Abstract
This is a report of seven-year-old male Akita mixed dog, with protein-losing enteropathy (PLE). He had a history of chronic vomiting and diarrhea with anorexia/hyporexia. Previously he suffered acute abdomen about eight months prior to this visit. Our dog showed uncommon combination of diseases that could cause PLE since it was affected by inflammatory bowel disease (IBD), intestinal lymphangiectasia (IL), and exocrine pancreatic insufficiency (EPI). The dog had most of the abnormalities found in IL, as well as hypoalbuminemia, hyperglobulinemia, lymphopenia, hypocalcemia, and hypercholesterolemia. During endoscopy exam, we found changes characteristic of IL such as irregular small white spots. We took biopsies from stomach, duodenum, and cecum. These biopsies showed infiltration by lymphocytes and plasmatic cells in the lamina propria also, the duodenal biopsies showed moderate dilation of the lymphatic vessels. The patient had 2.1 µg/mL of TLI, this result was compatible with EPI. We assume that the first pathology in this animal was IBD, which caused chronic pancreatitis (CP) that in turn progressed to EPI. It is also possible that IL was secondary to IBD. We have reported for the first time the correlation of IBD and EPI in dogs. This should change our approach to treating chronic diarrhea in dogs. Therefore, we propose that dogs diagnosed with EPI should also be subjected to endoscopy and intestinal biopsy. Similarly, to rule out secondary EPI, TLI should be measured routinely in dogs with IBD.
A 7-year-old male Akita mixed breed dog was brought to the University Veterinary Hospital. He had a history of chronic vomiting and diarrhea with anorexia/hyporexia and had suffered from an acute abdomen about eight months prior to admission. The animal was also experiencing abdominal pain relapses at least once a week, during which vomiting, diarrhea and anorexia would worsen. The body condition of the patient was 2/5 according to the Purina scale and weighed 26.6 kg (normal body weight estimated at 40 kg). During the physical exam, he seemed to experience mild abdominal pain and its mucosal membranes were slightly pale.
The patient had previously been treated with metronidazole, omeprazole, and oral metoclopramide. He had also been placed on a 10-week-long hypoallergenic commercial diet (Hill's Prescription Diet Z/D Dry). The owner explained that her dog would improve while on medication, but would relapse following suspension of medication. Blood biochemistry and hematology tests have been performed a week before admission ().
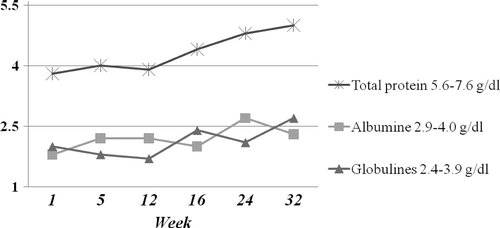
Figure 1. Serum protein concentration in a seven-year-old male Akita mixed breed dog over time showing a slight increase in total protein associated with a gradual but erratic increase of albumin and globulins.
Serial fecal exams (daily for three days) with zinc sulfate flotation showed no parasites, and ELISA tests for Giardia spp, Ehrlichia canis, and Dirofilaria immitis were negative. Abdominal X-rays and gastrointestinal transit appeared normal. In addition, abdominal ultrasound did not show hyperechoic mucosal striations indicative of intestinal lymphangiectasia or suggestive images of pancreatic abnormalities. Finally, urine test showed no abnormalities.
After receiving the laboratory report we sent the patient for a gastroduodenoscopy and colonoscopy. We also ran a test to measure trypsin-like immunoreactivity (TLI) (conducted by Carpermor Laboratories, Mexico City, Mexico). The patient had 2.1 µg/L of TLI (normal range 5.0–35.0 µg/L).
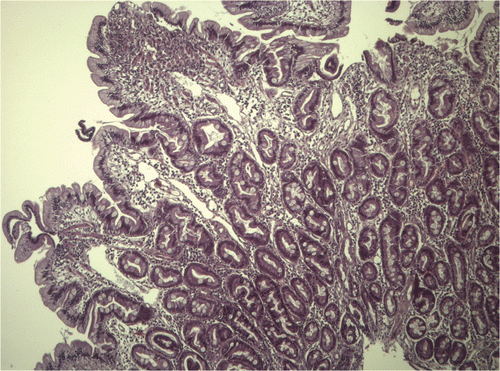
During the endoscopy, the esophagus and stomach showed no macroscopic changes. The lower esophageal sphincter had a normal tone, whereas the duodenum showed hyperemia and edema and bled more than normal. It also had white spots (). Colonic endoscopy showed no macroscopic changes. Eight biopsies were taken from each segment inspected. The gastric, duodenal, and colon biopsies showed inflammation caused by lymphocytes and plasma cells in the lamina propria (). The duodenal biopsies also showed moderate dilation of the lymphatic vessels ().
