A 14-month-old female ostrich (Struthio camelus), weighing approximately 115 kg and over 1.5 m tall was submitted to the Unit of Veterinary Histology and Pathology for necropsy evaluation. The ostrich was referred from a commercial farm located in Gran Canaria, Spain. Groups (n = 5) of young females (<18 months old) were housed in separate pens (1000 m2) with a shelter house (5 m × 8 m) and were fed daily with 1–1.2 kg/animal of ostrich layer pellets with free access to pasture and water. No reproductive activity (no male mating, no egg production) of these ostriches were reported.
The animal had showed anorexia, depression, and abdominal pain for two days duration. Then, the ostrich was separated from other females and signs of colic were observed for three days before death occurred. No medical treatment was provided to the ostrich and no blood tests were performed. The farmer pointed out that the animal had not suffered any trauma, and no signs of intoxication were evident.
At necropsy, the animal was in good body condition. The main gross finding was a dark purple mass of 8 × 8 × 8 cm in the left dorsal portion of the celomic cavity. This mass was determined to be a follicle of the left ovary. There was a 360-degree torsion around the follicular peduncle strangulating the artery, vein and the nerve within the peduncle (). There was also a deposition of fibrin material over the surface of the follicle. The content of the ovarian follicle was composed of a friable red to black material consistent with a coagulative necrosis (). The rest of the ovary had a normal appearance with a few post-vitellogenic follicles (1–3 cm). In addition, the oviduct was approximately 1.0 m in length and no lesions were observed in their anatomical components. Grossly, no predisposing factors for the torsion were observed.
Figure 1. Macroscopic appearance of the twisted follicle. Follicular peduncle (arrow). Uterus (arrow head). Scale bar 5 cm.
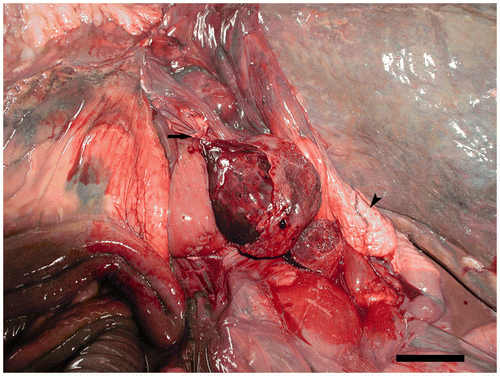
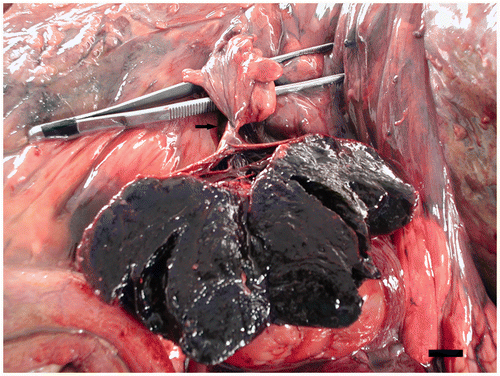
Figure 2. The content of the twisted follicle shows red firm material. The tweezers facilitate the visualization of the follicular torsion around the peduncle (arrow). Scale bar 1 cm.
A moderate amount of red to brown liquid was presented in the celomic cavity, and the intestinal serosa showed an opaque and fine granular aspect. The proventriculus and ventriculus showed no food content, and the intestine had a scant amount of content with scant dry feces, but no lesions were observed.
Figure 3. Inflammatory reaction with lymphocytes (right inset) and macrophages (left inset) besides variably sized, round, amphophilic to basophilic protein globules on the serosal surface of the intestine. Tunica muscularis is at the top of the figure and serosal surface at the bottom. Scale bar 200 µm.
