Abstract
Cattle can synthesize L-ascorbic acid (or Vitamin C) from either D-glucose or D-galactose through glucuronic acid pathway in the liver. L-Ascorbic acid present in cattle diet is almost totally destroyed by rumen microorganisms making them essentially dependent on its endogenous synthesis, which is assumed sufficient to meet the physiological requirement. Therefore, the role of vitamin C in cattle health and disease has remained widely overlooked. However, there is mounting evidence that the level of L-ascorbic acid in blood and other tissues decreases in association with stress and disease, and Vitamin C supplementation revealed favorable response as evident from early recovery. The present review is an attempt to summarize the existing literature pertaining to the physiological role of L-ascorbic acid and the scope of its supplementation in the prevention and treatment of diseases in cattle. It should be realized that the aqueous solution of vitamin C is highly acidic and subcutaneous or intramuscular administration may cause tissue irritation and inflammation, whereas the sodium ascorbate solution is less acidic and might be used for intramuscular administration.
1. Introduction
Animals have the ability to biosynthesize L-ascorbic acid through glucuronic acid pathway in liver (in mammals) or kidney (in reptile and birds) using either D-glucose or D-galactose (Basu and Schorah Citation1982). Cattle are essentially dependent on this endogenous synthesis as dietary vitamin C is almost totally destroyed by rumen microorganisms (Nockels Citation1988a). No published dietary requirement data of vitamin C are available for adult cattle since endogenous synthesis is assumed sufficient to meet the requirement (Nockels Citation1988b; Hemingway Citation1991). However, several studies have conclusively documented the decrease of vitamin C level in the blood during stress and diseases in cattle and other ruminants (Palludan and Wegger Citation1984; Ali Citation2000; Ranjan et al. Citation2005a). Decrease in the vitamin C level during stress and diseases may be the end result of decreased endogenous synthesis or increased demand or a combination of both. Any condition that decreases the availability of vitamin C precursors, i.e. glucose and galactose, may result in insufficient endogenous synthesis. For example, high milk-producing dairy cows have an elevated demand for glucose by the mammary gland in order to produce lactose, hence they may synthesize less vitamin C (Macleod, Zhang, Ozimeck, et al. Citation1999). However, few authors do not approve this hypothesis. Padilla et al. (Citation2005) observed that even ketotic cows have the ability to produce sufficient vitamin C and concluded that vitamin C synthesis is possibly given a high-metabolic priority. Santos et al. (Citation2001) also reported that high milk production in cattle is not associated with decrease in the plasma Vitamin C level.
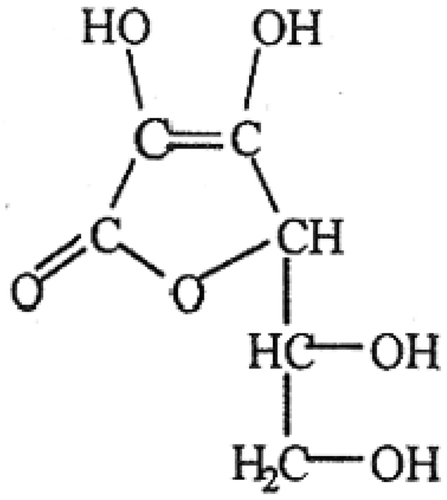
Only L-isomer of vitamin C () and its oxidized derivative L- dehydroascorbic acid has vitamin C activity. L-Dehydroascorbic acid is readily reduced back to the active form L-ascorbic acid in the bodyThe D-isomer, also called iso-ascorbic acid or erythroboric acid, does not have vitamin C-like activity (Basu and Schorah Citation1982).
2. Biosynthesis, distribution, and tissue concentrations
All ruminants including cattle have high endogenous capacity for the synthesis of L-ascorbic acid. However, calves are unable to synthesize vitamin C until two–three weeks of age, hence are totally dependent upon its supply through colostrum and milk. Fresh and frozen colostrums are satisfactory sources of vitamin C, but stored unfrozen colostrums rapidly lose their vitamin C content (Hemingway Citation1991).
