A 3.5-year-old, intact female Boer goat was presented to the University of Illinois Veterinary Teaching Hospital with a one-day history of being recumbent and unable to rise. The owners purchased the doe six months prior to presentation. The doe had been used by the previous owners as an embryo donor with two successful embryo collections and had a successful pregnancy in the breeding season immediately prior to purchase. The doe had been exposed to a buck five months prior to presentation and was diagnosed pregnant via ultrasound at 45 days post-breeding. In the month before presentation to the hospital, the owners noticed that the doe's abdomen was becoming progressively distended. The abdominal distention was attributed to pregnancy. After one day of being recumbent, the owners presented the doe to the hospital for suspected pregnancy toxemia.
On physical examination, the doe was recumbent but alert and responsive. She was tachypneic (40 breaths/min; reference [ref.] range: 16–34 breaths/min), tachycardic (148 beats/min; ref. range: 70–80 beats/min), and hypothermic (37.6 °C; ref. range: 38.5–39.7 °C). Thoracic auscultation revealed normal but shallow respirations bilaterally and no heart murmurs. The abdomen was large and distended ().
The primary differential diagnosis based on history and presenting symptoms was pregnancy toxemia. A STAT profileFootnote was performed initially on heparinized whole blood and revealed a hypoproteinemia (58 g/L; ref. range: 61–75 g/L), hyperglycemia (12.7 mmol/L; ref. range: 2.6–4.2 mmol/L), and hyperlactemia (3.6 mmol/L; ref. range: <1.0 mmol/L). These findings were inconsistent with pregnancy toxemia.
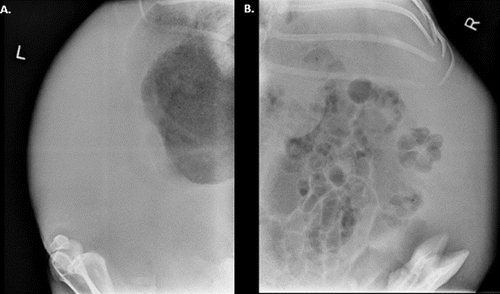
An abdominal ultrasound was performed to confirm pregnancy and determine the size and viability of fetuses. On abdominal ultrasound, a large amount of fluid was present in the abdomen but no fetuses were visualized. Abdominal radiographs were taken to verify that the doe was not pregnant. The abdominal radiographs confirmed there were no fetuses present and indicated the fluid seen on ultrasound was peritoneal free fluid and not intrauterine fluid ().
Figure 2. Abdominal radiographs. Loss of serosal detail in the abdomen confirms the presence of peritoneal free fluid.
Based on the diagnostic testing, pregnancy toxemia was excluded and the differential diagnosis list was expanded to include right-sided heart failure, peritonitis, uroabdomen, and neoplasia. At this point, further diagnostics were warranted. A complete blood cell count revealed an elevated number of segmented neutrophils (16.4 × 109 cells/L; ref. range: 1.2–7.2 × 109 cells/L) with normal numbers of lymphocytes (5.9 × 109 cells/L; ref. range: 2–9 × 109 cells/L) and monocytes (0.46 × 109 cells/L; ref. range: 0–550 × 109 cell/L). Erythrocyte parameters were within normal limits and included a packed cell volume of 31% (ref. range: 22–38%), hemoglobin of 109 g/L (ref. range: 80–120 g/L), and MCHC of 351 g/L (ref. range: 300–360 g/L). Fibrinogen was normal at 1.8 g/L (ref. range: 1–4 g/L) (Pugh Citation2002). Serum biochemistry abnormalities included hypoproteinemia (54 g/L; ref. range: 64–70 g/L) with a hypoalbuminemia (21 g/L; ref. range: 27–39 g/L), hypocalcemia (2.0 mmol/L; ref. range: 2.2–2.9 mmol/L), hypophosphatemia (0.87 mmol/L; ref. range: 1.4–2.9 mmol/L), hyperchloremia (113 mmol/L; ref. range: 99–110.3 mmol/L), hyperglycemia (11.9 mmol/L; ref. range: 2.75–4.13 mmol/L), decreased alkaline phosphatase activity (19 U/L; ref. range: 93–387 U/L), increased creatine kinase activity (715 U/L; ref. range: 0.8–9 U/L), and decreased cholesterol (1.64 mmol/L; ref. range: 2.08–3.38 mmol/L) (Pugh Citation2002).
