Abstract
Immune deficiency and altered immunity are among the best characterized and strongest known risk factors of non-Hodgkin lymphomas (NHL). For instance, chronic inflammation or certain disturbances in the immune system are associated with an increased lymphoma risk. Occupational and environmental factors (i.e., dioxin) as well as lifestyle factors (i.e., obesity) may contribute to these risk factors. The precise role of these factors in the etiology of NHL, however, is still not entirely clear. Although the existing epidemiologic studies have not revealed consistent patterns of perturbations of the immune system by these factors, the findings might suggest an adverse impact on both the humoral and cell-mediated immune system.
1. Introduction
Non-Hodgkin lymphomas (NHL), solid tumors of lymphocyte origin, are the most common hematopoietic cancers in both men and women in the developed world (Jaffe et al. Citation2001; Muller et al. Citation2005). The etiology of most NHL cases remains unclear, but the strongest and most consistent risk factors are related to altered immunity conditions. The overall aim of this review article is to describe the possible perturbations of the immune system preceding lymphomagenesis, resulting from known occupational and environmental risk factors of NHL. We will focus on dioxin exposure and obesity.
2. Lymphomagenesis: the role of the immune system
Severe immunodeficiency, including both hereditary immunodeficiency disorders and acquired conditions such as those observed in patients infected with the human immunodeficiency virus (HIV) and in transplant patients receiving immunosuppressive drugs, is a well-described and strong risk factor for NHL. Moreover, individuals with auto-immune conditions, such as rheumatoid arthritis, systemic lupus erythematosis, Sjogren's syndrome, Coeliac disease, and psoriasis, are at a higher risk of developing NHL (Hoover Citation1992; Mariette Citation2001; Engels et al. Citation2005; Grulich et al. Citation2007; Tio et al. Citation2012). Additional evidence of the integral role of the immune system in lymphomagenesis can be seen from epidemiologic studies that examined polymorphisms in genes coding for cytokines that modulate the inflammatory process or are linked to B cell activation (Wang et al. Citation2007; Ambinder et al. Citation2010; Skibola et al. Citation2010). In addition, recent genome-wide association studies (GWAS) of the follicular subtype of NHL identified association with two variants within the human leukocyte antigen (HLA) region (Skibola et al. Citation2009; Smedby et al. Citation2011).
Given the central role of the immune system in lymphomagenesis, it is hypothesized that moderate perturbations of the immune system may be a risk factor as well (Grulich et al. Citation2007).
3. Methodological issues
3.1. Complementary study designs: the ‘‘meet in the middle’’ concept
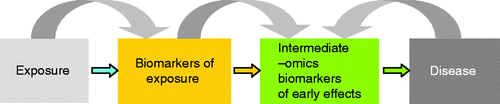
The US National Institute for Environmental Health Sciences (NIEHS) has suggested to integrate environmental exposures within the study of the natural history of disease. Indeed, the study of how an environmental agent affects molecular targets, cellular function, tissue function, and survival ultimately informs us about the etiology, pathogenesis, and distribution of the disease (Vineis and Perera Citation2007). The recently introduced “meet in the middle approach” has the potential to open new avenues for prevention by identifying the specific environmental factors involved in the disease process (Vineis and Perera Citation2007). In this approach, the relationship between putative intermediate markers and disease outcome is explored. In parallel, associations between exposure estimates and intermediate markers are assessed as well. Subsequently, the overlap between markers of exposure and predictive markers of disease outcome would classify relevant intermediate markers of environmental-driven diseases (). An example of this approach was recently published by Chadeau-Hyam et al. (Citation2011). They identified a dietary colon cancer biomarker (a derivative of benzoic acid produced by fiber-digesting gut bacteria) by correlating a prospectively measured metabolic profile with both dietary fiber intake and reduced colon cancer risk.
Prospective studies which study the disease process from exposure to preclinical response and subsequently to a clinically diagnosed disease are conceptually suitable for the identification of new biomarkers of both exposure and early biological effect, since they are based on pre-clinical biological samples that are not influenced, if the disease occurs years later, by the inherent metabolic changes due to the disease itself. However, one prospective sample might not reflect both the exposure and outcome of interest in an optimal way. Moreover, exposure measurements cannot be optimized in prospective studies due to some inherent limitations including (1) if the exposure does not occur in isolation from other hazardous exposures; (2) if a sufficient induction period since exposure has not yet passed; or (3) if the observed biomarker does not reflect the exposure of interest due to the short half-life of the marker. Therefore, use of other study designs with optimally designed exposure measurements (i.e., cross-sectional or semi-longitudinal molecular epidemiological studies) combined with prospective studies has been recommended (Vlaanderen et al. Citation2010).
3.2. Use of biomarkers in cross-sectional molecular epidemiological studies
Cross-sectional studies can be used to evaluate intermediate biologic effects from a wide range of exposures in the diet, environment and lifestyle factors. Results of these studies are interpreted based on the assumption that the intermediate endpoints reflect biologic changes considered relevant to a particular disease development (Marchand Citation2005). However, these studies are not capable of directly establishing or refuting a causal relation between an exposure or a level of exposure and risk for developing a disease. Cross-sectional studies could, however, provide mechanistic insight into well-established exposure–disease relations and supplement suggestive but inconclusive evidence of the carcinogenicity of an exposure. For intermediate endpoints with etiologic fractions (proportion of cases that the marker or the pathway that is reflected by the marker had played a causal role in its development) that are close to 1.0, either positive or negative results are particularly informative, while for intermediate endpoints linked to the risk of developing cancer but with a substantially lower etiologic fraction, the interpretation is more circumspect. Specifically, a positive association between an exposure and an intermediate biomarker is informative, but a null association does not rule out that the exposure is carcinogenic as the exposure may act through a mechanism not reflected by the particular endpoint under study (Garcia_Closas et al. Citation2006).
Given the limitations in both the cross-sectional and prospective “meet in the middle” study designs, it seems that more than one study design is warranted for biomarker discovery in environmentally driven diseases. As a consequence, combination of these two different approaches to study the possible perturbations of the immune system by occupational and environmental risk factors of NHL seems appropriate and can yield new relevant information.
4. Predictive blood markers of lymphomas
Current advances in the understanding of the pathogenesis of hematopoietic malignancies and the introduction of high-throughput technologies facilitate translational research toward the discovery, development, and clinical validation of novel biomarkers for the early detection as well as for disease progression and recurrence (Lotze and Rees Citation2004). As early detection of hematopoietic malignancies has been shown to improve the survival rates, there is an obvious benefit from early identification of at-risk individuals for disease development (Swerdlow Citation2003). Early studies that examined pre-diagnosis materials for lymphoma biomarkers are those among HIV patients who due to severe immunosuppression were found at high risk of lymphoma development.
4.1. Lymphoma blood biomarkers among HIV patients
The CD4+ lymphocyte count was among the first biomarkers that have been studied in relation to AIDS-NHL. Early studies suggested that nadir (lowest ever) CD4+ lymphocyte count was an important predictor of acquired immunodeficiency syndrome related to non-Hodgkin lymphoma (AIDS-NHL) (Ambinder et al. Citation2010). Two recent studies reported that the most recent CD4+ lymphocyte count, rather than the nadir lymphocyte count, is predictive of AIDS-NHL (Bower et al. Citation2009; Zoufaly et al. Citation2009). Interestingly, it has been shown that HIV viremia was related to NHL risk independently of CD4+ lymphocyte count (Zoufaly et al. Citation2009; Engels et al. Citation2010). HIV virions that carry the CD40 ligand, an activator of induced cytidine deaminase (AID), might stimulate polyclonal B cell activation and generate the molecular lesions that contribute to the development of NHL (Ambinder et al Citation2010).