Vitamin C circulates freely in plasma, leukocytes, and red blood cells and enters into all tissues and body fluids in human beings (Iqbal et al. Citation2004). It has been recorded that vitamin C is actively transported into human blood leukocytes, thereby maintaining a high vitamin C concentration unless vitamin C deficiency occurs (Washko et al. Citation1989). High vitamin C concentration can be recorded in pituitary and adrenal glands, followed by spleen, liver, brain, and kidneys of adult cattle (Kolb et al. Citation1989). Thymus, corpus-luteum, retina, testicles, thyroid glands, lymph nodes, pancreas, and salivary glands also have high vitamin C concentration ( Citation2002 L- L- Citation1997
Measurement of vitamin C levels has been largely confined to different biological fluids, plasma, and whole blood. Vitamin C concentration in leukocytes seems to be an accurate index of body status in man (Washko et al. Citation1989). Normal vitamin C concentration in the blood of cattle and other ruminant species as reported by different authors is given in . Blood vitamin C level does not change in response to lactation or the number of lactations (Santos et al. Citation2001). However, certain other factors such as season in bulls (Kolb et al. Citation1991), stage of oestrus cycle and pregnancy in cows (Ataman et al. Citation2010), and the type of housing system in calves (Cummins and Brunner Citation1991) may have significant influence.
Table 1. Plasma concentration (mg/L) of ascorbic acid in healthy cattle and other domestic ruminants.
3. Biochemical functions
Vitamin C is a powerful reducing agent required in the hydroxylation of proline and lysine for the biosynthesis of collagen in human beings (Gaby and Singh Citation1991). Collagen and hence vitamin C support wound healing and the formation of blood vessels, bone, and teeth. In addition, vitamin C plays a protective role at many cellular and sub-cellular levels by preventing free radical-induced damage ( Citation1986). Vitamin C concentration in the aqueous humor of cattle is several times higher than its plasma concentration and the level is maintained both by active transport (Helbig et al. Citation1990) and regeneration by ciliary body (Bode et al. Citation1993).
Vitamin C Vitamin C Citation1986 is required for the synthesis of several hormones and neurotransmitters. It acts as a true co-substrate for dopamine beta-monooxygenase for the biosynthesis of norepinephrine from dopamine in bovine granulosa cells (Dhariwal et al. Citation1991). It also acts as a reducing agent for the synthesis of aldosterone in the adrenal gland (Yanagibashi et al. Citation1990) and oxytocin in granulosa cells of corpus luteum (Luck and Jungclas Citation1988). Vitamin C regulates oxytocin secretion through direct interaction with catecholamines (Luck and Jungclas Citation1987). In humans, vitamin C is required for the biosynthesis of carnitine and the catecholamines that regulate the nervous system (Iqbal et al. Citation2004). In in vitro cell culture, vitamin C has been demonstrated to play a critical role in the production and release of corticosteroids in the bovine adrenal glands (Finn and Johns Citation1980).
In myeloid leukemia cell culture, the addition of Vitamin C in high doses has been reported to induce oxidative damage inside the cell, suggesting that vitamin C in high concentration may play a pro-oxidant role (Osiecki et al. Citation2010).
4. Ascorbic acid and immunity
Vitamin C is required for collagen synthesis and hence maintenance of skin and epithelial linings of the body orifices (Gaby and Singh Citation1991). Neutrophils contain about 40–60 times higher vitamin C concentration than plasma. Motility and phagocytic capacity of neutrophils are enhanced following vitamin C supplementation (Roth and Kaeberle Citation1985). Vitamin C concentration in neutrophils is rapidly depleted in acute disease, infection, corticosteroid administration, and trauma in human beings (Basu and Schorah Citation1982). Vitamin C protects neutrophils from oxidative damage and promotes oxidative killing of bacteria inside neutrophils (Goldschmidt et al. Citation1988; Wang et al. Citation1997). Vitamin C also supports normal lymphocyte proliferation, stimulates interferon production (Gerber Citation1975), and reduces the suppressor activity of mononuclear leukocytes (Gaby and Singh Citation1991). Low blood vitamin C level in camels may compromise their resistance against infectious diseases (Mohamed et al. Citation2011).
5. Ascorbic acid in cattle diseases
5.1. Heat stress
The plasma vitamin C level in cattle declines during heat stress (Padilla et al. Citation2006). Few research reports also document the beneficial effects of Vitamin C supplementation in goats (Kobeisy Citation1997; Sivakumar et al. Citation2010). Supplementation of ascorbic acid polyphosphate along with salts (sodium bicarbonate and potassium carbonate) was found effective in reducing heat stress in buffaloes (Kumar et al. Citation2011). In goats, vitamin C supplementation has been reported to ameliorate the stress-induced hemolysis of erythrocytes after road transportation for 12 hours during hot-dry season (Minka and Ayo Citation2010).
