Abstract
Background: Dioctophyma renale is a large nematode distributed worldwide that may cause progressive and severe destruction of renal parenchyma.
Objectives: The present study aimed to evaluate pre- and post-operatively dogs submitted to right nephrectomy due to D. renale and to assess the histopathological damage of the removed kidney.
Animals and methods: Eight crossbred dogs, aged from 12 to 48 months that were unilaterally nephrectomized due to the presence of D. renale were evaluated. Physical examination, urinalysis, complete blood count, serum biochemistry, and abdominal ultrasound were performed immediately before and one month after nephrectomy. The nephrectomized right kidneys were submitted to macroscopic and microscopic evaluations.
Results: Urinalysis preoperatively detected occult blood in all dogs and D. renale eggs in five cases. Complete blood count showed all parameters within the reference range, except one dog post-operatively. Serum biochemistry performed before and after surgery verified that urea, creatinine and sodium were within the reference range values in all dogs. Other findings varied among the dogs. The length and arterial resistive index mean values of the left kidney were similar pre- and post-operatively.
Conclusions: Thus, the inconsiderable change in laboratory findings pre- and post-operatively was attributable to compensation by left kidney function for the removed abnormal right kidney. Right kidney histology revealed chronic nephropathy due to D. renale.
Clinical importance: Imaging diagnosis should be performed on dogs suspected as carrying the disease or on those from an enzootic area since the laboratory findings are not specific except eggs in the urine.
1. Introduction
The most common indications for nephrectomy in dogs include severe trauma, neoplasm, renal or ureteral calculi, infections, severe hydronephrosis and ureteral abnormalities (Gookin et al. Citation1996; MacPhail Citation2013). Another less frequent cause is Dioctophyma renale, a large nematode also known as giant kidney worm distributed worldwide that may cause progressive and severe destruction of the renal parenchyma (Soler et al. Citation2008; Secchi et al. Citation2010; Liu Citation2012). Infestation in man is rare, but might also result in kidney destruction (Fernando Citation1983).
D. renale is more likely to develop in a dog after ingesting its infected paratenic hosts, including fishes and frogs (Liu Citation2012). In many clinical cases the aetiology of the infestation could not be determined (Sousa et al. Citation2011), although contamination had been hypothesized due to the presence of aquatic environments (Pereira et al. Citation2006; Nakagawa et al. Citation2007; Ferreira et al. Citation2010), or to the occurrence of another paratenic host such as rats, especially in stray dogs (Kommers et al. Citation1999). In general, the adult worms are found more frequently in the right kidney, probably due to its anatomic proximity to the duodenum (Leite et al. Citation2005; Nakagawa et al. Citation2007; Soler et al. Citation2008; Secchi et al. Citation2010; Cottar et al. Citation2012). After infection, acquisition by ingestion, the larvae penetrate the duodenal wall, and later enter the abdominal cavity and migrate to the kidney where they mature into adult worms (Liu Citation2012). However, free worms have also been reported in the canine abdominal cavity, and sporadically at other bodily sites (Leite et al. Citation2005; Nakagawa et al. Citation2007; Sousa et al. Citation2011; Maia et al. Citation2012; Veiga et al. Citation2012). The location of the adult worms may be correlated with the site where the infecting larvae penetrate the digestive tract (Osborne et al. Citation1969; Kommers et al. Citation1999). An erratic cycle was also described in a dog with the presence of eggs in lung parenchyma, and cardiac and hepatic vascular bed (Lemos et al. Citation2010).
The clinical signs are, in general, unspecific or sometimes absent (Kommers et al. Citation1999; Nakagawa et al. Citation2007; Lemos et al. Citation2010; Sousa et al. Citation2011; Cottar et al. Citation2012; Liu Citation2012). Therefore, in most canine cases, the diagnosis is made when eggs are observed in the urine, or during necropsy, or occasionally during surgical interventions (Kommer et al. Citation1999; Leite et al. Citation2005; Pereira et al. Citation2006; Nakagawa et al. Citation2007; Soler et al. Citation2008; Silveira et al. Citation2009; Lemos et al. Citation2010; Secchi et al. Citation2010). Imaging studies including ultrasound and computed tomography (CT) have been used to confirm the presence of the worms in the kidney as well as to identify the occurrence of the parasite inside the abdominal cavity or other organs (Soler et al. Citation2008; Silveira et al. Citation2009; Secchi et al. Citation2010; Cottar et al. Citation2012; Rahal et al. Citation2014). The basic treatment of the disease has been the surgical removal of the parasites and nephrectomy in cases where the worms are in the kidney (Soler et al. Citation2008; Ferreira et al. Citation2010; Secchi et al. Citation2010; Sousa et al. Citation2011; Cottar et al. Citation2012; Maia et al. Citation2012). Apparently, there is not an effective drug that acts against D. renale yet (Liu Citation2012).