Figure 2. Endoscopic image of the duodenum in a seven-year-old male Akita mixed breed dog showing scattered white spots on the mucosa.
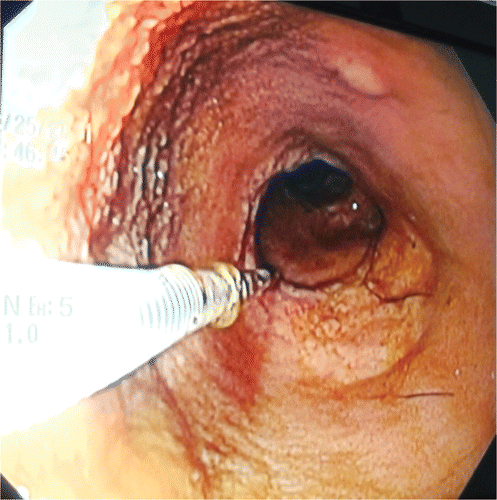
Figure 3. Microphotograph of gastric mucosa showing infiltration of lymphocytes and plasma cells in the lamina propia with intermingling fibrosis. (Haematoxylin-eosin, original magnification ×100).
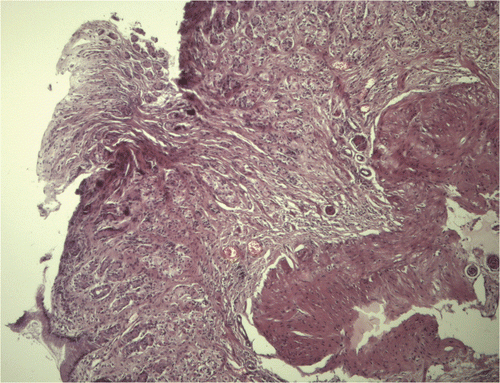
Figure 4. Microphotograph of duodenal mucosa showing moderate dilatation of lymphatic vessels in addition to moderate infiltration of lymphocytes and plasma cells in the lamina propria. Also note the marked thickening and shortening of the mucosal villi. (Haematoxylin-eosin, original magnification ×100).
The animal subsequently received oral prednisolone, starting with 60 mg every 24 hours for 15 days, then 30 mg every 24 hours for 15 days, and then 30 mg every 48 hours for 10 more days. He was also administered metronidazole (500 mg every 12 hours for 10 days) and omeprazole (20 mg every 24 hours) orally. His diet was replaced by a low-fat diet comprising one–two cups of spaghetti without salt, baked potatoes, and one–two cans of tuna (in water). The homemade diet was changed gradually to commercial low-fat diet (Hill's Prescription Diet Canine w/d). The dog was also administered pancreatic enzyme tablets with every meal (Viokase-V).
The dog had a good appetite and appeared to adapt well to the commercial low-fat diet, but was still having episodes of vomiting and diarrhea twice a week. The patient was checked 10 days after the endoscopy and had gained 1.4 kg of body weight up to 28 kg in total. The recommended treatment was continued. The animal was weighed every week and by the third week he weighed 33 kg. However, vomiting, diarrhea, and abdominal pain recommenced, once or twice a week. By the fifth week, the patient's weight had declined to 27 kg. As a consequence, azathioprine was administered orally for 20 days (50 mg every 24 hours). The patient's body weight was still monitored every week and he was administered omeprazole and metoclopramide orally when he vomited, two or three times a week. Diet was changed to commercial intestinal diet (Hill's Prescription Diet Canine i/d). Since then, the dog was eating alternately w/d and i/d.
The patient has continued to gain weight since then, but vomiting episodes occur about once a week, although in small amounts only. The diarrhea has ceased and he has normal eating habits. The maximum body weight attained was 33 kg. However, after suspension of azathioprine administration he lost weight again, decreasing to about 29–30 kg. For this reason, this drug was reinstated and at the time of writing has been administered for the last 30 days.