5.2. Neonatal diseases
Milk is a poor source of vitamin C as the average concentration in whole milk of cow and goat is about 1–2 mg/100 mL (Renner Citation1983), which is inadequate to fulfill the requirement of newborn animals. The recommended level of vitamin C in dry milk replacer for calves is 2000 mg/kg. Endogenous vitamin C production in the calf does not start until two–three weeks of age and does not reach a maximum until 8–16 weeks (Palludan and Wegger Citation1984). As a consequence, vitamin C level in blood decreases after birth up to three months of age (Kolb Citation1992). Furthermore, vitamin C level in blood is also influenced by several factors, such as genetic potential of the individual animal to produce vitamin C (Palludan and Wegger Citation1984), the presence of environmental stressors (Cummins and Brunner Citation1989), and management factors (Cummins and Brunner Citation1991).
Decrease in vitamin C level has been noted in calves in association with many diseases. Dermatosis in calves commencing on the ears and spreading to neck and chest has been associated with low level of vitamin C (Radostits et al. Citation1994). Scurvy-like disease has also been reported occasionally in adult cattle (Duncan et al. Citation1944). Several studies have documented significant decrease in plasma vitamin C level in pneumonia (Jagos et al. Citation1977) and enteric infections (Hemingway Citation1991; Seifi et al. Citation1996) in calves. It has been hypothesized that the demand of vitamin C increases in various infections due to increased tissue requirements (Hemingway Citation1991). Beneficial effects of vitamin C supplementation on calf diarrhea cases further consolidate this hypothesis (Cummins and Brunner Citation1989; Sahinduran and Albay Citation2004). It may also be possible that the decrease in vitamin C concentration leads to compromise in immunity and predisposes the animal to various diseases. An immune-stimulatory effect of vitamin C supplementation has already been reported in dairy calves (Cummins and Brunner Citation1991). Response of vitamin C supplementation on immune function, however, varies with age. It increases the plasma concentration of IgG in calves less than two months old (Cummins and Brunner Citation1989) and IgM in calves older than two months (Hidiroglou et al. Citation1995).
5.3. Reproductive problems
Perhaps, the first use of vitamin C supplementation for therapeutic purposes in adult ruminant was for the treatment of infertility. Subcutaneous administration of 2.2–4.4 mg/kg BW vitamin C on every third–fourth day claimed to improve the performance of bulls with poor breeding records (Daykin Citation1960). Similar dosages were also recommended in cows to improve the conception rate. The administration of vitamin C during the period of hormonal stimulation in women has showed a positive though statistically insignificant impact in terms of higher number of pregnancies (Crha et al. Citation2003). Vitamin C concentrations vary according to the follicle size, functional status, and the stage of estrous cycle, suggesting a role in the process of follicular development during the estrous cycle in buffalo (Khan and Das Citation2011). In ovaries collected from slaughterhouse, it was found that small- and medium-sized follicles of acyclic buffaloes had lower vitamin C concentrations than the corresponding size estrogen-active follicles of their cyclic counterparts (Khan and Das Citation2012). Addition of vitamin C in the in vitro culture medium has been reported to increase the rate of buffalo embryo development (Saikhun et al. Citation2008). However, the exact mechanism by which vitamin C supplementation improves breeding performance in cattle still remains inconclusive. Beneficial effects of vitamin C supplementation on male fertility in humans have also been investigated by several researchers. It prevents sperm agglutination, resulting into poor fertility in men (Dawson et al. Citation1983). Bull semen also has little endogenous antioxidants. Addition of optimum quantity of vitamin C to semen extender can help protect spermatozoa from damages during storage and handling induced by free radicals (Foote et al. Citation2002). However, vitamin C is also reported to enhance iron-induced oxidative damage to spermatozoa (Dawra et al. Citation1983) and impairing the success rate of bovine in vitro fertilization (Daivit et al. Citation1998). Thus, debate continues over its role in male fertility.