Therefore, the present study aimed to evaluate pre- and post-operatively dogs submitted to unilateral nephrectomy due to D. renale, and to assess the histopathological damage of the removed kidney. The hypothesis was that in most of the cases the function of left kidney usually is capable of compensating for the removed abnormal right kidney, which would account for inconsiderable change between pre- and post-operative laboratory findings.
2. Materials and methods
Eight crossbred dogs, three males and five females, aged from 12 to 48 months (mean 31.5 ± 11 SD) and weighing from 3.4 to 13.7 kg (mean 8.5 ± 3.5) that were unilaterally nephrectomized (right kidney) due to the presence of D. renale were evaluated. Only dogs that had D. renale located exclusively in the kidney were included, excluding dogs with worms freely in the abdominal cavity.
At the moment of the nephrectomy, male dogs were castrated and the females submitted to ovariohysterectomy. All animals had been living in the same farm and had been receiving a diet based on fish viscera. They were rescued and adopted by a non-governmental organization (NGO). According to the NGO the dogs were asymptomatic.
Physical examination, urinalysis (urine collected by cystocentesis), complete blood count, serum biochemistry [creatinine, urea, total protein, albumin, phosphorus, sodium, potassium, alanine aminotransferase (ALT), and alkaline phosphatase (ALP), gamma-glutamyltransferase (GGT)], and abdominal ultrasound were performed immediately before and one month after nephrectomy. The abdominal ultrasound exams in B-mode scan (MyLab Alpha, Esaote, Italy) were realized with the dogs positioned in dorsal recumbency. Sonographic images of the kidneys were acquired on sagittal and transversal planes. The maximum length of the left kidney was measured, and resistive index (RI) values of the main renal and interlobar arteries were evaluated in the left kidney as follows: (peak systolic velocity − end diastolic velocity)/peak systolic velocity.
During and after the treatment, commercial feed was prescribed for the dogs. After unilateral nephrectomy, the right kidneys were submitted for macroscopic and microscopic evaluations.
3. Results
Physical examination of the dogs before surgery revealed heart and respiratory rates, body temperature, color of mucous membranes, level of hydration, and size of peripheral lymph nodes within the normal range. These data did not change one month post-operatively.
Complete urinalysis before surgery showed that five dogs (1, 2, 3, 4, and 6) had D. renale eggs. Physical examination of urine showed specific gravity ranging from 1.020 to 1.056 (mean 1.038 ± 0.013), with values in two dogs (4 and 8) higher than 1.045. Four dogs (2, 3, 7, and 8) had alkaline urine (mean pH 7.1 ± 1.6). Urine occult blood was detected in all dogs, but microscopic hematuria occurred in six dogs (1, 2, 3, 4, 6, and 8). No dog showed macroscopic hematuria (reddish color). Complete urinalysis after surgery showed that physical, chemical, and microscopic examination of urine were within the reference range, and D. renale eggs were no longer detected. The exception was dog number 3 who remained with mild microscopic hematuria.
Although preoperative complete hemogram revealed that all animals presented mean red blood cell counts within reference range values (5.5–8.5 × 1012/L) (Meyer & Harvey Citation2004), hemoglobin (mean 141 ± 18 g/L) was decreased in dog 3, and mean corpuscular hemoglobin concentration (34.6 ± 2.4%) was decreased in this animal and dog 1. Three dogs (2, 3, and 5) presented mature neutrophils (mean 9.5 ± 0.4 × 109/L), but leukocytosis (mean 14.0 ± 3.6 × 109/L) was detected in only one animal (5). Dog 8 had monocytosis (mean 0.97 ± 0.27 × 109/L). Post-operative complete blood count showed all parameters within the reference range, except dog 4 who presented thrombocytopenia (8.0 × 109 platelets/L).