Early studies among HIV patients have shown that serum levels of some cytokines and chemokines, including interleukin (IL) 6 (Pluda et al. Citation1993; Breen et al. Citation1999), IL10 (Breen et al. Citation2003), and C-X-C motif chemokine 13 (CXCL13)(Widney et al. Citation2005), are related to the occurrence of lymphoma among HIV patients. Recent studies not only confirm these previous findings of IL6 (Epeldegui et al. Citation2007; Breen et al. Citation2011; Rabkin et al. Citation2011), IL10 (Breen et al. Citation2011), and CXCL13 (Hussain et al. Citation2010), but also reported associations for other cytokines. Rabkin et al. (Citation2011) reported significant associations between elevated levels of several cytokines, including IL1α, IL4, IL5, IL6, IL8, IL12p70, IL13, granulocyte-macrophage colony-stimulating factor (GMCSF), transforming growth factor alpha (TGF-α), vascular endothelial growth factor (VEGF), and interferon-induced protein 10 (IP10) measured in pre-diagnostic blood samples of HIV patients and the risk of developing NHL. They concluded that cytokine-mediated hyperstimulation of B cell proliferation (T helper 2 cytokines) may play a role in AIDS-NHL.
Moreover, in several studies elevated levels of soluble CD23 (a B cell activation molecule) (Yawetz et al. Citation1995; Schroeder, Saah, Ambinder, et al. Citation1999; Schroeder, Saah, Hoover, et al. Citation1999; Epeldegui et al. Citation2007; Breen et al. Citation2011), sCD27 (Widney et al. Citation1999; Epeldegui et al. Citation2007; Breen et al. Citation2011), sCD30 (Breen et al. Citation2006, Citation2011; Epeldegui et al. Citation2007), and sCD44 (Breen et al. Citation2005; Epeldegui et al. Citation2007) were reported in prospective blood samples of AIDS-NHL patients as compared with HIV+ control and/or with AIDS control patients. Soluble CD30 level is indicative of a type 2 T cell and/or cytokine response, which would be expected to contribute to chronic B cell stimulation. Both sCD27 and sCD30, as a biomarker of immune activation, are members of the TNF receptor family, while sCD44 is a cell-surface adhesion molecule involved in inflammatory cell function as well as tumor cell growth and metastasis (Breen et al. Citation2005).
Abnormal blood levels of some immunoglobulins (Ig) due to B cell dysfunction also have been reported in some studies. These studies showed an association with either high levels of serum globulin (Grulich et al. Citation2000), IgE (Yawetz et al. Citation1995) and free light chains of kappa (κ) and lambda (λ) or a decreased level of IgG (Breen et al. Citation2006) with increased risk of AIDS-NHL, while other studies did not show changes in IgE (Breen et al. Citation2011), IgG, IgM, and IgA levels prior to the development of AIDS-NHL (Landgren et al. Citation2010).
Overall, the findings of studies on HIV patients support the idea that chronic B cell activation is preceding the AIDS-NHL occurrence. It has been shown that the B-cell activation is associated with DNA-modifying processes, including Ig class switch recombination (CSR) in Ig heavy chain genes (IgH) and somatic hypermutation (SHM) (Vendrame and Martinez-Maza Citation2011). Moreover, higher levels of cytidine deaminase, a DNA-mutating enzyme that plays a role in both IgH CSR and SHM, have been found to be related to AIDS-NHL development (Epeldegui et al. Citation2007). Therefore, it can be concluded that chronic-sustained B-cell stimulation leads to the accumulation of genetic changes implicated in B-cell lymphomagenesis (Breen et al. Citation2006).
4.2. Lymphoma blood biomarkers among the general population
Recently, the observations on the importance of chronic-sustained B-cell activation in AIDS-NHL led to subsequent studies investigating the possible role of B-cell activation pathways in lymphoma risk in the general population.
In a nested case-control study within the Italian European Prospective Investigation into Cancer and Nutrition cohort (EPIC-Italy) (Saberi Hosnijeh, Krop, Scoccianti, et al. Citation2010), a significant association was observed between NHL risk and lower plasma levels (collected on average 4.5 years prior to NHL diagnosis) of IL2 and tumor necrosis factor alpha (TNF-α) and higher levels of inter-cellular adhesion molecule (ICAM), which remained significant after excluding cases diagnosed within 2 years of follow-up. In another nested case-control study within the New York University – Women's Health Study cohort, 15 cytokines measured in blood samples collected a median 8.2 years prior to the NHL diagnosis were investigated. The results showed that increased serum levels of IL13 were associated with decreased NHL risk while elevated levels of soluble IL2 receptor (sIL2R) were associated with increased risk of NHL (Gu et al. Citation2010). They also reported a marginally positive association between TNF-α and sTNF-R2 and B-NHL as well as a negative association for IL5.
A recent case-control study, nested within the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial with a median length of 5 years follow-up from blood collection to case diagnosis, conducted by Purdue et al. (Citation2011) showed that elevated sTNF-R1 and sCD27 levels were associated with higher risk of developing NHL. These associations remained significant in analyses of cases diagnosed longer than 6 years following blood collection. They also reported that elevated levels of IL10, TNF-α, and sTNF-R2 were significantly associated with increased risk of NHL overall, but these associations weakened with increasing time from blood collection to case diagnosis and were null for cases diagnosed longer than 6 years post collection. Both sTNF-R1 and sTNF-R2 bind with TNF-α and may play a regulatory role by limiting its circulating levels in the blood. Moreover, an elevated level of sCD27, a member of the TNF receptor superfamily, involved in activation of both T-cells and B-cells, has been observed in relation with many infectious and autoimmune diseases and has been proposed as a marker of immune activation. These findings support the concept that sub-clinical inflammation and chronic B cell stimulation might play a role in lymphomagenesis in the general population.
Purdue et al. (Citation2009) also reported on the association between elevated circulating sCD30 levels in pre-diagnostic serum of healthy subjects and NHL risk. A recent replication study within the EPIC-Italy cohort (Vermeulen et al. Citation2011) provided support for an elevated risk of lymphoma with increasing plasma sCD30 levels. Soluble CD30 is cleaved from the cell surface of activated B- and T-cells during the process of immune activation and can be detected at low levels in normal serum. Most B-cell NHL tumors do not express CD30, and therefore sCD30 levels may serve as a surrogate for the activation status of the immune system, rather than reflecting tumor burden. As sCD30 is expressed by activated B-cells and the type 2 subset of T-cells that secrete B-cell stimulatory cytokines, increase in serum sCD30 might indicate increased B-cell activation as well as an immune environment conducive to chronic B-cell stimulation.
Although these studies have shown that dysregulation of cytokine production may precede the development of NHL in immunocompetent people, a clear pattern has not yet been established. Additional evidence from large prospective studies is therefore needed to clarify the relationship with NHL risk for these markers and related cytokines. However, taken together, findings to date provide considerable evidence that B-cell activation, chronic inflammation, and/or a shift in the balance of Th1/Th2 cytokines are important phenomena in NHL development.
5. Effect of possible environmental/occupational lymphomagens on blood immune markers of lymphomas
It has been postulated that possible risk factors of lymphoma, such as environmental and occupational exposures, may operate through perturbations of the immune system. Here, the possible effects of two potential risk factors of lymphoma – occupational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and a lifestyle risk factor (obesity) on (validated) markers of the immune system – will be discussed. A possible association between TCDD exposure and NHL risk is supported by several studies among occupationally and environmentally exposed individuals (Fingerhut et al. Citation1991; Bertazzi et al. Citation1993; Kogevinas et al. Citation1997; Hooiveld et al. Citation1998; Boers et al. Citation2010). In addition, recent studies have suggested that modifiable lifestyle factors such as obesity (Wolk et al. Citation2001; Skibola Citation2007; Willett et al. Citation2008) may have contributed to the rising rates of NHL.