Protein-losing enteropathy (PLE) is caused by a variety of gastrointestinal diseases causing the intestinal loss of albumin and globulin. If PLE is untreated, the final outcome is panhypoproteinemia with a reduction in intravascular oncotic pressure and the development of abdominal and pleural effusion, peripheral edema, and death. Chronic diarrhea, sporadic vomiting, lethargy, anorexia, and weight loss are the characteristic symptoms of PLE, all of which were presented by the dog described in this report. PLE can be caused by inflammatory bowel disease (IBD), intestinal lymphangiectasia (IL), parasites (e.g. Giardia sp.), food, ulcerations, and neoplasms (mainly malignant lymphoma). Moreover, exocrine pancreatic insufficiency (EPI) can also cause hypoproteinemia in dogs (Marks Citation2008) although not primarily, but only after secondary changes of the intestinal mucosa by bacterial overgrowth or inflammation due to abnormal bile-acids or unhydrolysed fatty acids. In our patient a combination of diseases may have caused PLE.
IBD is a common pathology in dogs and is clinically defined by the WSAVA as a spectrum of gastrointestinal disorders associated with inflammation of the gastrointestinal tract of unknown etiology (Washabau Citation2005). In dogs, lymphoplasmacytic infiltrates are the most common (German Citation2009; Guímaro Citation2010).
By definition, IL is a disorder characterized by dilated intestinal lacteals resulting in lymph leakage into the small bowel lumen being responsible for PLE in dogs (Van Kruiningen et al. Citation1984; Papendieck Citation2008). In addition to digestive symptoms, dogs can present edema alongside the body or limbs, ascites, or pleural effusion. The clinical presentation is usually chronic with development over weeks to months (Brooks Citation2005).
The dog presented here had most of the abnormalities found in IL, including hypoalbuminemia, hypoglobulinemia, lymphopenia, hypocalcemia, and hypercholesterolemia. Lymphopenia can be considered the hallmark of this entity that marks the difference with other causes of PLE (Branquinho et al. Citation2011). In addition, during endoscopy, we found changes in the characteristic of IL such as irregular small white spots (1 mm to 1 cm in diameter) similar to foam (Branquinho et al. Citation2011).
In dogs, IBD and IL can occur together and it is regarded that canine IL is most often secondary to IBD (Melzer and Sellon Citation2002). It is possible that the intestine of IBD patients has dense mural scarring that blocks lymph flow, therefore causing IL (Owens and Greenson Citation2007).
EPI is a disorder of maldigestion resulting from failure to secrete the pancreatic enzymes required for digestion. This disorder can lead to malabsorption if secondary intestinal disease develops (Owens and Greenson Citation2007). It is a common problem in dogs, especially in the German shepherd, but might also affect other breeds (Hall Citation2003). There are a number of probable causes of EPI in dogs with pancreatic acinar atrophy being the most common (Westermarck et al. Citation2010).
In humans, diseases involving the pancreas are frequently encountered in patients with IBD including acute and chronic pancreatitis (CP) (Navaneethan and Shen Citation2010), which may be subclinical (Barthet et al. Citation1999). Acute pancreatitis associated with IBD may be related to gallstones, papillary injury of the duodenum, or Crohn's disease-associated granulomatous inflammation (Spiess et al. Citation1992; Gschwantler et al. Citation1995). The etiology and pathogenesis of CP are vague in humans with IBD. However, some investigators suggest that epithelial cells of the gastrointestinal tract and pancreatic tissue share similar target molecular or cellular structures that are vulnerable to intolerable injury (Barthet et al. Citation2003). Some of the factors involved may include secretory acinar cells, the pro-inflammatory cytokines IL-1 and TNF-α, protease-activated receptor-2, pancreatitis-associated protein, pancreatic autoantibodies, and chronic stress. CP may be accompanied by clinically significant exocrine insufficiency (Triantafillidis and Merikas Citation2010).