Serum biochemistry performed before surgery verified that urea and creatinine were within the reference range values in all dogs. Only one dog (8) had a mild increase in serum ALP activity exceeding the reference range, but ALT and GGT were normal in all dogs. Total protein (mean 79 ± 7.0 g/L) was increased in six dogs (1, 2, 3, 4, 5, and 6), but albumin (mean 36 ± 3.0 g/L) was elevated in two dogs (6 and 8) and globulin (mean 43 ± 8.0 g/L) in four dogs (1, 2, 3, and 5). Sodium concentration was within the reference range values in all dogs, but phosphorus (mean 1.9 ± 0.55 mmol/L) was increased in three dogs (2, 3, and 8). Only two dogs (1 and 5) showed slight hypokalemia. Serum biochemistry realized one month after surgery showed that ALP was still increased in dog 8. Total protein remained increased in dogs 1 and 5, while phosphorus was still increased in dog 8. The other biochemical parameters were within the reference range values in all dogs.
Ultrasonography of all dogs revealed several ring-like structures with a double-layer wall or arrayed as bands inside the right kidney. Three dogs (1, 4, and 6) showed a loss of medullary anatomic details and partial loss of the cortical anatomic details; four dogs (2, 3, 5, and 7) had a kidney completely occupied by parasites and loss of morphologic aspect. In dog 8, only medullary anatomic details were lost. The left kidney did not show any signs of worms, and the areas of renal cortex, medulla and sinus were well defined. The average size of the left kidneys was 5.43 ± 0.85 cm before right nephrectomy versus 5.48 ± 0.91 cm post-operatively. These kidneys had resistive index mean values of 0.64 ± 0.04 and 0.65 ± 0.04 for the main renal artery, and 0.66 ± 0.04 and 0.67 ± 0.03 for the interlobar artery, respectively, pre- and post-operatively.
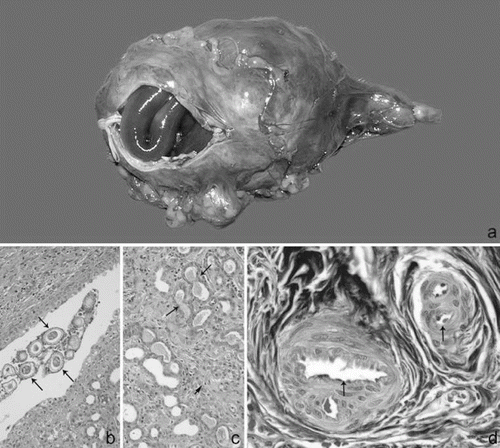
Macroscopically, all dogs had worms inside the right kidney. Three dogs (1, 4, and 6) showed total destruction of the renal medulla, and partial destruction of the renal cortex. Four (2, 3, 5, and 7) presented with total destruction of the cortex and medullar tissue, and kidney totally occupied by parasites (). Dog 8 presented corticomedullary differentiation. Histology revealed chronic nephropathy due to D. renale. The amount of eggs was high without predilection for any of the various kidney structures, such as cortex, medullary or pelvis (). As a result of worm infestation most of the cortical parenchyma showed irregularity. The glomerular size and morphology were small and atrophic, and sometimes exhibited areas of fibrinoid necrosis and capillary wall thickening. In addition, mesangial areas were observed to be expanded by amyloid substances. Both cortical and medullary stroma presented tubules with tortuous aspect, sometimes filled with acidophilic amorphous material, and hyaline cylinders. Structures similar to thyroid follicles were observed (). Both Hematoxylin Eosin and Masson's Trichrome also revealed wall thickening of the arteries – of both large and small calibers – surrounded by layers of fibroblasts (). In addition, an extensive inflammatory cell infiltration was observed, composed mainly of mononuclear cells mixed with some neutrophils and eosinophils.