5.1. TCDD exposure
We assessed a broad range of immunologic parameters directed toward detecting changes in both the humoral and cellular arms of the immune system among Dutch workers occupationally exposed to high levels of TCDD approximately 35 years after last exposure (Saberi Hosnijeh et al. Citation2011; Saberi Hosnijeh, Boers, et al. Citation2012; Saberi Hosnijeh, Lenters, et al. Citation2012). The Dutch herbicide cohort consists of workers from two chemical factories in the Netherlands involved in the production and formulation of chlorophenoxy herbicides (Hooiveld et al. Citation1998). In factory A (workers employed between 1955 and 1985), one of the main products was 2,4,5-trichlorophenoxyacetic acid (2,4,5-T). Other pesticides manufactured in factory A were 2,4,5-trichlorophenol (2,4,5-TCP), lindane, dichlobenil, and tetradifon. Contamination with TCDD and other dioxins is possible during the production of 2,4,5-T and 2,4,5-TCP. In March 1963, an uncontrolled reaction occurred in an autoclave in factory A where 2,4,5-TCP was synthesized at the time. After the explosion, the contents of the autoclave were released into the factory hall, including dioxins such as TCDD. In factory B (workers employed between 1965 and 1986), the main products were 4-chloro-2-methylphenoxyacetic acid (MCPA), 4-chloro-2-methylphenoxy propanoic acid (MCPP), and 2,4-dichlorophenoxyacetic acid (2,4-D), which are unlikely to be contaminated with TCDD (Boers et al. Citation2010). Increased risks of all cancer mortality and NHL were reported previously by Hooiveld et al. (Citation1998) for factory A. A possible association between TCDD exposure and NHL risk was recently confirmed in a follow-up of this cohort (Boers et al. Citation2010). It was found that most lymphocyte subsets, in particular the B cell compartment, decreased in absolute counts with increasing TCDD exposure levels (Saberi Hosnijeh, Lenters, et al. Citation2012). Moreover, blood levels of most cytokines, chemokines, and growth factors had a negative association with TCDD levels with a formal statistical significance for fractalkine, transforming growth factor alpha (TGF-α), and fibroblast growth factor 2 (FGF2) (Saberi Hosnijeh, Boers, et al. Citation2012). These changes were independent from the described changes in blood cell counts. As such, TCDD exposure in humans seems to influence both function and the number of immune cells.
In addition, possible changes in immunoglobulins and complement factors in relation to plasma TCDD levels were reported. Plasma TCDD levels were not associated with markers of humoral immunity with the possible exception of a borderline significant decrease in complement factor 4 (C4) levels (Saberi Hosnijeh et al. Citation2011). Overall, these findings support that dioxin exposure could have an adverse impact on the immune system, in particular indicative of a suppression of the immune system. Moreover, these results provided support to the hypothesis that changes in cell-mediated immunity by TCDD may play a role in TCDD toxicity and associated health effects.
The studied participants were among the highest exposed occupational individuals, based on blood TCDD concentrations, and certainly higher than the levels that have been found in environmental settings (Saberi Hosnijeh et al. Citation2011; Saberi Hosnijeh, Boers, et al. Citation2012; Saberi Hosnijeh, Lenters, et al. Citation2012). The main findings of the relevant published studies that investigated immunological changes in relation to dioxin exposure are summarized in .
Table 1. Qualitative assessment of blood immune marker changes related to TCDD exposure.
5.1.1. Studies on industrial workers and/or exposed subjects of industrial accidents
A study on persons exposed to TCDD due to an industrial accident in England (17 years after exposure) reported no differences between exposed workers and controls in T- and B-cell lymphocyte counts and subsets, but the number of natural killer cells was significantly higher in exposed workers (Jennings et al. Citation1988). Another study among workers exposed to TCDD in Germany (more than 35 years before blood collection) showed a non-significant decrease of lymphocyte counts, B-, T-, and T-helper cells and a significant increase of IgA, IgG, and C4 for exposed workers compared to a reference group (Ott et al. Citation1994). Results of a study on TCDD-exposed subjects of Seveso, Italy, 20 years after the industrial accident, showed that plasma IgG levels of a random sample of the population in the most highly exposed zones decreased with increasing TCDD plasma concentration, but IgM, IgA, C3, and C4 plasma concentrations did not exhibit any consistent association with TCDD levels (Baccarelli et al. Citation2002). Nagayama et al. (Citation2001) reported no significant changes on serum levels of IgG, IgA, and IgM and lymphocyte subsets in exposed subjects of the Yusho industrial accident.
Neubert et al. (Citation1993) reported no significant change in immunological cell subsets and multiple monoclonal antibody markers among workers exposed to polychlorinated dibenzo-p-dioxins (PCDD)/polychlorinated dibenzofurans (PCDFs) in a German study on workers involved in the decontamination of a chemical plant, who had moderately increased dioxin body burdens. However, in a follow-up study, they reported a significant decrease in plasma concentrations of IgG1 in exposed workers (Neubert et al. Citation2000). No differences were reported for other immunoglobulins (IgM, IgA, IgD, IgG2, IgG3, and IgG4) and cytokines of IL1α, IL1ß, IL6, and TNF-α. No significant differences could be detected between 11 exposed workers and 10 age-matched, healthy controls for lymphocyte subsets in another study among German industrial workers exposed to high levels of TCDD 20 years before blood collection (Tonn et al. Citation1996). Halperin et al. (Citation1998) reported a decrease in CD26+ cells (activated T-cells) among subjects occupationally exposed to TCDD compared to a non-exposed reference group. Phenotype analysis of peripheral blood mononuclear cells revealed no significant differences in the proportion of CD3+, CD4+, CD8+ T lymphocytes, CD16+ natural killer cells, or CD19+ B lymphocytes of industrial workers exposed to high concentration of TCDD in another independent study (Ernst et al. Citation1998). A study among former workers of a pesticide-producing plant exposed to PCDDs and PCDFs in Germany showed that all blood cell counts, IgA, IgG, and IgM, were not significantly correlated with exposure to PCDD/PCDF (Jung et al. Citation1998). However, another study among workers exposed to TCDD during the manufacture of trichlorophenol (TCP) showed that the level of gamma globulins decreased with increasing TCDD concentration (Jansing and Korff Citation1994).
5.1.2. Studies on Vietnam War veterans exposed to Agent Orange
In a study on US Air force veterans of the Vietnam War, who were exposed to herbicides contaminated with TCDD, Michalek et al. (Citation1999) reported no significant differences in lymphocyte/subsets counts and immunoglobulin levels (IgA, IgG, and IgM) among a high-exposed group as compared to a non-exposed control group. However, a non-significant decrease in most lymphocyte subsets was reported among the high-exposed group. Another study among Vietnam War Korean veterans conducted by Kim et al. (Citation2003) showed lower plasma IgG levels as compared to controls, with a significant decrease in the IgG1 levels. In contrast, increased plasma levels of IgE were observed among exposed veterans.
5.1.3. Studies on waste incineration workers
A study in South Korea investigated the effects of dioxin exposure on three different immune parameters: leukocyte sub-populations (CD3+, CD4+, CD8+, CD19+, and CD69+), plasma immunoglobulin levels (IgA, IgG, IgM, and IgE), and cytokines (IL4 and Interferon gamma (INF-γ)) among waste incineration workers. There was no significant difference in T- and B-cell profiles between waste incineration workers and control subjects. However, T-cell activation was found to be significantly higher in the waste incineration workers than in the control subjects, but B-cell activation did not exhibit this trend. Immunoglobulins and cytokines were found in lower amounts in the waste incineration workers, but this difference was not statistically significant except for IL4 (Oh et al. Citation2005).