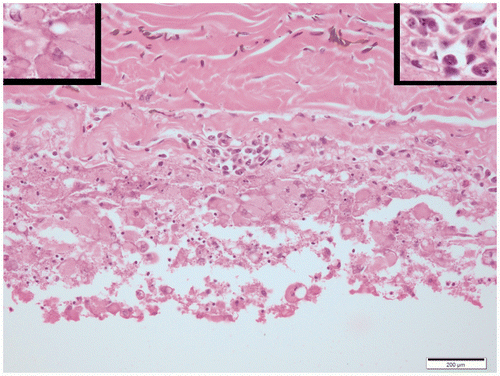
Histopathological examination of the twisted follicle revealed coagulative necrosis of follicular tissue compatible with ischemia, hemorrhage, coagulated blood, and fibrin. A moderate inflammatory reaction denoted by the presence of macrophages with abundant clear eosinophilic cytoplasm, some of them with clear cytoplasmic vacuoles, and lymphocytes, associated with different sized, round, amphophilic to basophilic protein globules, was observed on the serosal surface of the small and large intestine (). No bacteria were observed in the haematoxylin-eosin and Gram-stained samples. Based on macroscopic and histological findings, a diagnosis of follicular ovarian torsion (follicular OT) and aseptic peritonitis was made.
Ostriches are large flightless birds, raised commercially for their meat, eggs, hide, and feathers (Ajudi et al. Citation2009). Ostriches are considered to be seasonal breeders (Degen et al. Citation1994) and, consequently, the size and appearance of the reproductive organs vary considerably, depending on the physiological state of the animal (Hicks-Alldredge Citation1996). In the female ostrich, only the left ovary and oviduct are developed (Fowler Citation1991). The active ovary is composed of stroma and numerous follicles, which exhibit a wide range of sizes. In appearance it could be compared to a bunch of grapes, with the stalked post-vitellogenic follicles hanging into the body cavity. Each peduncle contains a vein, an artery, and a nerve (Bronneberg and Taverne Citation2003).
In farm-breeding ostriches, a good reproductive efficiency is the basic aim to reach a satisfactory rate of production (Bronneberg and Taverne Citation2003). Different factors and diseases have been reported to reduce the fertility and hatchability in farmed ostriches, and may result in reproductive dysfunction, either failure to produce eggs, ovulatory failure, or production of abnormal or contaminated eggs (Cabassi et al. Citation2004; Dzoma Citation2010). Anatomical causes of infertility include anomalies of the oviduct, resulting in internal ovulation or abnormal eggs. In addition, cystic ovaries may develop as a consequence of an excessive follicular activity (Hicks-Alldredge Citation1996). However, to the best of our knowledge, torsion of the follicular peduncle in ostriches has not been previously reported.
Ovarian torsion has been defined as a total or partial rotation of the adnexa (ovary and/or the fallopian tube) around its vascular axis (Descargues et al. Citation2001; Tsafrir et al. Citation2012). In women, ovarian torsion is a well-known, uncommon, but important cause of abdominal and pelvic pain and yet poorly recognized clinical entity that can involve the tube, ovary, and ancillary structures either separately or together (Houry and Abbot Citation2001; White and Stella Citation2005; Tsafrir et al. Citation2012). The risk factors of ovarian torsion include conditions such as previous abdominal surgery, ovarian hyperstimulation, previous or current ovarian cyst, and ovarian tumors (Houry and Abbot Citation2001; Karnik et al. Citation2006). However, it can also occur in normal ovarian tissue, basically in young children (Kokoska et al. Citation2000; Hoey et al. Citation2005). It is the fifth most common gynecologic emergency, with a reported incidence of 3%, not only being more frequent in women of childbearing age but also occurs in childhood and in the postmenopausal period (Descargues et al. Citation2001). In the veterinary literature, OT-follicular OT (depending on the anatomy of the ovary and the species involved) is an uncommon condition and has been reported in a foal (Evard et al. Citation1988; Valk et al. Citation1998), a mare (Sedrish et al. Citation1997), a bitch (Seyrek-Intas et al. Citation2011), and a green iguana (Mehler et al. Citation2002). No information on follicular OT has been reported in pet birds and poultry.