In females, vitamin C has multiple roles in reproductive physiology. During ovarian folliculogenesis, it plays an important role in collagen biosynthesis, steroidogenesis, and apoptosis (Luck and Zhao Citation1993). In vitro study has highlighted the role of vitamin C in the maintenance of ovarian follicle health and basement membrane remodeling in bovines (Thomas et al. Citation2001). As discussed earlier, ascorbate regulates the biosynthesis and release of oxytocin. Of interest, low vitamin C level in placental tissues has been reported in retained placenta cases (Kankofer Citation2001). Significant decline in plasma total vitamin C concentration in dairy cows has been reported during the periparturient period (Tanaka et al. Citation2011).
5.4. Mastitis
Several research reports document decreased vitamin C concentration in milk and plasma in bovine and caprine mastitis (Steffert Citation1993; Gupta et al. Citation1999; Ranjan et al. Citation2005a). Parenteral administration of vitamin C alone or along with intramammary antibiotics improves the cure rate in spontaneous cases of bovine mastitis (Chaiyotwittayakun et al. Citation2002; Naresh et al. Citation2002; Ranjan et al. Citation2005b). Vitamin C along with cupric ions as teat dip or intra-mammary infusion also helps in the prevention and treatment of mastitis in dairy cows (Upton Citation1988). Weiss et al. (Citation2004) observed large decrease in vitamin C level in Escherichia coli-induced mastitis. However, in another study, intravenous administration of vitamin C in cows that received an intramammary challenge of endotoxin had limited positive effects on clinical signs (Chaiyotwittayakun et al. Citation2002). Santos et al. (Citation2001) reported that plasma vitamin C concentrations in dairy cows do not correlate with somatic cell counts in milk.
5.5. Parasitic diseases
Decrease in vitamin C level in few parasitic diseases including theileriosis (Sangwan and Sangwan Citation1999) and fasciolosis (Curca and Tanasescu Citation1985) are on record. Lower level of plasma vitamin C was also reported in sheep infected with Fasciola hepatica (Gameel Citation1982).
6. Ascorbic acid supplementation
L-Ascorbic acid is extremely unstable in rumen environment, where it is quickly degraded with a half-life of 3.5 hours (Macleod, Zhang, Kennelly, et al. Citation1999). Sodium and calcium ascorbyl 1-2-phosphate are more stable with a half-life of 6.9 hours (Macleod, Zhang, Kennelly, et al. Citation1999) and greater bioavailability after oral dosing (Weiss Citation2001). Oral supplementation of ascorbyl-2-polyphosphate at 40 g/day for five days has been reported to increase the plasma vitamin C concentration in cattle (Macleod, Zhang, Ozimeck, et al. Citation1999). Supplementation of vitamin C at 100 mg/kg BW to goats before road transportation has been reported to mitigate the risk of adverse effects of high-temperature humidity index values and other stress factors (Minka and Ayo Citation2012). Coating of vitamin C with ethyl cellulose, silicone, or hydrogenated soybean oil also enhances its oral bioavailability in cattle (Hidiroglou Citation1999; Padilla et al. Citation2007). Parenteral and perhaps intravenous administration is the best route for vitamin C supplementation in ruminants. Of note, the aqueous solution of vitamin C acid is highly acidic and subcutaneous or intramuscular administration may cause tissue irritation and inflammation (Roth and Kaeberle Citation1985). However, sodium ascorbate solution is less acidic and can be used for intramuscular administration (The Pharmaceutical Press Citation1979).
7. Conclusion
Ascorbic acid is a strong antioxidant nutrient present in blood plasma, neutrophils, and other body tissues. It has diverse physiological functions and plays an important role in the regulation of immune function and offers protection from oxidative damage at many cellular and sub-cellular levels. Though cattle can synthesize vitamin C from D-glucose or D-galactose, several reports suggest the decrease in its tissue concentrations during various diseases and stress and beneficial effects of its supplementation on the maintenance of health and fertility. However, extensive research is required to explore the need and effect of ascorbic acid supplementation to cattle. Alleviation of heat stress, management of calf diarrhea, mastitis, and infertility are some of the potential conditions in which vitamin C supplementation may be helpful. Development of rumen stable vitamin C supplements and dose standardization is essential for its large-scale use regarding preventive and therapeutic purposes.
Notes
.
Hediger
). In a study in mice, it was found that
ascorbic acid does not cross the blood
–
brain barrier, but
dehydroascorbic acid easily crosses the blood
–
brain barrier through glucose transporters (
Agus et al.