In felines, some studies have shown an association between CP and IBD. However, in dogs the pathogenetic association between these two conditions is less clear (Xenoulis et al. Citation2008). An association between IBD and CP has not been reported in canines yet, but CP may have been under-recognized in dogs with studies suggesting that it may be more frequent than previously thought (Newman et al. Citation2006; Watson et al. Citation2007). However, it has been suggested that some dogs with IBD have elevated pancreatic lipase immunoreactivity (cPLI) and that this may be suggestive of IBD-associated pancreatitis (Kathrani et al. Citation2009). The prevalence of cases of EPI due to CP in dogs is not clear as reported by Watson et al. (Citation2010). Pancreatic cells of either the endocrine or exocrine pancreas might be affected by fibrosis (Xenoulis et al. Citation2008). For this reason, dogs with EPI resulting from CP may also suffer from diabetes mellitus (Watson Citation2003). However, the animal presented here showed no signs of glucosuria or diabetes mellitus.
Drugs could be a definite cause of acute pancreatitis in patients with IBD, with mesalamine being the most frequently encountered although cases due to corticosteroids and metronidazole have also been described (Navaneethan and Shen Citation2010; Triantafillidis and Merikas Citation2010). Of note, the animal in this report only received metronidazole.
Pancreatic enzymes are required to treat EPI in dogs. However, in humans, proton pump inhibitors or H2-blockers could be administered in order to alter the duodenal pH and ensure the optimal effectiveness of pancreatic enzymes. Currently, there is limited scientific evidence about the true effectiviness of probiotics. However, Pezilli (Citation2009) described that the use of some specific probiotics may attenuate intestinal inflammation, thereby enabling the optimal efficacy of pancreatic extracts and the control of clinical signs. Based on this information, we administered omeprazole and probiotics with apparently acceptable results.
In the current case, it was difficult to establish which of the three conditions occurred first with two possibilities likely. First, it is probable that IBD was the entity triggering PLE in the current case. It might also be hypothesized that the dog was suffering from CP with acute flare-ups which ultimately led to IBD. In both cases it cannot be excluded that IL was secondary to IBD.
In this report, the dog showed a poor response to therapy. It is thus essential to establish a quick diagnosis in dogs with chronic diarrhea in order to prevent a disease such as IBD ultimately leading to secondary conditions that are difficult to treat and have a poor prognosis like IL.
Unfortunately, the correlation between IBD and EPI has not been proven in this case-report. On the other hand, we wanted to emphasize the importance of an affected dog simultaneously presenting with three different pathologies causing PLE. As a consequence, this might change our approach to treatment of chronic diarrhea in dogs. Traditionally, dogs with EPI (TLI values lower than 2.1 µg/L) are not subjected to endoscopy and biopsy.
Therefore, we propose that if the standard treatment in dogs with EPI does not lead to improvement of clinical signs, additional examinations such endoscopy and intestinal biopsy might be considered. Similarly, to rule out secondary EPI, TLI should be measured in dogs with IBD that have a poor response to habituated treatment.
Acknowledgments
The authors would like to thank the residents of the University Veterinary Hospital for their contribution in the management of the case. We would also like to thank to Guadalupe Diaz, DVM for the translation of this manuscript. Finally, we want to express that the videoendoscopes were acquired with FOMIX-CONACyT sponsorship.
References
- Barthet , M , Dubucquoy , L , Garcia , S , Gasmi , M , Desreumaux , P , Colombel , JF , Grimaud , JC , Iovanna , J and Dagorn , JC . 2003 . Pancreatic changes in TNBS-induced colitis in mice . Gastroenterol Clin Biol , 27 : 895 – 900 .
- Barthet , M , Hastier , P , Bernard , JP , Bordes , G , Frederick , J , Allio , S , Mambrini , P , Saint-Paul , MC , Delmont , JP Salducci , J . 1999 . Chronic pancreatitis and inflammatory bowel disease: true or coincidental association? . Am J Gastroenterol , 94 : 2141 – 2148 .
- Branquinho J, Mestrinho L, Faísca P, Morais de Almeida P. 2011. A case of intestinal lymphangiectasia with lipogranulomatous lymphangitis in a female rottweiler dog. Revista Lusófona de Ciência e Medicina Veterinária [internet]. Available from: <http://revistas.ulusofona.pt/index.php/rlcmv/article/view/2410/1896 (19 October 2012)
- Brooks , T . 2005 . Case study in canine intestinal lymphangiectasia . Can Vet J , 46 : 1138 – 1142 .
- German , AJ . 2009 . “ Gastrointestinal diseases: inflammatory bowel disease ” . In Kirk's current veterinary therapy , 14th , Edited by: Bonagura , JD and Twedt , DC . 501 – 506 . Saint Louis (MO) : Saunders Elsevier .