Regarding the predisposing causes in veterinary medicine, in a mare (Sedrish et al. Citation1997) ovarian torsion was associated with a large granulosa-theca cell tumor and was in accordance with the findings in human medicine. However, in the current case, no signs of neoplastic transformation were observed. In a bitch, it was consequence of an abnormal disposition of the uterine horns (Seyrek-Intas et al. Citation2011). In two foals (Evard et al. Citation1988; Valk et al. Citation1998), OT developed as a consequence of the larger relative size of the ovaries and their supporting structures, compared with the same structures in adults, in addition to their relative mobility predispose them to torsion or strangulation. In the case of follicular OT in a green iguana (Mehler et al. Citation2002), a full torsion around the common axis was observed in two follicles of the left ovary, while the remaining follicles were completely normal. The macroscopic and histopathological findings were totally comparable with those observed in the ostrich. In the current case, predisposing factors for the torsion, as neoplasm or anatomical malformations, were not observed. In accord, a possible explanation may be that the large size of follicles in the ratites species could predispose the ovarian torsion in the ostrich. This hypothesis is consistent with the findings observed in ovarian torsion in foals (Evard et al. Citation1988; Valk et al. Citation1998).
Peritonitis due to the passage of yolk material into the peritoneal cavity is a common condition in avian species (Hicks-Alldredge Citation1996). Egg yolk peritonitis may occur from trauma, salpingitis, rupture of the oviduct, neoplasia, ovarian cystic hyperplasia, and ectopic ovulation due to reverse peristalsis of the oviduct (Schmidt et al. Citation2003). In addition, egg yolk peritonitis may be sterile or contaminated by bacteria (Hicks-Alldredge Citation1996). In the current case, the macroscopic findings and the histopathological examination of the peritoneal cavity confirmed the development of a generalized peritonitis without sepsis. In women, when ovarian torsion and acute peritonitis occur concomitantly, it is often not evident which is the primary event (Hoey et al. Citation2005). Probably, in the current case report the torsion of the peduncle caused rupture of the follicular membrane with the consequent passage of yolk material into the body cavity, resulting in an inflammatory response and the development of peritonitis.
Ovarian torsion-follicular ovarian torsion is considered as a surgical emergency that requires immediate clinical intervention. If there is no resolution of this condition in the 24 hours after the appearance of the first clinical symptoms, it is practically assured to cause unviability of the tissue involved (Sedrish et al. Citation1997; Mehler et al. Citation2002). However, the clinical features of torsion can be non-specific and can be difficult to distinguish clinically from appendicitis and other causes of acute abdominal pain (Evard et al. Citation1988; Argenta et al. Citation2000; White and Stella Citation2005). On the other hand, egg peritonitis in birds requires rapid and appropriate antibiotic therapy. However, if large amount of yolk is present, surgical intervention should be performed (Hicks-Alldredge Citation1996). Unfortunately, no medical or surgical treatment was administered to the ostrich after the beginning of the clinical signs. In the absence of other significant findings, the death of the animal should be attributed to a multisystemic failure as a result of the follicular ovarian torsion and peritonitis related to the presence of egg yolk material in the body cavity.
References
- Ajudi , G , Nicassio , M , Pagana , G , Silvestre , F and Lacalandra , GM . 2009 . Induction of sexual activity in male and female Ostriches (Struthio camelus) with GnRH implant . Ital J Anim Sci , 8 : 789 – 792 .
- Argenta , PA , Yeagley , TJ , Ott , G and Sondheimer , SJ . 2000 . Torsion of the uterine adnexa. Pathologic correlations and current management trends . J Reprod Med , 45 : 831 – 836 .
- Bronneberg , RGG and Taverne , MAM . 2003 . Ultrasonography of the female reproductive organs in farmed Ostriches (Struthio camelus spp.) . Theriogenology , 60 : 617 – 633 .