).
Englard and Seifter
is regarded to have critical functions in the brain including its role as a cofactor of dopamine β-hydroxylase, and is thereby involved in catecholamine biosynthesis.
inhibits peroxidation of membrane phospholipids and acts as a scavenger of free radicals in
the
brain (
Englard and Seifter
) and
References
- Agus , DB , Gambhir , SS , Pardridge , WM , Spielholz , C , Baselga , J , Vera , JC and Golde , DW . 1997 . Vitamin C crosses the blood–brain barrier in the oxidized form through the glucose transporters . J Clin Invest , 100 : 2842 – 2848 .
- Ali , AA . 2000 . Influence of some diseases conditions on blood serum levels of antioxidant vitamins and some trace elements of Egyptian Balady sheep in Assuit Governorate . Assuit Vet Med J , 42 : 120 – 133 .
- Ataman , MB , Erdem , H , Bulbul , B , Haliloglu , S , Cinar , M and Akoz , M . 2010 . Plasma β-carotene, vitamin A and vitamin C levels in cyclic and pregnant cows . Kafkas Univ Vet Fak Derg , 16 : 579 – 584 .
- Basu , TK and Schorah , CJ . 1982 . Vitamin C in health and disease , London and Canberra : Croom Helm .
- Bode , AM , Green , E , Yavarow , CR , Wheeldon , SL , Bolken , S , Gomez , Y and Rose , RC . 1993 . Ascorbic acid regeneration by bovine iris-ciliary body . Curr Eye Res , 12 : 593 – 601 .
- Chaiyotwittayakun , A , Erskine , RJ , Bartlett , TH , Herdt , PM and Harmon , RJ . 2002 . The effect of ascorbic acid and L-histidine therapy on acute mammary inflammation in dairy cattle . J Dairy Sci , 85 : 60 – 67 .
- Chanda , R . 1958 . The effect of thyroxine on phosphatise, ascorbic acid and tocopherol content in the blood and milk of the cow and the buffalo . Curr Sci India , 3 : 102 – 103 .
- Crha , I , Hruba , D , Ventruba , P , Fiala , J , Totusek , J and Visnova , H . 2003 . Ascorbic acid and infertility treatment . Cent Eur J Public Health , 11 : 63 – 67 .
- Cummins , KA and Brunner , CJ . 1989 . Dietary ascorbic acid and immune response in dairy calves . J Dairy Sci , 72 : 129 – 134 .
- Cummins , KA and Brunner , CJ . 1991 . Effect of calf housing on plasma ascorbate and endocrine and immune function . J Dairy Sci , 74 : 1582 – 1588 .
- Curca , D and Tanasescu , TC . 1985 . Ascorbic acid content in the liver and adrenal glands of cattle with parasitic liver disease . Med Vet , 28 : 55 – 59 .
- Daivit , GC , Cetica , PD and Beconi , MT . 1998 . Effect of alpha-tocopherol and ascorbic acid on bovine in vitro fertilization . Theriogenology , 49 : 619 – 627 .
- Dawra , RK , Sharma , OP and Makkar , HP . 1983 . Lipid peroxidation in bovine semen . Int J Fertil , 28 : 231 – 234 .
- Dawson , EB , Harris , WA and McGanity , WJ . 1983 . Effect of ascorbic acid on sperm fertility . Fed Proc , 42 : 531
- Daykin , PW . 1960 . Veterinary applied pharmacology and therapeutics , 619 – 621 . London : Bailliere Tindall and Cassell .
- Dhariwal , KR , Black , CD and Levine , M . 1991 . Semidehydroascorbic acid as an intermediate in norepinephrine biosynthesis in chromaffin granules . J Biol Chem , 226 : 12908 – 12914 .
- Duncan , CW , Huffman , CF , Mitchell Jr , R and Reid , JT . 1944 . Symptom of scurvy observed in a herd of cattle . J Dairy Sci , 24 : 636
- Englard , S and Seifter , S . 1986 . The biochemical functions of ascorbic acid . Annu Rev Nutr , 6 : 365 – 406 .
- Finn , FM and Johns , PA . 1980 . Ascorbic acid transport by isolated bovine adrenal cortical cells . Endocrinology , 106 : 811 – 817 .