Figure 1. (a) Macroscopic aspect of the right kidney showing destruction of the renal tissue, and presence of worms. (b) Histological aspect of the chronic nephropathy of the right kidney due to Dioctophyma renale. Eggs of the worms into the renal pelvis show different phases of maturation (arrows). HE, 20×. (c) Tubular dilatation or tubular atrophy, and hyaline cylinders suggesting thyroidization (arrows); stroma with cicatrization tissue and inflammation (short arrow). HE, 20×. (d) Artery occlusion associated with intima-media thickness and several layers of connective tissue (arrows). Masson's Trichrome, 40×.
4. Discussion
Hematuria, urinary infection, polydipsia/polyuria, and dysuria are the most significant signs of urinary tract abnormality described in some canine cases of D. renale (Kommers et al. Citation1999; Nakagawa et al. Citation2007; Soler et al. Citation2008; Secchi et al. Citation2010; Cottar et al. Citation2012). In the present study the dogs did not show any significant clinical signs according to the NGO, as observed in other reports (Kommers et al. Citation1999; Nakagawa et al. Citation2007; Lemos et al. Citation2010; Sousa et al. Citation2011; Cottar et al. Citation2012; Liu Citation2012), while three dogs did not present D. renale eggs in urinalysis probably due to absence of a gravid female worm (Liu Citation2012). Urinary specific gravity was higher than 1.045 in dogs 4 and 8, a finding probably associated with microscopic hematuria (Stockman & Scott Citation2008). In addition, occult blood in urine was the only finding that was observed in all dogs just preoperatively. Thus, imaging diagnosis, such as ultrasound and CT (Soler et al. Citation2008; Silveira et al. Citation2009; Secchi et al. Citation2010; Cottar et al. Citation2012; Rahal et al. Citation2014) should be performed on dogs suspected as carrying the disease or on those from an enzootic area.
Complete blood count was performed on some dogs with D. renale, but the results varied and included normal value ranges (Nakagawa et al. Citation2007; Soler et al. Citation2008; Secchi et al. Citation2010), or anemia (Nakagawa et al. Citation2007; Cottar et al. Citation2012), or leukocytosis (Ferreira et al. Citation2010). In the present study, one dog presented decreased hemoglobin and mean corpuscular hemoglobin concentration preoperatively, but the red blood cell count was normal, suggesting an iron-deficiency anemia that may be associated with hematuria due to renal injury (Stockkman et al. 2008). However, it is important to consider that anemia related to renal failure may take weeks to months to manifest (Osborne et al. Citation1969; Squires Citation2007). In the same manner, the presence of leukocytosis (in 12.5% of cases), increased segmented neutrophils (37.5%) and elevated monocytes (12.5%) were probably related to destruction of kidney tissue by the worms. In addition, these values returned to normal after surgery. The thrombocytopenia in dog 4 was probably related to Ehrlichia canis infection due to its presence in an area endemic for ticks. This dog received doxycycline (Doxifin®, Ouro Fino, Cravinhos, Brasil) at 10 mg/kg BW, PO, q 24 h for 30 days, as soon as the CBC results were known.
Moreover, serum biochemical values were also reported in some cases, but the results varied and included no alteration (Soler et al. Citation2008; Ferreira et al. Citation2010; Secchi et al. Citation2010), or azotemia (Cottar et al. Citation2012). The cases herein reported showed values within the normal range not only for urea but also creatinine, which suffers less interference from diet and other non-renal factors, indicating compensation by the left kidney (Squires Citation2007). The increased serum ALP activity in dog 8 may have been related to distress that increases the corticosteroid-induced alkaline phosphatase (Stockman & Scott Citation2008), or to the increase in bone alkaline phosphatase due to continuing growth, since this was the youngest animal (Stockman & Scott Citation2008).
In addition, 3 dogs had hyperphosphataemia that may suggest a reduced excretion of phosphorus due to renal parenchymal dysfunction (Tilley & Smith Citation2004; Squires Citation2007), or prerenal causes of decreased glomerular filtration rate (Stockman & Scott Citation2008). However, only dog 8 had hyperphosphataemia and hyperkalaemia post-operatively, suggesting chronic disease of the left kidney (Squires Citation2007; Stockman & Scott Citation2008). Also, this dog presented post-operative elevations of ALP and GGT, which are indicative of liver disease as well (Tilley & Smith, Citation2004). Other findings such as a preoperative increase of total protein may indicate that the patients (n = 6) had some degree of dehydration, but when associated with elevation of globulin may suggest inflammation (Stockman & Scott, Citation2008).