- Cabassi , CS , Taddei , S , Predari , G , Galvani , F , Ghidini , F , Schiano , E and Cavirani , S . 2004 . Bacteriologic findings in ostrich (Struthio camelus) eggs from farms with reproductive failures . Avian Dis , 48 : 716 – 722 .
- Degen , AA , Weil , S , Rosenstrauch , A , Kam , M and Dawson , A . 1994 . Seasonal plasma levels of luteinizing and steroid hormones in male and female domestic ostriches (Struthio camelus) . Gen Comp Endocrinol , 93 : 21 – 27 .
- Descargues , G , Tinlot-Mauger , F , Gravier , A , Lemoine , JP and Marpeau , L . 2001 . Adnexal torsion: a report on forty-five cases . Eur J Obstet Gynecol Reprod Biol , 98 : 91 – 96 .
- Dzoma , BM . 2010 . Some factors affecting fertility and hatchability in the farmed ostrich: a review . J Anim Vet Adv , 9 : 229 – 239 .
- Evard , JH , Fischer , AT and Greenwood , LD . 1988 . Ovarian strangulation as a cause of small colon obstruction in a foal . Equine Vet J , 20 : 217 – 218 .
- Fowler , ME . 1991 . Comparative clinical anatomy of ratites . J Wildlife Med , 22 : 204 – 227 .
- Hicks-Alldredge , KD . 1996 . “ Reproduction ” . In Ratite management, medicine and health , Edited by: Tully , TN and Shane , SM . 47 – 57 . Malabar , FL : Krieger Publishing Co .
- Hoey , BA , Stawicki , SP , Hoff , WS , Veeramasuneni , RK , Kovich , H and Grossman , MD . 2005 . Ovarian torsion associated with appendicitis in a 5-year-old girl: a case report and review of the literature . J Pediatr Surg , 40 : 17 – 20 .
- Houry , D and Abbott , JT . 2001 . Ovarian torsion: a fifteen-year review . Ann Emerg Med , 38 : 156 – 159 .
- Karnik , AS , Sainami , NI and Kamat , NN . 2006 . Sequential bilateral torsion of normal ovaries in a prepubertal child . J Clin Ultrasound , 34 : 33 – 37 .
- Kokoska , ER , Keller , MS and Weber , TR . 2000 . Acute ovarian torsion in children . Am J Surg , 180 : 462 – 465 .
- Mehler , SJ , Rosenstein , DS and Patterson , JS . 2002 . Imaging diagnosis-follicular torsion in a green iguana (Iguana iguana) with involvement of the left adrenal gland . Vet Radiol Ultrasound , 43 : 343 – 345 .
- Schmidt RE, Reavill DR, Phalen DN, editors. 2003. Peritoneum and mesenteries. In: Pathology of pet and aviary birds, 1st ed. Iowa: Wiley-Blackwell, p. 216–217
- Sedrish , SA , McClure , JR , Pinto , C , Oliver , J and Burba , DJ . 1997 . Ovarian torsion associated with granulosa-theca cell tumor in a mare . J Am Vet Med Assoc , 211 : 1152 – 1154 .
- Seyrek-Intas , K , Kumru , IH , Seyrek-Intas , D , Salci , H and Wehrend , A . 2011 . Utero-ovarian torsion in a bitch and subsequent fertility after unilateral ovariohysterectomy . Tierarztl Prax K H. , 39 : 268 – 270 .
- Tsafrir Z, Hasson J, Levin I, Solomon E, Lessing JB, Azem F. 2012. Adnexal torsion: cystectomy and ovarian fixation are equally important in preventing recurrence. Eur J Obstet Gynecol Reprod Biol. 162:203–205
- Valk , N , Davis , EW and Blackford , JT . 1998 . Ovarian torsion as a cause of colic in a neonatal foal . J Am Vet Med Assoc , 213 : 1454 – 1456 .
- White , M and Stella , J . 2005 . Ovarian torsion: 10-year perspective . Emerg Med Australas , 17 : 231 – 237 .