A small amount of the peritoneal fluid was collected via abdominocentesis and submitted for analysis. The creatinine concentration from the abdominal fluid was 61.9 µmol/L, nearly equivalent with the serum creatinine concentration (70.7 µmol/L), which ruled out uroabdomen. Cytological analysis of the fluid revealed a sample of low cellularity with a nucleated cell count of 202 × 106 cells/L and RBC count of <1000 × 106 cells/L with a differential count of 43% small lymphocytes, 29% neutrophils, and 28% macrophages. The fluid had a moderate protein concentration of 25 g/L and a specific gravity of 1.015. It was interpreted as a modified transudate and no microorganisms or neoplastic cells were observed. The low cellularity and lack of abnormal cells ruled out peritonitis and made neoplasia less likely.
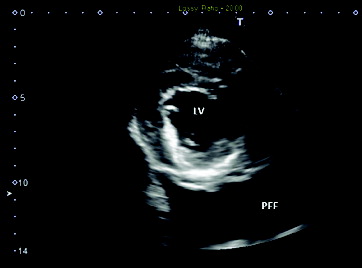
To rule out cardiac disease, a thoracic ultrasound was performed. The ultrasound revealed a structurally and functionally normal heart with a large volume of slightly echogenic pleural effusion (). A thoracocentesis was performed to stabilize the patient overnight. Approximately 600 mL of fluid was recovered from the left hemithorax. Thoracocentesis was repeated on the right hemithorax but it was complicated by the profound abdominal distension which led to the needle being in the abdomen for a portion of the procedure. The following morning, a large amount of pleural fluid was still visible within the thoracic cavity via ultrasound. While preparing for another thoracocentesis, the patient went into respiratory and cardiac arrest. Attempts at resuscitation were unsuccessful and the patient was pronounced dead. The animal was submitted for postmortem examination.
Figure 3. Ultrasonographic image of the heart: right parasternal transverse image of the heart revealed a normal image of the left ventricle (LV) with a large amount of slightly echogenic pleural free fluid (PFF).
At necropsy, the abdominal cavity contained approximately 22 L of pale yellow, clear, and watery fluid mixed with abundant amounts of fibrin. The serosal surfaces along the gastrointestinal tract including rumen, reticulum, omasum, abomasum, small intestine, and cecum were rough and irregular with numerous scattered, slightly raised, firm, pale tan to white foci, ranging from pinpoint to 3 mm in diameter. The omentum was markedly thickened (up to 1 cm) and firm, with dozens of soft, raised, tan to white nodules similar to those identified on the serosal surface of the gastrointestinal tract.
The pleural cavities contained approximately 3 L (right side) and 1 L (left side) of fluid similar to the fluid within the abdomen. The lungs were diffusely mottled pink to red, wet, and heavy. The lungs filled less than 20 volume% of the thoracic cavity.
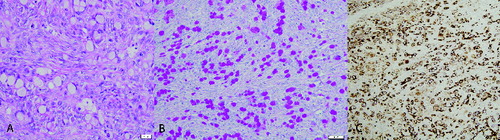
Microscopically, the serosal and subserosal regions of the gastrointestinal tract (rumen, reticulum, omasum, abomasum, and small intestine) and omentum were infiltrated by individual and occasional small acini of round to polygonal neoplastic cells. Cells were expanded by a vacuolated cytoplasm and had a peripheralized crescent-shaped, vesiculated nucleus with a single, often prominent, nucleolus ((A)). There was one mitotic figure within 10 random 400× fields. Glandular structures were not identified within the neoplastic cell population and there were large accumulations of richly collagenous connective tissue (scirrhous reaction). Only one ovary was examined microscopically and within the ovary, there was effacement of approximately 50% of the ovarian tissue by similar neoplastic cells resulting in formation of a mass. Cytoplasmic vacuoles in neoplastic cells throughout the ovary and mesentery stained red (positive) for periodic acid-Schiff stain (PAS) with and without diastase ((B)). Neoplastic cells exhibited positive cytoplasmic immunoreactivity for cytokeratin and did not exhibit immunoreactivity for vimentin ((C)). Based on the findings of this case, a diagnosis of metastatic signet-ring cell carcinoma was made.