- Gschwantler , M , Kogelbauer , G , Klose , W , Bibus , B , Tscholakoff , D and Weiss , W . 1995 . The pancreas as a site of granulomatous inflammation in Crohn's disease . Gastroenterology , 108 : 1246 – 1249 .
- Guímaro JOM. 2010. Chronic inflammatory bowel disease: a comparative study between endoscopic imaging and histopathology in 73 canines [Master thesis]. [Lisboa (Portugal)] Universidad técnica de Lisboa. Available from: http://hdl.handle.net/10400.5/1711 (19 October 2012)
- Hall EJ. 2003. Exocrine pancreatic insufficiency. Paper presented at: 28th WSAVA World Congress; Bangkok, Thailand
- Kathrani , A , Steiner , JM , Suchodolski , J , Eastwood , J , Syme , H , Garden , OA and Allenspach , K . 2009 . Elevated canine pancreatic lipase immunoreactivity concentration in dogs with inflammatory bowel disease is associated with a negative outcome . J Small Anim Pract , 50 : 126 – 132 .
- Marks SL. 2008. PLE= Protein-losing enteropathies diagnosis and management. Paper presented at: International Congress of the Italian Companion Animal Veterinarians. Rimini, Italia
- Melzer , KJ and Sellon , R . 2002 . Canine intestinal lymphangiectasia . Compend Contin Educ Pract Vet , 24 : 953 – 961 .
- Navaneethan , U and Shen , B . 2010 . Hepatopancreatobiliary manifestations and complications associated with inflammatory bowel disease . Inflamm Bowel Dis , 16 ( 9 ) : 1598 – 1619 .
- Newman , SJ , Steiner , JM , Woosley , K , Williams , DA and Barton , L . 2006 . Histologic assessment and grading of the exocrine pancreas in the dog . J Vet Diagn Invest , 18 : 115 – 118 .
- Owens , SR and Greenson , JK . 2007 . The pathology of malabsorption: current concepts . Histopathology , 50 : 64 – 82 .
- Papendieck , CM . 2008 . Lymphedema in pediatrics. From signs to diagnosis and treatment . SFLB Lect Vascul , 3 ( 7 ) : 379 – 387 .
- Pezzilli , R . 2009 . Chronic pancreatitis: maldigestion, intestinal ecology and intestinal inflammation . World J Gastroenterol , 15 ( 14 ) : 1673 – 1676 .
- Spiess , SE , Braun , M , Vogelzang , RL and Craig , RM . 1992 . Crohn's disease of the duodenum complicated by pancreatitis and common bile duct obstruction . Am J Gastroenterol , 87 : 1033 – 1036 .
- Triantafillidis , JK and Merikas , E . 2010 . Pancreatic involvement in patients with inflammatory bowel disease . Ann Gastroenterol , 23 ( 2 ) : 105 – 112 .
- Van Kruiningen , HJ , Lees , GE and Hayden , DW . 1984 . Lipogranulomatosus lymphangitis in canine intestinal lymphangiectasia . Vet Pathol , 21 : 377 – 383 .
- Washabau RJ. 2005. Report from WSAVA Gastrointestinal Standardization Group
- Watson , PJ . 2003 . Exocrine pancreatic insufficiency as an end stage of pancreatitis in four dogs . J Small Anim Pract , 44 ( 7 ) : 306 – 312 .
- Watson , PJ , Archer , J , Roulois , AJ , Scase , TJ and Herrtage , ME . 2010 . Observational study of 14 cases of chronic pancreatitis in dogs . Vet Rec , 167 ( 25 ) : 968 – 976 .
- Watson , PJ , Roulois , AJ , Scase , T , Johnston , PE , Thompson , H and Herrtage , ME . 2007 . Prevalence and breed distribution of chronic pancreatitis at post-mortem examination in first-opinion dogs . J Small Anim Pract , 48 : 609 – 618 .
- Westermarck , E , Saari , S and Wiberg , M . 2010 . Heritability of exocrine pancreatic insufficiency in German Shepherd dogs . J Vet Internal Med , 24 : 450 – 452 .
- Xenoulis , PG , Suchodolski , JS and Steiner , JM . 2008 . Chronic pancreatitis in dogs and cats . Compend Contin Educ Vet , 30 ( 3 ) : 166 – 180 .