger , WJ , Nordlund , KV and Collins , MT . 1996 . Effect of milk sample collection strategy on the sensitivity and specificity of bacteriologic culture and somatic cell count for detection of Staphylococcus aureus intramammary infection in dairy cattle . Prev Vet Med , 26 : 1 – 8 .
- Castro-Alonso , A , Rodríguez , F , De la Fé , C , Espinosa de los Monteros , A , Poveda , JB , Andrada , M and Herráez , P . 2009 . Correlating the immune response with the clinical-pathological course of persistent mastitis experimentally induced by Mycoplasma agalactiae in dairy goats . Res Vet Sci , 86 : 274 – 280 .
- Contreras , A , Corrales , JC , Sánchez , A and Sierra , D . 1997 . Persistence of subclinical intramammary pathogens in goats throughout lactation . J Dairy Sci , 80 : 2815 – 2819 .
- Contreras , A , Luengo , C , Sánchez , A and Corrales , JC . 2003 . The role of intramammary pathogens in dairy goats . Livest Prod Sci , 79 : 273 – 283 .
- Contreras , A , Sierra , D , Corrales , JC , Sánchez , A and Marco , J . 1996 . Physiological threshold of somatic cell count and California Mastitis Test for diagnosis of caprine subclinical mastitis . Small Rumin Res , 21 : 259 – 264 .
- Contreras , A , Sierra , D , Sánchez , A , Corrales , JC , Marco , JC , Paape , MJ and Gonzalo , C . 2007 . Mastitis in small ruminants . Small Rumin Res , 68 : 145 – 153 .
- Corrales , JC , Sanchez , A , Luengo , C , Poveda , JB and Contreras , A . 2004 . Effect of clinical contagious agalactia on the bulk tank milk somatic cell count in Murciano-Granadina goat herds . J Dairy Sci , 87 : 3165 – 3171 .
- Deinhofer , M and Pernthaner , A . 1995 . Staphylococcus spp. as mastitis-related pathogens in goat milk . Vet Microbiol , 43 : 161 – 166 .
- Dohoo , IR , Smith , J , Andersen , S , Kelton , DF and Godden , S . 2011 . Diagnosing intramammary infections: evaluation of definitions based on a single milk sample . J Dairy Sci , 94 : 250 – 261 .
- Dulin , AM , Paape , MJ , Schultze , WD and Weinland , BT . 1983 . Effect of parity, stage of lactation, and intramammary infection on concentration of somatic cells and cytoplasmic particles in goat milk . J Dairy Sci , 66 : 2426 – 2433 .
- Erskine , RJ and Eberhart , RJ . 1988 . Comparison of duplicate and single quarter milk samples for the identification of intramammary infections . J Dairy Sci , 71 : 854 – 856 .
- Gomes , V , Melville Paiva Della Libera , AM , Paiva , M , Madureira , KM and Araujo , WP . 2006 . Effect of the stage of lactation on somatic cell counts in healthy goats (Caprae hircus) breed in Brazil . Small Rumin Res , 64 : 30 – 34 .
- Haenlein , GFW . 2002 . Relationship of somatic cell counts in goat milk to mastitis and productivity . Small Rumin Res , 45 : 163 – 178 .
- Hall , SM and Rycroft , AN . 2007 . Causative organisms and somatic cell counts in subclinical intramammary infections in milking goats in the UK . Vet Rec , 160 : 19 – 22 .
- Hogan , SJ , González , RN , Harmon , RJ , Nickerson , SC , Oliver , SG , Pankey , JW and Smith , KL . 1999 . Laboratory handbook on bovine mastitis , Revised , Verona, WI, USA : National Mastitis Council .
- Hunter , AC . 1984 . Microflora and somatic cell content of goat milk . Vet Rec , 114 : 318 – 320 .
- Kalogridou-Vassiliadou , D . 1991 . Mastitis-related pathogens in goat milk . Small Rumin Res , 4 : 203 – 212 .
- Koop , G , Dik , N , Nielen , M and Lipman , LJA . 2010a . Short communication: repeatability of differential goat bulk milk culture and associations with somatic cell count, total bacterial count, and standard plate count . J Dairy Sci , 93 : 2569 – 2573 .
- Koop , G , Nielen , M and van Werven , T . 2009 . Bulk milk somatic cell counts are related to bulk milk total bacterial counts and several herd-level risk factors in dairy goats . J Dairy Sci , 92 : 4355 – 4364 .
- Koop , G , van Werven , T , Schuiling , HJ and Nielen , M . 2010b . The effect of subclinical mastitis on milk yield in dairy goats . J Dairy Sci , 93 : 5809 – 5817 .
- Koop , G , van Werven , T , Toft , N and Nielen , M . 2011 . Estimating test characteristics of somatic cell count to detect Staphylococcus aureus-infected dairy goats using latent class analysis . J Dairy Sci , 94 : 2902 – 2911 .