- Foote , RH , Brockett , CC and Kaproth , MT . 2002 . Motility and fertility of bull sperm in whole milk extender containing antioxidants . Anim Reprod Sci , 71 : 13 – 23 .
- Gaby , SK and Singh , VN . 1991 . “ Vitamin C ” . In Vitamin intake and health: a scientific review , Edited by: Gaby , SK , Bendich , A , Singh , VN and Machlin , L . 103 – 1043 . New York : Marcel Dedder .
- Gameel , AA . 1982 . Plasma ascorbic acid levels in sheep experimentally infected with Fasciola hepatica . Z Parasitenkd , 669 : 321 – 326 .
- Gerber , WF . 1975 . Effect of ascorbic acid, sodium salicylate and caffeine on the serum interferon level in response to viral infection . Pharmacology , 13 : 228
- Goldschmidt , MC , Masin , WJ , Brown , LR and Wyde , PR . 1988 . The effect of ascorbic acid deficiency on leukocyte phagocytosis and killing of Actinomyces viscosus . Int J Vitam Nutr Res , 58 : 326 – 334 .
- Gupta , VK , Kumar , A and Sharma , SD . 1999 . Plasma ascorbic acid in healthy and subclinical mastitic goats . Indian J Vet Med , 19 : 83 – 85 .
- Hediger , MA . 2002 . New view at vitamin C . Nat Med , 8 : 445 – 446 .
- Helbig , H , Korbmacher , C and Wiederholt , M . 1990 . Mechanism of ascorbic acid transport in the aqueous humor . Fortschr Ophthalmol , 87 : 421 – 424 .
- Hemingway , DC . 1991 . Vitamin C in the prevention of neonatal calf diarrhoea . Can Vet J , 32 : 184
- Hidiroglou , M , Batra , TR , Ivan , M and Markham , F . 1995 . Effect of supplemental vitamins E and C on the immune response of calves . J Dairy Sci , 78 : 1578 – 1583 .
- Hidiroglou , M . 1999 . Technical note: forms and route of vitamin C supplementation for cows . J Dairy Sci , 82 : 1831 – 1833 .
- Iqbal , K , Khan , A and Khattak , MAK . 2004 . Biological significance of ascorbic acid (vitamin C) in human health – a review . Pakistan J Nutr , 3 : 5 – 13 .
- Jagos , P , Bouda , J and Dvorrak , R . 1977 . Ascorbic acid levels in the bronchopneumonia of calves . Vet Med (Praha) , 22 : 133 – 136 .
- Kankofer , M . 2001 . Non-enzymatic antioxidative defense mechanisms against reactive oxygen species in bovine-retained and not-retained placenta: vitamin C and glutathione . Reprod Domest Anim , 36 : 203 – 206 .
- Khan , FA and Das , GK . 2011 . Follicular fluid nitric oxide and ascorbic acid concentrations in relation to follicular size, functional status and stage of estrous cycle in buffalo . Anim Reprod Sci , 125 : 62 – 68 .
- Khan , FA and Das , GK . 2012 . Follicular characteristics and intrafollicular concentrations of nitric oxide and ascorbic acid during ovarian acyclicity in water buffalo (Bubalus bubalis) . Trop Anim Health Prod , 44 : 125 – 131 .
- Kobeisy . 1997 . Effect of vitamin C and E on rectal temperature and respiratory rates in heat stressed goats . Assiut Vet Med J , 37 : 120 – 132 .
- Kolb , E , Dittrich , H , Dobeleit , G , Schmalfuss , R , Siebert , P , Stauber , E and Wahren , M . 1991 . The content of beta-carotene, vitamin A and ascorbic acid in different tissues of bulls, short-scrotum bulls and oxen of different body weights . Berl Munch Tierarztl , 104 : 423 – 427 .
- Kolb , E , Wahren , M , Dobeleit , G and Grundel , G . 1989 . The content of ascorbic acids in different tissues of cattle, normally developed piglets, splay-legged piglets, adult swine and dogs . Ach Exp Vet Med , 43 : 327 – 334 .
- Kolb , E . 1992 . Recent findings of the significance of ascorbic acid for domestic animals and its use in veterinary medicine . Tierarztl Umsch , 47 : 163 – 175 .