5.1.4. Studies on a population at risk from environmental contamination of TCDD
A study among Missouri residents (Times beach area) exposed to TCDD-contaminated soil reported no significant changes in T-cell numbers, T-helper cells, T-suppressor cells, and T4+/T8+ ratio. However, there were a greater percentage of individuals with a T4+/T8+ ratio ≤1 in the high-exposed group (Knutsen Citation1984). Studies on community residents of Quail Run Mobile Home Park (Missouri residents, USA), environmentally exposed to contaminated industrial sludge, showed a significantly decreased percentage of lymphocytes expressing CD3+, CD4+, and CD2+, but the mean numbers of each of the T-cell subsets was comparable between the groups (Hoffman et al. Citation1986; Knutsen et al. Citation1987). Subsequent studies (Evans et al. Citation1988; Webb et al. Citation1989) showed significant increases associated with higher concentrations of TCDD for IgG, %CD3+, %CD8+, number of T-cells, percentages of CD2+, and CD4+/Leu-8 position.
Svensson et al. (Citation1994) indicated that high consumption of fatty fish from the Baltic Sea, contaminated with persistent organochlorine compounds, is associated with lower number of natural killer cells. Another study among employees who worked in day-care centers where the floor had been treated with wood preservatives containing pentachlorophenol (PCP) and γ-hexachloro-cyclohexane (contaminated with PCDD and furans) showed no association between inhalation dioxin exposure and the number of peripheral CD4, CD8 cells, or their ratio (Wolf and Karmaus Citation1995).
All together, the existing epidemiologic studies in humans have not revealed consistent patterns of perturbations of the immune system by TCDD (). This can be due to several factors that affected the results of the above-mentioned studies, including: (1) the effect assessment of acute exposure in some studies compared to the effects of long-term (chronic) exposure to TCDD, (2) heterogeneity of the applied exposure assessment methods (lack of individual quantification of the body burden), (3) different statistical analysis (between-group differences instead of dose–effect or dose–response relationship), (4) differences in time between blood measurements and exposure, (5) differences in the magnitude of the exposure, (6) correlated (confounding) exposures, (7) residual confounding, and (8) survival bias.
Nevertheless, taken together, the observed findings might suggest that dioxin exposure can have an adverse impact on both the humoral and cell-mediated immune system and as such may provide support for lymphomagenesis of TCDD.
According to the ‘‘meet in the middle’’ concept, the overlap between the observed markers of TCDD exposure and the putative markers of NHL could identify relevant intermediate markers. However, to date, most of the markers have not been validated in relation to NHL risk in prospective studies (Saberi Hosnijeh, Krop, Scoccianti, et al. Citation2010; Gu et al. Citation2010; Purdue et al. Citation2009, Citation2011). Therefore, the value of these observations is not clear. Nevertheless, the possible effects of TCDD on suppression of the immune system (Saberi Hosnijeh et al. Citation2011; Saberi Hosnijeh, Boers, et al. Citation2012; Saberi Hosnijeh, Lenters, et al. Citation2012) cannot be excluded, a process which has been linked to NHL in prospective studies (Grulich et al. Citation2007).
5.2. Obesity
Results of our study within the EPIC-Italy (Taghavi Azar Sharabiani et al. Citation2011) suggested that IL8, IL10, IFN-α, and IP10 are related to obesity (Body mass index; BMI). Among these predictors, IP10 had the highest prognostic value for obesity. Changes in these immunological factors are likely to be mediated by leptin and adiponectin. There is evidence that leptin may promote an optimal pro-inflammatory response because, on one hand, it plays a pivotal role in the systemic inflammatory response and, on the other hand, restrains the inflammatory response via IL10 production (Bracho-Riquelme et al. Citation2008).
Results of previous studies have also shown that obesity causes alterations to the immune system (). A community-based cross-sectional study showed that obesity is linked to elevated leukocyte and lymphocyte subsets, IL6 and IL1α levels (Nieman et al. Citation1999). Two other cross-sectional studies showed that the levels of C-reactive protein (CRP), and concentrations of the pro-inflammatory cytokines IL6 and TNF-α, were related to all measures of obesity (Yudkin et al. Citation1999; Park et al. Citation2005). In a study on 189 untreated asymptomatic men in Canada, IL6 appeared to be clearly associated with visceral adiposity while TNF-α showed an association with indices of total body fatness (Cartier et al. Citation2008). Elevated CRP levels were associated to obesity among participants of the Third National Health and Nutrition Examination Survey (Visser et al. Citation1999). In addition, an association between IL8, (Straczkowski et al. Citation2002; Kim et al. Citation2006), sTNF-R2 (Straczkowski et al. Citation2002), MCP-1 (Kim et al. Citation2006), and BMI has been reported. In another cross-sectional study among 148 non-diabetic subjects, serum concentrations of sCD40L, an inflammatory marker, were significantly higher in obese subjects compared to normal weight subjects (Unek et al. Citation2010). In contrast, Ciftci et al. (Citation2004) showed that serum IL6 and TNF-α levels were not correlated with BMI in both patients and controls of a case-control study. Results of the Acute Respiratory Distress Syndrome Network (ARDSNet) trials showed that plasma IL6 and IL8 levels were inversely related to BMI, and that white blood cell count increased proportionally with BMI (Stapleton et al. Citation2010).
Table 2. Qualitative assessment of blood immune marker changes related to obesity.
Recently, adipose tissue is recognized as an endocrine organ that secretes adipokines, including cytokines and chemokines (Mohamed-Ali et al. Citation1998; Antuna-Puente et al. Citation2008). Both IL6 and TNF-α, which are of the most important pro-inflammatory cytokines, are expressed in adipose tissues (Yudkin et al. Citation1999). Overall, obesity seems to promote chronic inflammation and increased production of pro-inflammatory cytokines (Skibola Citation2007).
An important issue in analyzing immune function/parameters in obese individuals is that the effect of obesity itself on the immune system can be confounded by nutritional status, physical activity, and the coexistence of metabolic conditions.
Our studies (Saberi Hosnijeh, Krop, Scoccianti, et al. Citation2010; Taghavi Azar Sharabiani et al. Citation2011) are among the first studies that used prospectively collected samples, thus avoiding inverse causation bias in that some biomarkers might be affected by the disease process or treatment of the disease. These studies only had one prospectively collected blood sample for each individual to characterize their immune profile. To increase the accuracy of the biomarker assessment, repeated measures of the biomarkers might be necessary, because biomarker levels may vary substantially from day to day. However, when the inter- and intra-individual variability in cytokine levels was considered (Saberi Hosnijeh, Krop, Portengen et al. Citation2010), it was found that cytokine levels did not vary much over time within an individual as compared to the variance between individuals over at least a 2-week period. Therefore, in this particular context, a single cytokine measurement is expected to represent an individual's immune profile adequately.
In the two studies described previously (Saberi Hosnijeh, Krop, Scoccianti, et al. Citation2010; Taghavi Azar Sharabiani et al. Citation2011), no overlap was observed between marker levels of obesity and NHL (as based on the original concept of “meet in the middle” approach). However, a potential role of obesity in lymphomagenesis cannot be excluded because, on one hand, several studies showed that obesity is a low-grade chronic inflammatory state and, on the other hand, a chronic inflammatory condition has been linked to NHL risk in previous epidemiological studies (Smedby et al. Citation2011). It therefore seems that a broader investigation of markers of the immune system, such as, for instance, markers of chronic B cell activation (sCD30), might be warranted to further investigate the possible immune mediated association between obesity and NHL.