- Koskinen , MT , Holopainen , J , Pyörälä , S , Bredbacka , P , Pitkälä , A , Barkema , HW , Bexiga , R , Roberson , J , Sølverød , L Piccinini , R . 2009 . Analytical specificity and sensitivity of a real-time polymerase chain reaction assay for identification of bovine mastitis pathogens . J Dairy Sci , 92 : 952 – 959 .
- Koskinen , MT , Wellenberg , GJ , Sampimon , OC , Holopainen , J , Rothkamp , A , Salmikivi , L , van Haeringen , WA , Lam , TJGM and Pyörälä , S . 2010 . Field comparison of real-time polymerase chain reaction and bacterial culture for identification of bovine mastitis bacteria . J Dairy Sci , 93 : 5707 – 5715 .
- Leitner , G , Merin , U , Lavi , Y , Egber , A and Silanikove , N . 2007 . Aetiology of intramammary infection and its effect on milk composition in goat flocks . J Dairy Res , 74 : 186 – 193 .
- Lerondelle , C , Richard , Y and Issartial , J . 1992 . Factors affecting somatic cell counts in goat milk . Small Rumin Res , 8 : 129 – 139 .
- Luengo , C , Sánchez , A , Corrales , JC , Fernández , C and Contreras , A . 2004 . Influence of intramammary infection and non-infection factors on somatic cell counts in dairy goats . J Dairy Res , 71 : 169 – 174 .
- Maisi , P and Riipinen , I . 1991 . Pathogenicity of different species of staphylococci in caprine udder . Br Vet J , 147 : 126 – 132 .
- Manlongat , N , Yang , TJ , Hinckley , LS , Bendel , RB and Krider , HM . 1998 . Physiologic-chemoattractant-induced migration of polymorphonuclear leukocytes in milk . Clin Diagn Lab Immunol , 5 : 375 – 381 .
- McDougall , S and Voermans , M . 2002 . Influence of estrus on somatic cell count in dairy goats . J Dairy Sci , 85 : 378 – 383 .
- Merin , U , Silanikove , N , Shapiro , F , Bernstein , S and Leitner , G . 2004 . Changes in milk composition as affected by subclinical mastitis in sheep and goats . S Afr J Anim Sci , 34 : 188 – 191 .
- Min , BR , Tomita , G and Hart , SP . 2007 . Effect of subclinical intramammary infection on somatic cell counts and chemical composition of goats' milk . J Dairy Res , 74 : 204 – 210 .
- Moroni , P , Pisoni , G , Antonini , M , Ruffo , G , Carli , S , Varisco , G and Boettcher , P . 2005 . Subclinical mastitis and antimicrobial susceptibility of Staphylococcus caprae and Staphylococcus epidermidis isolated from two Italian goat herds . J Dairy Sci , 88 : 1694 – 1704 .
- Moroni , P , Pisoni , G , Savoini , G , van Lier , E , Acuna , S , Damian , JP and Meikle , A . 2007 . Influence of estrus of dairy goats on somatic cell count, milk traits, and sex steroid receptors in the mammary gland . J Dairy Sci , 90 : 790 – 797 .
- Olde Riekerink , RG , Barkema , HW , Scholl , DT , Poole , DE and Kelton , DF . 2010 . Management practices associated with the bulk-milk prevalence of Staphylococcus aureus in Canadian dairy farms . Prev Vet Med , 97 : 20 – 28 .
- Olde Riekerink , RG , Barkema , HW , Veenstra , S , Poole , DE , Dingwell , RT and Keefe , GP . 2006 . Prevalence of contagious mastitis pathogens in bulk tank milk in Prince Edward Island . Can Vet J , 47 : 567 – 572 .
- Olechnowicz , J and Sobek , Z . 2008 . Factors of variation influencing production level, SCC and basic milk composition in dairy goats . J Anim Feed Sci , 17 : 41 – 49 .
- Paape , MJ and Capuco , AV . 1997 . Cellular defense mechanisms in the udder and lactation of goats . J Anim Sci , 75 : 556 – 565 .
- Paape , MJ , Poutrel , B , Contreras , A , Marco , JC and Capuco , AV . 2001 . Milk somatic cells and lactation in small ruminants . J Dairy Sci , 84 : E237 – E244 .
- Paape , MJ , Wiggans , GR , Bannerman , DD , Thomas , DL , Sanders , AH , Contreras , A , Moroni , P and Miller , RH . 2007 . Monitoring goat and sheep milk somatic cell counts . Small Rumin Res , 68 : 114 – 125 .
- Park , YW . 1991 . Interrelationships between somatic cell counts, electrical conductivity, bacteria counts, percent fat and protein in goat milk . Small Rumin Res , 5 : 367 – 375 .
- Perrin , GG and Baudry , C . 1993 . Somatic cell counts in goats milk . Le Lait , 73 : 489 – 497 .
- Pirisi , A , Lauret , A and Dubeuf , JP . 2007 . Basic and incentive payments for goat and sheep milk in relation to quality . Small Rumin Res , 68 : 167 – 178 .
- Rota , AM , Gonzalo , C , Rodriguez , PL , Rojas , AI , Martin , L and Tovar , JJ . 1993 . Somatic cell types in goats milk in relation to total cell count, stage and number of lactation . Small Rumin Res , 12 : 89 – 98 .