- Kumar , A , Singh , G , Kumar , BVS and Meur , SK . 2011 . Modulation of antioxidant status and lipid peroxidation in erythrocyte by dietary supplementation during heat stress in buffaloes . Livest Sci , 138 : 299 – 303 .
- Luck , MR and Jungclas , B . 1987 . Catecholamines and ascorbic acid as stimulators of bovine ovarian oxytocin secretion . J Endocrinol , 114 : 423 – 430 .
- Luck , MR and Jungclas , B . 1988 . The time-course of oxytocin secretion from cultured bovine granulose cells, stimulated by ascorbate and catecholamines . J Endocrinol , 116 : 247 – 258 .
- Luck , MR and Zhao , Y . 1993 . Identification and measurement of collagen in the bovine corpus luteum and its relationship with ascorbic acid and tissue development . J Reprod Fertil , 99 : 647 – 652 .
- Macleod , DD , Zhang , X , Kennelly , JJ and Ozimek , L . 1999 . Pharmacokinetics of ascorbic acid and ascorby L-2-polyphosphate in the rumen fluid of dairy cows . Milchwissenschaft , 54 : 63 – 65 .
- Macleod , DD , Zhang , X , Ozimeck , L and Kennelly , JJ . 1999 . Ascorby L-2-polyphosphate as a source of ascorbic acid for dairy cattle . Milchwissenschaft , 54 : 123 – 126 .
- Mac-Pherson , A . 1983 . Plasma ascorbic acid in sheep as affected by cobalt status . Proceedings of Workshop on Ascorbic Acid in Domestic Animals . 1983 , Skjoldenaesholm . pp. 148 – 151 .
- Minka , NS and Ayo , JO . 2010 . Physiological responses of erythrocytes of goats to transportation and the modulatory role of ascorbic acid . J Vet Med Sci , 72 : 875 – 881 .
- Minka , NS and Ayo , JO . 2012 . Assessment of thermal load on transported goats administered with ascorbic acid during the hot-dry conditions . Int J Biometerol , 56 : 333 – 341 .
- Mohamed , HE , Alhaidary , A and Beynen , AC . 2011 . Ascorbic acid status of female camels during different phases of reproduction . Trop Anim Health Pro , 43 : 279 – 281 .
- Mohamed , HE , Mousa , HM and Beynen , AC . 2004 . Vitamin C status of Sudanese cattle and sheep . J Biol Sci , 4 : 778 – 779 .
- Naresh , R , Dwivedi , SK , Swarup , D and Patra , RC . 2002 . Evaluation of ascorbic acid treatment in clinical and subclinical mastitis of dairy cows . Asian Austral J Anim Sci , 15 : 905 – 911 .
- Nockels , CF . 1988a . The role of vitamin in modulating disease resistance . Vet Clin N Am Food A , 4 : 531 – 532 .
- Nockels , CF . 1988b . Immunoenhancing vitamins for cattle . Agri Pract , 9 : 10 – 17 .
- Osiecki , M , Ghanavi , P , Atkinson , K , Nielsen , LK and Doran , MR . 2010 . The ascorbic acid paradox . Biochem Bioph Res Co , 400 : 466 – 470 .
- Padilla , L , Matsui , T , Ikeda , S , Kitagawa , M and Yano , H . 2007 . The effect of vitamin C supplementation on plasma concentration and urinary excretion of vitamin C in cattle . J Anim Sci , 85 : 3367 – 3370 .
- Padilla , L , Matsui , T , Kamiya , Y , Kamiya , M , Tanaka , M and Yano , H . 2006 . Heat stress decreases plasma vitamin C concentration in lactating cows . Livest Sci , 101 : 300 – 304 .
- Padilla , L , Shibano , K , Inoue , J , Matsui , T and Yano , H . 2005 . Plasma vitamin C concentration is not related to incidence of ketosis in dairy cows during the early lactation period . J Vet Med Sci , 67 : 883 – 886 .
- Palludan , B and Wegger , I . 1984 . “ Plasma ascorbic acid in calves ” . In Ascorbic acid in domestic animals , Edited by: Wegger , I , Tagwerker , F and Moustgaard , J . 131 – 138 . Copenhagen , , Denmark : Royal Danish Agricultural Society .
- Radostits , OM , Blood , DC and Gay , CC . 1994 . Veterinary medicine: a textbook of diseases of cattle, sheep, pigs, goats and horses , London : ELBS with Bailliere Tindall .