6. Next steps in environmental lymphoma research
Biomarkers have contributed greatly to our understanding of mechanistic pathways from early exposures to cancer development as well as early detection of cancers. Although these findings are informative on the immunological changes that precede the development of lymphomas and their possible relations with putative lymphomagens, the precise role of each individual biomarker in the etiology of these cancers is still not completely clear. More studies with larger sample sizes are needed to confirm the clinical relevance of the observed changes in biomarkers.
As many large prospective cohort and case-control studies with biologic samples have now been established, more-and-more biomarkers are becoming available for epidemiologic studies. The first challenge, that molecular epidemiology faces, is standardization of biomarker measurement including standardization of sample collection, processing, banking, and analysis. Proper handling of biological samples from the time of collection to the analysis protects the quality of the specimens and the validity of the results (Holland et al. Citation2003). Standard procedures and quality check schemes are necessary because there is a lack of definition to guarantee reproducibility of new procedures (Gallo et al. Citation2011). Second, standardization of exposure measurements across studies is of critical importance. Misclassification of exposure can severely affect estimates of disease risks, and even in some extreme situations, cause misleading interpretations about exposure–disease associations. Accurate assessment of exposure to occupational and environmental risk factors is needed to ensure that epidemiological studies meet their objectives in investigating the exposure–disease relationship.
References
- Ambinder , RF , Bhatia , K , Martinez-Maza , O and Mitsuyasu , R . 2010 . Cancer biomarkers in HIV patients . Curr Opin HIV AIDS , 5 ( 6 ) : 531 – 537 .
- Antuna-Puente , B , Feve , B , Fellahi , S and Bastard , JP . 2008 . Adipokines: the missing link between insulin resistance and obesity . Diabetes Metab , 34 ( 1 ) : 2 – 11 .
- Baccarelli , A , Mocarelli , P , Patterson , DG Jr , Bonzini , M , Pesatori , AC , Caporaso , N and Landi , MT . 2002 . Immunologic effects of dioxin: new results from Seveso and comparison with other studies . Environ Health Perspect , 110 ( 12 ) : 1169 – 1173 .
- Bertazzi , PA , Pesatori , AC , Consonni , D , Tironi , A , Landi , MT and Zocchetti , C . 1993 . Cancer incidence in a population accidentally exposed to 2,3,7,8-tetrachlorodibenzo-para-dioxin . Epidemiology , 4 ( 5 ) : 398 – 406 .
- Boers , D , Portengen , L , Bueno-de-Mesquita , HB , Heederik , DJJ and Vermeulen , R . 2010 . Cause-specific mortality of Dutch chlorophenoxy herbicide manufacturing workers . Occup Environ Med , 67 : 24 – 31 .
- Bower , M , Fisher , M , Hill , T , Reeves , I , Walsh , J , Orkin , C , Phillips , AN , Bansi , L , Gilson , R Easterbrook , P . 2009 . CD4 counts and the risk of systemic non-Hodgkin's lymphoma in individuals with HIV in the UK . Haematologica , 94 ( 6 ) : 875 – 880 .
- Bracho-Riquelme , RL , Reyes-Romero , MA , Pescador , N and Flores-García , AI . 2008 . A leptin serum concentration less than 10 ng/ml is a predictive marker of outcome in patients with moderate to severe secondary peritonitis . Eur Surg Res , 41 ( 2 ) : 238 – 244 .
- Breen , EC , Boscardin , WJ , Detels , R , Jacobson , LP , Smith , MW , O'Brien , SJ , Chmiel , JS , Rinaldo , CR , Lai , S and Martínez-Maza , O . 2003 . Non-Hodgkin's B cell lymphoma in persons with acquired immunodeficiency syndrome is associated with increased serum levels of IL10, or the IL10 promoter-592 C/C genotype . Clin Immunol , 109 ( 2 ) : 119 – 129 .
- Breen , EC , Epeldegui , M , Boscardin , WJ , Widney , DP , Detels , R and Martínez-Maza , O . 2005 . Elevated levels of soluble CD44 precede the development of AIDS-associated non-Hodgkin's B-cell lymphoma . AIDS , 19 ( 15 ) : 1711 – 1712 .
- Breen , EC , Fatahi , S , Epeldegui , M , Boscardin , W , Detels , R and Martínez-Maza , O . 2006 . Elevated serum soluble CD30 precedes the development of AIDS-associated non-Hodgkin's B cell lymphoma . Tumor Biol , 27 ( 4 ) : 187 – 194 .
- Breen , EC , Hussain , SK , Magpantay , L , Jacobson , LP , Detels , R , Rabkin , CS , Kaslow , RA , Variakojis , D , Bream , JH Rinaldo , CR . 2011 . B-cell stimulatory cytokines and markers of immune activation are elevated several years prior to the diagnosis of systemic AIDS-associated non-Hodgkin B-cell lymphoma . Cancer Epidemiol Biomarkers Prev , 20 ( 7 ) : 1303 – 1314 .
- Breen , EC , van der Meijden , M , Cumberland , W , Kishimoto , T , Detels , R and Martínez-Maza , O . 1999 . The Development of AIDS-associated Burkitt's/small noncleaved cell lymphoma is preceded by elevated serum levels of interleukin 6 . Clin Immunol , 92 ( 3 ) : 293 – 299 .
- Cartier , A , Lemieux , I , Alméras , N , Tremblay , A , Bergeron , J and Després , JP . 2008 . Visceral obesity and plasma glucose-insulin homeostasis: contributions of interleukin-6 and tumor necrosis factor-α in men . J Clin Endocrinol Metab , 93 ( 5 ) : 1931 – 1938 .
- Chadeau-Hyam , M , Athersuch , TJ , Keun , HC , De Iorio , M , Ebbels , TM , Jenab , M , Sacerdote , C , Bruce , SJ , Holmes , E and Vineis , P . 2011 . Meeting-in-the-middle using metabolic profiling – a strategy for the identification of intermediate biomarkers in cohort studies . Biomarkers , 16 ( 1 ) : 83 – 88 .
- Ciftci , TU , Kokturk , O , Bukan , N and Bilgihan , A . 2004 . The relationship between serum cytokine levels with obesity and obstructive sleep apnea syndrome . Cytokine , 28 ( 2 ) : 87 – 91 .
- Engels , EA , Cerhan , JR , Linet , MS , Cozen , W , Colt , JS , Davis , S , Gridley , G , Severson , RK and Hartge , P . 2005 . Immune-related conditions and immune-modulating medications as risk factors for non-Hodgkin's lymphoma: a case-control study . Am J Epidemiol , 162 ( 12 ) : 1153 – 1161 .
- Engels , EA , Pfeiffer , RM , Landgren , O and Moore , RD . 2010 . Immunologic and virologic predictors of AIDS-related non-Hodgkin lymphoma in the HAART era . J Acquir Immune Defic Syndr , 54 ( 1 ) : 78 – 84 .
- Epeldegui , M , Breen , EC , Hung , YP , Boscardin , WJ , Detels , R and Martínez-Maza , O . 2007 . Elevated expression of activation induced cytidine deaminase in peripheral blood mononuclear cells precedes AIDS-NHL diagnosis . AIDS , 21 ( 17 ) : 2265 – 2270 .
- Ernst , M , Flesch-Janys , D , Morgenstern , I and Manz , A . 1998 . Immune cell functions in industrial workers after exposure to 2,3,7,8-tetrachlorodibenzop-dioxin: dissociation of antigen-specific T-cell responses in cultures of diluted whole blood and of isolated peripheral blood mononuclear cells . Environ Health Perspect , 106 ( Suppl. 2 ) : 701 – 705 .