- Ruegg , PL . 2009 . The quest for the perfect test: phenotypic versus genotypic identification of coagulase-negative staphylococci associated with bovine mastitis . Vet Microbiol , 134 : 15 – 19 .
- Rysanek , D , Zouharova , M and Babak , V . 2009 . Monitoring major mastitis pathogens at the population level based on examination of bulk tank milk samples . J Dairy Res , 76 : 117 – 123 .
- Salama , AAK , Caja , G , Such , X , Casals , R and Albanell , E . 2005 . Effect of pregnancy and extended lactation on milk production in dairy goats milked once daily . J Dairy Sci , 88 : 3894 – 3904 .
- Sánchez , A , Contreras , A , Corrales , JC and Marco , JC . 2001 . Relationships between infection with caprine arthritis encephalitis virus, intramammary bacterial infection and somatic cell counts in dairy goats . Vet Rec , 148 : 711 – 714 .
- Sánchez , A , Contreras , A , Corrales , JC and Munoz , P . 2004 . Influence of sampling time on bacteriological diagnosis of goat intramammary infection . Vet Mic , 98 : 329 – 332 .
- Sánchez , A , Contreras , A , Jimenez , J , Luengo , C , Corrales , JC and Fernández , C . 2003 . Effect of freezing goat milk samples on recovery of intramammary bacterial pathogens . Vet Mic , 94 : 71 – 77 .
- Sanford , CJ , Keefe , GP , Sanchez , J , Dingwell , RT , Barkema , HW , Leslie , KE and Dohoo , IR . 2006 . Test characteristics from latent-class models of the California Mastitis Test . Prev Vet Med , 77 : 96 – 108 .
- Schaeren , W and Maurer , J . 2006 . Haufigkeiten subklinischer euterinfektionen und individuelle zellzahlen in drei ziegenherden im verlaufe einer gesamten laktation . Schweiz Arch Tierheilkd , 148 : 641 – 648 .
- Schuiling , HJ . 2007 . Prolonged lactations in goats. Report 97 , Wageningen UR : Animal Sciences Group .
- Sheldrake , RF , Hoare , RJ and Woodhouse , VE . 1981 . Relationship of somatic cell count and cell volume analysis of goat's milk to intramammary infection with coagulase-negative staphylococci . J Dairy Res , 48 : 393 – 403 .
- Smith , MC and Sherman , DM . 2009 . Mammary gland and milk productionGoat medicine , 2nd , Vol. Chap. 14 , 647 – 689 . Ames, IA, USA : Wiley-Blackwell .
- Sol , J , Sampimon , OC , Hartman , E and Barkema , HW . 2002 . Effect of preculture freezing and incubation on bacteriological isolation from subclinical mastitis samples . Vet Microbiol , 85 : 241 – 249 .
- Supré , K , Haesebrouck , F , Zadoks , RN , Vaneechoutte , M , Piepers , S and De Vliegher , S . 2011 . Some coagulase-negative Staphylococcus species affect udder health more than others . J Dairy Sci , 94 : 2329 – 2340 .
- Talafha , AQ , Lafi , SQ and Ababneh , MM . 2008 . The effect of estrus synchronization treatment on somatic cell count of transitional-anestrus local-Damascus cross breed goats' milk . Trop Anim Health Prod , 40 : 185 – 192 .
- Taponen , S , Salmikivi , L , Simojoki , H , Koskinen , MT and Pyörälä , S . 2009 . Real-time polymerase chain reaction-based identification of bacteria in milk samples from bovine clinical mastitis with no growth in conventional culturing . J Dairy Sci , 92 : 2610 – 2617 .
- Torres , AH , Rajala-Schultz , PJ and DeGraves , FJ . 2009 . Diagnosis of intramammary infections at dry-off based on sampling strategy, epidemiology of pathogens, and agreement beyond chance . J Vet Diag Inv , 21 : 427 – 436 .
- Turin , L , Pisoni , G , Giannino , ML , Antonini , M , Rosati , S , Ruffo , G and Moroni , P . 2005 . Correlation between milk parameters in CAEV seropositive and negative primiparous goats during an eradication program in Italian farm . Small Rumin Res , 57 : 73 – 79 .
- Valle , J , Piriz , S , de la Fuente , R and Vadillo , S . 1991 . Staphylococci isolated from healthy goats . Zentralbl Veterinarmed B , 38 : 81 – 89 .
- White , EC and Hinckley , LS . 1999 . Prevalence of mastitis pathogens in goat milk . Small Rumin Res , 33 : 117 – 121 .
- Wilson , DJ , Stewart , KN and Sears , PM . 1995 . Effects of stage of lactation, production, parity and season on somatic cell counts in infected and uninfected dairy goats . Small Rumin Res , 16 : 165 – 169 .
- Zadoks , RN and Watts , JL . 2009 . Species identification of coagulase-negative staphylococci: genotyping is superior to phenotyping . Vet Microbiol , 134 : 20 – 28 .