- Ranjan , R , Swarup , D , Naresh , R and Patra , RC . 2005a . Enhanced erythrocytic lipid peroxides and reduced plasma ascorbic acid, and alteration in blood trace elements level in dairy cows with mastitis . Vet Res Commun , 29 : 27 – 34 .
- Ranjan , R , Swarup , D , Naresh , R and Patra , RC . 2005b . Ameliorative potential of L-ascorbic acid in bovine clinical mastitis . Indian J Anim Sci , 75 : 174 – 177 .
- Renner , E . 1983 . Milk and dairy products in human nutrition , Munich , , Germany : WG Mott. University of Glessen .
- Roth , JA and Kaeberle , ML . 1985 . In vivo effect of ascorbic acid on neutrophil function in healthy and dexamethasone treated cattle . Am J Vet Res , 46 : 2434 – 2436 .
- Sahinduran , S and Albay , MK . 2004 . Supplemental ascorbic acid and prevention of neonatal calf diarrhoea . Acta Vet Brno , 73 : 221 – 224 .
- Saikhun , K , Faisaikam , T , Ming , Z , Lu , KH and Kitiyanant , Y . 2008 . Alfa-tocopherol and L-ascorbic acid increase the in vitro development of IVM/IVF swamp buffalo (Bubalus bubalis) embryos . Animal , 2 : 1486 – 1490 .
- Sangwan , N and Sangwan , AK . 1999 . Thiamin, riboflavin and ascorbic acid in relation to tropical theileriosis in cattle . Indian J Anim Sci , 69 : 316 – 317 .
- Santos , MV , Lima , FR , Rodrigues , PHM , Barros , SBM and daFonseca , LFL . 2001 . Plasma ascorbate concentrations are not correlated with milk somatic cell count and metabolic profile in lactating and dry cows . J Dairy Sci , 84 : 134 – 139 .
- Seifi , HA , Mokhber Dezfuly , MR and Bolurchi , M . 1996 . The effectiveness of ascorbic acid in the prevention of calf neonatal diarrhoea . J Vet Med B , 43 : 189 – 191 .
- Sivakumar , AVN , Singh , G and Varshney , VP . 2010 . Antioxidants supplementation on acid base balance during heat stress in goats . Asian Austral J Anim Sci , 23 : 1462 – 1468 .
- Steffert , IJ . (1993). Compositional changes in cows milk associated with health problem. Bull Milk Fat Flavour Forum, New Zealand Dairy Research Institute, Palmerston, pp. 119–125
- Tanaka , M , Kamiya , Y , Suzuki , T and Nakai , Y . 2011 . Changes in oxidative status in periparturient dairy cows in hot conditions . Anim Sci J , 82 : 320 – 324 .
- The Pharmaceutical Press . 1979 . The pharmaceutical codex , 11th , London : The Pharmaceutical Press .
- Thomas , FH , Leask , R , Srsen , V , Riley , SC , Spears , N and Telfer , EE . 2001 . Effect of ascorbic acid on health and morphology of bovine preantral follicle during long-term culture . Reproduction , 122 : 487 – 495 .
- Upton , P . (1988). Method for treating or preventing bovine mastitis. United States Patent, US, 4, 782, 048, p.4
- Wang , Y , Russo , TA , Kwon , O , Chanock , S , Rumsey , SC and Levine , M . 1997 . Ascorbate recycling in human neutrophils: induction by bacteria . Proc Natl Acad Sci USA , 94 : 13816 – 13819 .
- Washko , P , Rotrosen , D and Levine , M . 1989 . Ascorbic acid transport and accumulation in human neutrophils . J Biol Chem , 264 : 8996 – 9002 .
- Weiss , WP , Hogan , JS and Smith , KL . 2004 . Changes in vitamin C concentrations in plasma and milk from dairy cows after an intramammary infusion of Escherichia coli . J Dairy Sci , 87 : 32 – 37 .
- Weiss , WP . 2001 . Effect of dietary vitamin C on concentrations of ascorbic acid in plasma and milk . J Dairy Sci , 84 : 2302 – 2307 .
- Yanagibashi , K , Kobayashi , Y and Hall , PF . 1990 . Ascorbate as a source of reducing equivalents for the synthesis of aldosterone . Biochem Biophys Res Commun , 170 : 1256 – 1262 .