- Evans , R , Webb , K , Knutsen , A , Roodman , ST , Roberts , DW , Bagby , JR , Garrett , WA Jr and Andrews , JS Jr . 1988 . A medical follow-up of the health effects of long-term exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin . Arch Environ Health , 43 ( 4 ) : 273 – 278 .
- Fingerhut , MA , Halperin , WE , Marlow , DA , Piacitelli , LA , Honchar , PA , Sweeney , MH , Greife , AL , Dill , PA , Steenland , K and Suruda , AJ . 1991 . Cancer mortality in workers exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin . N Engl J Med , 324 ( 4 ) : 212 – 218 .
- Gallo , V , Egger , M , McCormack , V , Farmer , PB , Ioannidis , JPA , Kirsch-Volders , M , Matullo , G , Phillips , DH , Schoket , B Stromberg , U . 2011 . STrengthening the Reporting of OBservational studies in Epidemiology-Molecular Epidemiology (STROBE-ME): an extension of the STROBE statement . J Cli Epidemiol , 64 ( 12 ) : 1350 – 1363 .
- Garcia-Closas , M , Vermeulen , R , Sherman , ME , Moore , LE , Smith , MT and Rothman , N . 2006 . Application of biomarkers in cancer epidemiology , David Schottenfeld, Joseph F. . Fraumeni, editors. Cancer epidemiology and prevention. 3rd ed. Chap. 1.6. Oxford: Oxford University Press. p. 70–88
- Grulich , AE , Vajdic , CM and Cozen , W . 2007 . Altered immunity as a risk factor for non-Hodgkin lymphoma . Cancer Epidemiol Biomarkers Prev , 16 ( 3 ) : 405 – 408 .
- Grulich , AE , Wan , X , Law , MG , Milliken , ST , Lewis , CR , Garsia , RJ , Gold , J , Finlayson , RJ , Cooper , DA and Kaldor , JM . 2000 . B-cell stimulation and prolonged immune deficiency are risk factors for non-Hodgkin's lymphoma in people with AIDS . AIDS , 14 ( 2 ) : 133 – 140 .
- Gu , Y , Shore , R , Arslan , A , Koenig , K , Liu , M , Ibrahim , S , Lokshin , A and Zeleniuch-Jacquotte , A . 2010 . Circulating cytokines and risk of B-cell non-Hodgkin lymphoma: a prospective study . Cancer Causes Control , 21 ( 8 ) : 1323 – 1333 .
- Halperin , W , Vogt , R , Sweeney , MH , Shopp , G , Fingerhut , M and Petersen , M . 1998 . Immunological markers among workers exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin . Occup Environ Med , 55 ( 11 ) : 742 – 749 .
- Hoffman , RE , Stehr-Green , PA , Webb , KB , Evans , RG , Knutsen , AP , Schramm , WF , Staake , JL , Gibson , BB and Steinberg , KK . 1986 . Health effects of long-term exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin . JAMA , 255 ( 15 ) : 2031 – 2038 .
- Holland , NT , Smith , MT , Eskenazi , B and Bastaki , M . 2003 . Biological sample collection and processing for molecular epidemiological studies . Mutat Res-Rev Mut Res , 543 ( 3 ) : 217 – 234 .
- Hooiveld , M , Heederik , DJJ , Kogevinas , M , Boffetta , P , Needham , LL , Patterson , DG Jr and Bueno-de-Mesquita , HB . 1998 . Second follow-up of a Dutch cohort occupationally exposed to phenoxy herbicides, chlorophenols, and contaminants . Am J Epidemiol , 147 ( 9 ) : 891 – 899 .
- Hoover , RN . 1992 . Lymphoma risks in populations with altered immunity—a search for mechanism . Cancer Res , 52 ( Suppl. 19 ) : 5477s – 5478s .
- Hussain , SK , Widney , D , Jacobson , L , Breen , EC , Levine , A , Detels , R , Zhang , ZF and Martínez-Maza , O . 2010 . Elevated serum levels of CXCL13 precede HIV-associated non-Hodgkin's lymphoma . Infect Agent Cancer , 5 ( Suppl. 1 ) : A24
- Jaffe , ES , Harris , NL , Stein , H and Vardiman , JW . 2001 . world health organization classification of Tumours: pathology and genetics of tumours of haematopoietic and lymphoid tissues , Lyon , , France : IARC Press .
- Jansing , PJ and Korff , R . 1994 . Blood levels of 2,3,7,8-tetrachlorodibenzo-p-dioxin and gamma-globulins in a follow-up investigation of employees with chloracne . J Dermatol Sci , 8 ( 2 ) : 91 – 95 .
- Jennings , AM , Wild , G and Ward , JD . 1988 . Immunological abnormalities 17 years after accidental exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin . Br J Ind Med , 45 ( 10 ) : 701 – 704 .
- Jung , D , Berg , PA , Edler , L , Ehrenthal , W , Fenner , D , Flesch-Janys , D , Huber , C , Klein , R , Koitka , C Lucier , G . 1998 . Immunologic findings in workers formerly exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin and its congeners . Environ Health Perspect , 106 ( Suppl. 2 ) : 689 – 695 .
- Kim , HA , Ki , EM , Park , YC , Yu , JY , Hong , SK , Jeon , SH , Park , KL , Hur , SJ and Heo , Y . 2003 . Immunotoxicological effects of agent orange exposure to the Vietnam War Korean veterans . Ind Health , 41 ( 3 ) : 158 – 166 .
- Kim , CS , Park , HS , Kawada , T , Kim , JH , Lim , D , Hubbard , NE , Kwon , BS , Erickson , KL and Yu , R . 2006 . Circulating levels of MCP-1 and IL-8 are elevated in human obese subjects and associated with obesity related parameters . Int J Obes (Lond) , 30 ( 9 ) : 1347 – 1355 .
- Knutsen , AP . 1984 . Immunologic effects of TCDD exposure in humans . Environ Contam Toxicol , 33 ( 1 ) : 673 – 681 .
- Knutsen , AP , Roodman , ST , Evans , RG , Mueller , KR , Webb , KB , Stehr-Green , P , Hoffman , RE and Schramm , WF . 1987 . Immune studies in dioxin-exposed Missouri residents: quail run . Environ Contam Toxicol , 39 ( 3 ) : 481 – 489 .
- Kogevinas , M , Becher , H , Benn , T , Bertazzi , PA , Boffetta , P , Bueno-de-Mesqurta , HB , Coggon , D , Colin , D , Flesch-Janys , D Fingerhut , M . 1997 . Cancer mortality in workers exposed to phenoxy herbicides, chlorophenols, and dioxins an expanded and updated international cohort study . Am J Epidemiol , 145 ( 12 ) : 1061 – 1075 .
- Landgren , O , Goedert , JJ , Rabkin , CS , Wilson , WH , Dunleavy , K , Kyle , RA , Katzmann , JA , Rajkumar , SV and Engels , EA . 2010 . Circulating serum free light chains as predictive markers of AIDS-related lymphoma . J Clin Oncol , 28 ( 5 ) : 773 – 779 .
- Lotze , MT and Rees , RC . 2004 . Identifying biomarkers and surrogates of tumors (cancer biometrics): correlation with immunotherapies and immune cells . Cancer Immunol Immunother , 53 ( 3 ) : 256 – 261 .
- Marchand , LL . 2005 . Epidemiological approach to studying cancer II: molecular epidemiology , Peter G. . Shields, editor. Cancer risk assessment. Chap. 3. New York: Taylor & Francis group. p. 39–148
- Mariette , X . 2001 . Lymphomas complicating Sjögren's syndrome and hepatitis C virus infection may share a common pathogenesis: chronic stimulation of rheumatoid factor B cells . Ann Rheum Dis , 60 ( 11 ) : 1007 – 1010 .