The resistive index obtained during Doppler sonography has been used to estimate alterations in vascular resistance provoked by renal disease, unilateral ureteral obstruction induced by surgery, and diuretic actions among others (Nyland et al. Citation1993; Rivers et al. Citation1997; Choi et al. Citation2003). Mean renal resistive indices of 0.64 ± 0.03 (Choi et al. Citation2003), and 0.72 (Novellas et al. Citation2007) have been reported in dogs with normal kidneys. In the present study, the values were similar to the first report. Thus, the resistance of blood flow in the evaluated arteries was normal before surgery as well as post-operatively, indicating good function of the left kidney in spite of the right kidney disease.
The structural abnormalities observed macroscopically in the right kidney were similar to ultrasound images in most of the dogs in the present study. These findings confirm that nephrectomy was the best option in all cases. Thus, the survival of dogs with renal dioctophymosis depends on the function of the remaining kidney (Osborne et al. Citation1969). In addition, microscopic necrosis, mononuclear inflammatory reaction, atrophy, fibrosis, and hyperplasia of the pelvis lining epithelium were found in a retrospective study of necropsy of 13 cases in 18 years (Kommers et al. Citation1999). Another retrospective study (Leite et al. Citation2005) of 11 necropsy cases in 25 years reported proliferation of fibrous connective tissue, tubular dilation, lymphoplasmocitary infiltration, glomerular atrophy, and epithelial hyperplasia of the renal pelvis. Most of these findings were also observed in residual renal tissue in the present study. However, the chronicity of the process was characterized by thyroidization. Worm eggs were observed in interstitial connective tissue and tubular lumens according to a previous report (Osborne et al. Citation1969). In the present study, no predilection for the presence of eggs was observed.
In conclusion, in these dogs submitted to right nephrectomy due to D. renale, the left kidney function compensated for the abnormal right kidney, which accounts for the finding of inconsiderable change between the pre- and post-operative laboratory findings. Histopathological analysis of the right kidney detected chronic nephropathy due to D. renale.
References
- Choi H, Won S, Chung W, Lee K, Chang D, Lee H, Eom K, Lee Y, Yoon J. 2003. Effect of intravenous mannitol upon the resistive index in complete unilateral renal obstruction in dogs. J Vet Intern Med. 17:158–162.
- Cottar BH, Dittrich G, Ferreira AA, Carvalho ACP, Albernaz VG, Luz MT, Tasqueti UI. 2012. Ultrasound findings in dogs with Dioctophyma renale – a retrospective study. Vet Zootec. 19: 8–11.
- Fernando SS. 1983. The giant kidney worm (Dioctophyma renale) infection in man in Australia. Am J Surg Pathol. 7:281–284.
- Ferreira VL, Medeiro FP, July JR, Raso TF. 2010. Dioctophyma renale in a dog: clinical diagnosis and surgical treatment. Vet Parasitol. 168:151–155.
- Gookin JL, Stone EA, Spaulding KA, Berry CR. 1996. Unilateral nephrectomy in dogs with renal disease: 30 cases (1985-1994). J Am Vet Med Assoc. 208:2020–2026.
- Kommers GD, Ilha MRS, Barros CSL. 1999. Dioctophymosis in dogs: 16 cases. Cienc Rural. 29:517–522.
- Leite LC, Círio SM, Diniz JMF, Luz E, Navarro-Silva MA, Silva AWC, Leite SC, Zadorosnei AC, Musiat KC, Veronesi EM, Pereira CC. 2005. Anatomopathologic lesions found in Dioctophyma renale (Goeze, 1782) infections in domestic dogs (Canis familiaris, LINNAEUS, 1758). Arch Vet Sci. 10:95–101.
- Lemos LS, Santos ASO, Rodrigues ABF, Goulart MLVS, Almeida LG, Silveira LS. 2010. Extrarenal lesion caused by Dioctophyma renale eggs in an erratic cycle in a dog. Int J Morphol. 28:1031–1034.