Figure 4. (A) Histopathology of the omentum. Neoplastic cells are present with severe desmoplasia. Hematoxylin and eosin. (B) Histochemical staining of the omentum. Neoplastic cells contain PAS positive material within the cytoplasm. PAS without diastase. (C) Immunohistochemistry of the omentum, goat; signet-ring carcinoma. Neoplastic cells are positive for cytokeratin (AE1/AE3) (200×).
Cases of gastric carcinomas in domestic animals are classified according to the growth pattern and are divided into five types: tubular adenocarcinoma, mucinous adenocarcinoma, signet-ring cell carcinoma, tubulopapillary adenocarcinoma, and papillary carcinoma (Head et al. Citation2003). In veterinary medicine, signet-ring cell carcinomas most frequently arise from the stomach in dogs (Sugihara et al. 1991; Carrasco et al. Citation2011). Signet-ring carcinomas arising from the ovary have not been reported in veterinary medicine but have been reported rarely in human medicine (El-Safadi et al. Citation2010). The current report describes a metastatic signet-ring cell carcinoma in a Boer goat.
Neoplastic disease is less frequently diagnosed in the goat as compared to other domestic animals with 8.7%–11% prevalence in recent retrospective studies (Howerth & Butler Citation2011; Löhr Citation2013). Tumors commonly reported in goats include lymphoma, squamous cell carcinoma, thymoma, mammary carcinoma, pheochromocytoma, melanocytic tumors, and vascular neoplasms (Brandly & Migaki Citation1963; Zubaidy Citation1976; Bildfell et al. Citation2002). While its occurrence is much higher in sheep, intestinal adenocarcinoma has been previously described in Toggenburg, Guaderrama, and Pygora goats (Haibel Citation1990; Pérez et al. Citation1998; Löhr Citation2013). An intestinal adenocarcinoma with signet-ring appearance was reported in a Pygora goat but a primary tumor was not identified (Löhr Citation2013). Although rare, a variety of primary ovarian tumors have also been described in the doe including granulosa cell tumors and dysgerminoma. There is a single report of a mucinous adenocarcinoma of the ovary of a Nubian doe that had metastasized to the parietal peritoneum, pericardium, and lung (Memon et al. Citation1995). To our knowledge, this is the first case report of a metastatic signet-ring cell carcinoma with ovarian involvement in a Boer goat.
Primary signet-ring cell carcinoma of the ovary is extremely rare with only a few cases reported in human medicine (McCluggage & Young Citation2008; El-Safadi et al. Citation2010; Leen & Singh Citation2012). Several reports in older literature have previously described a primary signet-ring cell carcinoma of the ovary (Krukenberg tumor) in humans. It was later shown that a substantial proportion of these tumors were not ovarian primaries, but actually represented metastases from tumors elsewhere in the body, specifically the gastrointestinal tract (Young Citation2006). True primary ovarian carcinomas of mucinous morphology are typically low grade and low stage, where the tumors are limited to the ovaries and have very low likelihood of aggressive clinical behavior (McCluggage & Young Citation2008; El-Safadi et al. Citation2010; Leen & Singh Citation2012). Features that favor primary ovarian origin include unilateral localization, large size (>12 cm), background endometriosis, and association with other ovarian pathology, such as adenofibroma or cystadenoma.
In contrast to primary ovarian carcinomas of mucinous morphology in humans, metastatic mucinous carcinoma in the ovary has a population of signet-ring cells (McCluggage & Young Citation2008; Leen & Singh Citation2012). Other characteristics highly suggestive of a metastatic mucinous carcinoma, especially in combination, include bilateral localization, small size (<10 cm), surface deposition, nodular growth pattern, destructive stromal invasion with desmoplasia, and extensive extraovarian spread. Primary sites of signet-ring carcinomas reported to metastasize to the ovary in humans include the stomach, pancreas, biliary system, appendix, and colorectum. Immunohistochemistry is of limited value for the differentiation between primary and metastatic neoplasms due to overlap of immunophenotypes, as primary ovarian mucinous neoplasms frequently express enteric markers (McCluggage & Young Citation2008). Therefore, it is often necessary to distinguish a signet-ring cell carcinoma of the ovary from ovarian metastasis by extensive examination of the gastrointestinal tract, excluding the occurrence of a primary tumor.