- Michalek , JE , Ketchum , NS and Check , IJ . 1999 . Serum dioxin and immunologic response in veterans of Operation Ranch Hand . Am J Epidemiol , 149 ( 11 ) : 1038 – 1046 .
- Mohamed-Ali , V , Pinkey , JH and Coppack , SW . 1998 . Adipose tissue as an endocrine and paracrine organ . Int J Obes Relat Metab Disord , 22 ( 12 ) : 1145 – 1158 .
- Müller , AMS , Ihorst , G , Mertelsmann , R and Engelhardt , M . 2005 . Epidemiology of non-Hodgkin's lymphoma (NHL): trends, geographic distribution, and etiology . Ann Hematol , 84 ( 1 ) : 1 – 12 .
- Nagayama , J , Tsuji , H , Iida , T , Hirakawa , H , Matsueda , T and Ohki , M . 2001 . Effects of contamination level of dioxins and related chemicals on thyroid hormone and immune response systems in patients with “Yusho” . Chemosphere , 43 ( 4-7 ) : 1005 – 1010 .
- Neubert , R , Maskow , L , Triebig , G , Broding , HC , Jacob-Müller , U , Helge , H and Neubert , D . 2000 . Chlorinated dibenzo-p-dioxins and dibenzofurans and the human immune system: 3. Plasma immunoglobulins and cytokines of workers with quantified moderately-increased body burdens . Life Sci , 66 ( 22 ) : 2123 – 2142 .
- Neubert , R , Maskow , L , Webb , J , Jacob-Müller , U , Nogueira , AC , Delgado , I , Helge , H and Neubert , D . 1993 . Chlorinated dibenzo-p-dioxins and dibenzofurans and the human immune system. 1. Blood cell receptors in volunteers with moderately increased body burdens . Life Sci , 53 ( 26 ) : 1995 – 2006 .
- Nieman , DC , Henson , DA , Nehlsen-Cannarella , SL , Ekkens , M , Utter , AC , Butterworth , DE and Fagoaga , OR . 1999 . Influence of obesity on immune function . J Am Diet Assoc , 99 ( 3 ) : 294 – 299 .
- Oh , E , Lee , E , Im , H , Kang , HS , Jung , WW , Won , NH , Kim , EM and Sul , D . 2005 . Evaluation of immuno- and reproductive toxicities and association between immunotoxicological and genotoxicological parameters in waste incineration workers . Toxicology , 210 ( 1 ) : 65 – 80 .
- Ott , MG , Zober , A and Germann , C . 1994 . Laboratory results for selected target organs in 138 individuals occupationally exposed to TCDD . Chemosphere , 29 ( 9 ) : 2423 – 2437 .
- Park , HS , Park , JY and Yu , R . 2005 . Relationshi of obesity and viseral adiposity with serum concentrations of CRP, TNF-a and IL-6 . Diabetis Res Clin Pract , 69 ( 1 ) : 29 – 35 .
- Pluda , JM , Venzon , DJ , Tosato , G , Lietzau , J , Wyvill , K , Nelson , DL , Jaffe , ES , Karp , JE , Broder , S and Yarchoan , R . 1993 . Parameters affecting the development of non-Hodgkin's lymphoma in patients with severe human immunodeficiency virus infection receiving antiretroviral therapy . J Clin Oncol , 11 ( 16 ) : 1099 – 1107 .
- Purdue , MP , Lan , Q , Bagni , R , Hocking , WG , Baris , D , Reding , DJ and Rothman , N . 2011 . Prediagnostic serum levels of cytokines and other immune markers and risk of non-Hodgkin lymphoma . Cancer Res , 71 ( 14 ) : 4898 – 4907 .
- Purdue , MP , Lan , Q , Martinez-Maza , O , Oken , MM , Hocking , W , Huang , WY , Baris , D , Conde , B and Rothman , N . 2009 . A prospective study of serum soluble CD30 concentration and risk of non-Hodgkin lymphoma . Blood , 114 ( 13 ) : 2730 – 2732 .
- Rabkin , CS , Engels , EA , Landgren , O , Schuurman , R , Camargo , MC , Pfeiffer , R and Goedert , JJ . 2011 . Circulating cytokine levels, Epstein-Barr viremia, and risk of acquired immunodeficiency syndrome-related non-Hodgkin lymphoma . Am J Hematol , 86 ( 10 ) : 875 – 878 .
- Saberi Hosnijeh , F , Boers , D , Portengen , L , Bueno-de-Mesquita , HB , Heederik , D and Vermeulen , R . 2011 . Long-term effects on humoral immunity among workers exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) . Occup Environ Med , 68 : 419 – 424 .
- Saberi Hosnijeh , F , Boers , D , Portengen , L , Bueno-de-Mesquita , HB , Heederik , D and Vermeulen , R . 2012 . Plasma cytokine concentrations in workers exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) . Front Oncol , 2 : 37
- Saberi Hosnijeh , F , Krop , EJM , Portengen , L , Rabkin , CS , Linseisen , J , Vineis , P and Vermeulen , R . 2010 . Stability and reproducibility of simultaneously detected plasma and serum cytokine levels in asymptomatic subjects . Biomarkers , 15 ( 2 ) : 140 – 8 .
- Saberi Hosnijeh , F , Krop , EJM , Scoccianti , C , Krogh , V , Palli , D , Panico , S , Tumino , R , Sacredote , C , Nawroly , N Portengen , L . 2010 . Plasma cytokines and future risk of non-Hodgkin lymphoma (NHL): a case-control study nested in the Italian European prospective investigation into cancer and nutrition . Cancer Epidemiol Biomarkers Prev , 19 ( 6 ) : 1577 – 1584 .
- Saberi Hosnijeh F, Lenters V, Boers D, Portengen L, Baeten E, Bueno-de-Mesquita HB, Heederik D, Bloem AC, Vermeulen R. 2012. Changes in lymphocyte subsets in workers exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Occup Environ Med. In press
- Schroeder , JR , Saah , AJ , Ambinder , RF , Martinez-Maza , O , Breen , EC , Variakojis , D , Margolick , JB , Jacobson , LP , Rowe , DT and Hoover , DR . 1999 . Serum sCD23 level in patients with AIDS-related non-Hodgkin's lymphoma is associated with absence of Epstein–Barr virus in tumor tissue . Clin Immunol , 93 ( 3 ) : 239 – 244 .
- Schroeder , JR , Saah , AJ , Hoover , DR , Margolick , JB , Ambinder , RF , Martinez-Maza , O , Breen , EC , Jacobson , LP , Variakojis , D and Rowe , DT . 1999 . Serum soluble CD23 level correlates with subsequent development of AIDS-related non-Hodgkin's lymphoma . Cancer Epidemiol Biomarkers Prev , 8 ( 11 ) : 979 – 984 .
- Skibola , CF . 2007 . Obesity, diet and risk of non-Hodgkin lymphoma . Cancer Epidemiol Biomarkers Prev , 16 ( 3 ) : 392 – 395 .
- Skibola , CF , Bracci , PM , Halperin , E , Conde , L , Craig , DW , Agana , L , Iyadurai , K , Becker , N , Brooks-Wilson , A Curry , JD . 2009 . Genetic variants at 6p21.33 are associated with susceptibility to follicular lymphoma . Nat Genet , 41 ( 8 ) : 873 – 875 .
- Skibola , CF , Bracci , PM , Nieters , A , Brooks-Wilson , A , de Sanjose , S , Hughes , AM , Cerhan , JR , Skibola , DR , Purdue , M Kane , E . 2010 . Tumor necrosis factor (TNF) and lymphotoxin-alpha (LTA) polymorphisms and risk of non-Hodgkin lymphoma in the InterLymph Consortium . Am J Epidemiol , 171 ( 3 ) : 267 – 276 .