- Liu D. 2012. Dioctophyme. In: Liu D, editor. Molecular detection of human parasitic pathogens. Boca Raton, FL: Taylor & Francis; p. 535–538.
- MacPhail CM. 2013. Surgery of the kidney and ureter. In: Fossum TW, editor. Small animal surgery. Philadelphia, MO: Elsevier Mosby; p. 705–733.
- Maia VCC, Vieira SL, Oliveira PC, Campos FS, Horta V, Veiga CCP. 2012. Dioctofimosis inguinal in dog – case report. Vet Zootec. 19:86–88.
- Meyer D, Harvey JW. 2004. Veterinary laboratory medicine interpretation and diagnosis Philadelphia (PA): Saunders.
- Nakagawa TL, Bracarense AP, Reis AC, Yamamura MH, Headley SA. 2007. Giant kidney worm (Dioctophyma renale) infections in dogs from Northern Paraná, Brazil. Vet Parasitol. 145:366–370.
- Novellas R, Espada Y, Gopegui RR. 2007. Doppler ultrasonographic estimation of renal and ocular resistive and pulsatility indices in normal dogs and cats. Vet Radiol Ultrasound. 48:69–73.
- Nyland TG, Fisher PE, Doverspike M, Hornof WJ, Olander HJ. 1993. Diagnosis of urinary tract obstruction in dogs using duplex Doppler ultrasonography. Vet Radiol Ultrasound. 34:348–352.
- Osborne CA, Stevens JB, Hanlon GF, Rosin E, Bemrick WJ. 1969. Dioctophyma renale in a dog. J Am Vet Med Assoc. 155:605–620.
- Pereira BJ, Girardelli GL, Trivilin LO, Lima VR, Nunes LC, Martins IVF. 2006. The occurrence of dioctophymosis in dogs from Municipality of Cachoeiro do Itapemirim in the State of Espírito Santo, Brazil, from May to December of 2004. Rev Bras Parasitol Vet. 15:123–125.
- Rahal SC, Mamprim MJ, Oliveira HS, Mesquita LR, Faria LG, Takahira RK, Matsubara LM, Agostinho FS. 2014. Ultrasound, computed tomographic and operative findings in dogs with Dioctophyma renale. J Am Vet Med Assoc. 244:555–558.
- Rivers BJ, Walter PA, Polzin DJ, King VL. 1997. Duplex Doppler estimation of intrarenal pourcelot resistive index in dogs and cats with renal disease. J Vet Int Med. 11:250–260.
- Secchi P, Valle SF, Brun MV, Motta AC, Rausch SF, Messina SA, Vieira MIB. 2010. Videolaparoscopic nephrectomy in the treatment of canine dioctophymosis. Acta Sci Vet. 38:85–89.
- Silveira LL, Lemos LS, Ferreira FS, Freitas MV, Pereira MAC, Carvalho CB. 2009. Comparative analysis among the techniques of sedimentation-centrifugal and fast sedimentation (Paratest®) on identification of eggs of Dioctophyma renale in piss of dogs. J Bras Cienc An. 2:150–158.
- Soler M, Cardoso L, Teixeira M, Agut A. 2008. Imaging diagnosis – Dioctophyma renale in a dog. Vet Radiol Ultrasound. 49:307–308.
- Sousa AAR, Sousa AAS, Coelho MCOC, Quessada AM, Freitas MVM, Moraes RFN. 2011. Dioctophymosis in dogs. Acta Sci Vet. 39:1–4.
- Squires RA. 2007. Uraemia. In: Elliot J, Grauer GF, editors. BSAVA manual of canine and feline nephrology and urology. Gloucester: British Small Animal Veterinary Association; p. 54–68.
- Stockman SL, Scott MA. 2008. Fundamentals of veterinary clinical pathology. Ames, IA: Wiley-Blackwell.
- Tilley LP, Smith FWK. 2004. In: Tilley LP, Smith FWK, editors. The five-minute veterinary consult canine and feline. Philadelphia, PA: Lippincott Williams and Wilkins; p. 1487.
- Veiga CCP, Oliveira PC, Ferreira AMR, Azevedo FD, Vieira SL, Paiva MGA. 2012. Dioctophimosis in pregnant uterus in dog – case report. Rev Bras Med Vet. 34:188–191.