- Smedby , KE , Foo , JN , Skibola , CF , Darabi , H , Conde , L , Hjalgrim , H , Kumar , V , Chang , ET , Rothman , N Cerhan , JR . 2011 . GWAS of follicular lymphoma reveals allelic heterogeneity at 6p21.32 and suggests shared genetic susceptibility with diffuse large B-cell lymphoma . PLoS Genet , 7 ( 4 ) : e1001378
- Stapleton , RD , Dixon , AE , Parsons , PE , Ware , LB , Suratt , BT and Network NHLBI Acute Respiratory Distress Syndrome . 2010 . The association between BMI and plasma cytokine levels in patients with acute lung injury . Chest , 138 ( 3 ) : 568 – 577 .
- Straczkowski , M , Dzienis-Straczkowska , S , Stêpieñ , A , Kowalska , I , Szelachowska , M and Kinalska , I . 2002 . Plasma interleukin-8 concentrations are increased in obese subjects and related to fat mass and tumor necrosis factor-alpha system . J Clin Endocrinol Metab , 87 ( 10 ) : 4602 – 4606 .
- Svensson , BG , Hallberg , T , Nilsson , A , Schütz , A and Hagmar , L . 1994 . Parameters of immunological competence in subjects with high consumption of fish contaminated with persistent organochlorine compounds . Int Arch Occup Environ Health , 65 ( 6 ) : 351 – 358 .
- Swerdlow , AJ . 2003 . Epidemiology of Hodgkin's disease and non-Hodgkin's lymphoma . Eur J Nucl Med Mol Imaging , 30 ( Suppl. 1 ) : S3 – S12 .
- Taghavi Azar Sharabiani , M , Vermeulen , R , Scoccianti , C , Saberi Hosnijeh , F , Minelli , L , Sacerdote , C , Palli , D , Krogh , V , Tumino , R Chiodini , P . 2011 . Immunologic profile of excessive body weight . Biomarkers , 16 ( 3 ) : 243 – 51 .
- Tio , M , Cox , MR and Eslick , GD . 2012 . Meta-analysis: coeliac disease and the risk of all-cause mortality, any malignancy and lymphoid malignancy . Aliment Pharmacol Ther , 35 ( 5 ) : 540 – 551 .
- Tonn , T , Esser , C , Schneider , EM , Steinmann-Steiner-Haldenstatt , W and Gleichmann , E . 1996 . Persistence of decreased T-helper cell function in industrial workers 20 years after exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin . Environ Health Perspect , 104 ( 4 ) : 422 – 426 .
- Unek , IT , Bayraktar , F , Solmaz , D , Ellidokuz , H , Sisman , AR , Yuksel , F and Yesil , S . 2010 . The levels of soluble CD40 ligand and C-reactive protein in normal weight, overweight and obese people . Clin Med Res , 8 ( 2 ) : 289 – 95 .
- Vendrame , E and Martinez-Maza , O . 2011 . Assessment of pre-diagnosis biomarkers of immune activation and inflammation: insights on the etiology of lymphoma . J Proteome Res , 10 ( 1 ) : 113 – 119 .
- Vermeulen , R , Saberi Hosnijeh , F , Portengen , L , Krogh , V , Palli , D , Panico , S , Tumino , R , Sacredote , C , Purdue , M Lan , Q . 2011 . Circulating soluble CD30 and future risk of lymphoma; evidence from two prospective studies in the general population . Cancer Epidemiol Biomarkers Prev , 20 ( 9 ) : 1925 – 1927 .
- Vineis , P and Perera , F . 2007 . Molecular epidemiology and biomarkers in etiologic cancer research: the new in light of the old . Cancer Epidemiol Biomarkers Prev , 16 ( 10 ) : 1954 – 1965 .
- Visser , M , Bouter , LM , McQuillan , GM , Wener , MH and Harris , TB . 1999 . Elevated C-reactive protein levels in overweight and obese adults . JAMA , 282 ( 22 ) : 2131 – 2135 .
- Vlaanderen , J , Moore , LE , Smith , MT , Lan , Q , Zhang , L , Skibola , CF , Rothman , N and Vermeulen , R . 2010 . Application of OMICS technologies in occupational and environmental health research: current status and projections . Occup Environ Med , 67 ( 2 ) : 136 – 143 .
- Wang , SS , Cozen , W , Cerhan , JR , Colt , JS , Morton , LM , Engels , EA , Davis , S , Severson , RK , Rothman , N Chanock , SJ . 2007 . Immune mechanisms in non-Hodgkin lymphoma: joint effects of the TNF G308A and IL10 T3575A polymorphisms with non-Hodgkin lymphoma risk factors . Cancer Res , 67 ( 10 ) : 5042 – 5054 .
- Webb , KB , Evans , RG , Knutsen , AP , Roodman , ST , Roberts , DW , Schramm , WF , Gibson , BB , Andrews , JS Jr , Needham , LL and Patterson , DG . 1989 . Medical evaluation of subjects with known body levels of 2,3,7,8-tetrachlorodibenzo-p-dioxin . J Toxicol Environ Health , 28 ( 2 ) : 183 – 193 .
- Widney , DP , Breen , EC , Boscardin , WJ , Kitchen , SG , Alcantar , JM , Smith , JB , Zack , JA , Detels , R and Martínez-Maza , O . 2005 . Serum levels of the homeostatic B cell chemokine, CXCL13, are elevated during HIV infection . J Interferon Cytokine Res , 25 ( 11 ) : 702 – 706 .
- Widney , D , Gundapp , G , Said , JW , van der Meijden , M , Bonavida , B , Demidem , A , Trevisan , C , Taylor , J , Detels , R and Martínez-Maza , O . 1999 . Aberrant expression of CD27 and soluble CD27 (sCD27) in HIV infection and in AIDS-associated lymphoma . Clin Immunol , 93 ( 2 ) : 114 – 123 .
- Willett , EV , Morton , LM , Hartge , P , Becker , N , Bernstein , L , Boffetta , P , Bracci , P , Cerhan , J , Chiu , BC Cocco , P . 2008 . Non-Hodgkin lymphoma and obesity: a pooled analysis from the InterLymph Consortium . Int J Cancer , 122 ( 9 ) : 2062 – 2070 .
- Wolf , N and Karmaus , W . 1995 . Effect of inhalative exposure to dioxins in wood preservatives on cell-mediated immunity in day-care center teachers . Environ Res , 68 ( 2 ) : 96 – 105 .
- Wolk , A , Gridley , G , Svensson , M , Nyrén , O , McLaughlin , JK , Fraumeni , JF and Adami , HO . 2001 . A prospective study of obesity and cancer risk (Sweden) . Cancer Causes Control , 12 ( 1 ) : 13 – 21 .
- Yawetz , S , Cumberland , W , van der Meyden , M and Martinez-Maza , O . 1995 . Elevated serum levels of soluble CD23 (sCD23) precede the appearance of acquired immunodeficiency syndrome-associated non-Hodgkin's lymphoma . Blood , 85 ( 7 ) : 1843 – 1849 .
- Yudkin , JS , Stehouwer , CDA , Emeis , JJ and Coppack , SW . 1999 . C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction a potential role for cytokines originating from adipose tissue? . Arterioscler Thromb Vasc Biol , 19 ( 4 ) : 972 – 978 .
- Zoufaly , A , Stellbrink , HJ , Kollan , C , Hoffmann , C and van Lunzen , J . 2009 . Cumulative HIV viremia during highly active antiretroviral therapy is a strong predictor of AIDS‐related lymphoma . J Infect Dis , 200 ( 1 ) : 79 – 87 .