Signet-ring cell carcinomas are uncommon in domestic species other than dogs and are generally associated with gastric origin. Tumors of the glandular stomach in dogs frequently result in metastasis of regional lymph nodes, and less likely carcinomatosis to the omentum, mesentery, and peritoneum (Head et al. Citation2002). Infiltration by tumor cells often induces excessive fibrosis (scirrhous reaction) from growth factor secretion. The pronounced fibrous reaction at sites of metastasis is similar to intestinal adenocarcinomas of domestic animals, including sheep and goats. Intestinal adenocarcinomas of the sheep and goats are usually solitary and located in the distal small intestine as an annular stenosing lesion (Pearson & McCaughey Citation1978; Haibel Citation1990; Pérez et al. Citation1998). In addition to extensive transcoelomic spread on peritoneal surfaces, there are also reported cases of metastasis to the ovary and oviducts in ewes (Pearson & McCaughey Citation1978). Histologically, the primary tumor in the intestine is typically composed of variably mucin-rich cuboidal or columnar epithelial cells arranged in an acinar pattern with minimal fibrosis. Sites of metastases have similar neoplastic cells but are mainly composed of abundant reactive fibrous tissue.
The only previous report of an epithelial tumor in the ovary of a goat (mucinous adenocarcinoma), to our knowledge, is that of a six-year-old Nubian doe with marked bilateral abdominal distension (Memon et al. Citation1995). The goat had progressive ascites and extensive fibrous transcoelomic deposits along the peritoneal and pleural surfaces.The neoplasm in the Nubian goat was presented as an indistinct, firm, slightly raised, gray-tan mass of the left ovary. Microscopically, the left ovary was partially effaced by an infiltrative mass composed of neoplastic columnar and goblet cells arranged in tubuloacinar structures within a dense hypercellular fibrous stroma. Neoplastic epithelial cells contained PAS positive and alcian blue positive droplets of mucin and a final diagnosis of mucinous adenocarcinoma of the ovary was given. The main pathologic findings are generally consistent with what was observed in the Boer goat. However, in our case, neoplastic cells were of signet-ring cell morphology. Based on the World Health Organization's system of classification of tumors of the alimentary tract in domestic animals, mucinous adenocarcinoma is reserved for lesions where more than half the tumor is formed by large pools of extracellular mucin. In contrast, a signet-ring cell carcinoma implies more than half of the tumor is composed of cells with eccentric nuclei and intracellular mucin (Head et al. Citation2003).
In the case of the Boer goat in this report, the presence of neoplastic cells throughout the abdominal cavity was consistent with carcinomatosis. Although a single small neoplastic mass was identified within an ovary, the extensive transcoelomic spread of signet-ring cells in an infiltrative pattern with a marked scirrhous response was highly suggestive of a primary neoplastic site within the gastrointestinal tract with subsequent metastasis to the ovary.
Clinical differential diagnoses for bilateral abdominal distension/ascites in adult do include right-sided heart failure, hepatopathy, protein-losing enteropathy, protein-losing nephropathy, infectious peritonitis, uroabdomen, and neoplasia. Most of these differentials (hepatopathy, enteropathy, nephropathy, peritonitis, and uroabdomen) were ruled out by routine diagnostics such as obtaining a thorough history, physical examination, bloodwork, and peritoneal free fluid analysis. Ruling out heart failure required the use of echocardiogram performed by trained professionals. After performing diagnostics, the only differential remaining was a non-exfoliative neoplasia.
Intra-abdominal adenocarcinomas have been asso-ciated with accumulation of as much as 30 L of ascitic fluid in the abdomen of female goats (Haibel Citation1990; Memon et al. Citation1995; Pérez et al. Citation1998). Ascites in these does, as well as other domestic species with intra-abdominal carcinomas, have been attributed to lymphatic obstruction by neoplastic emboli. In mice, it has been shown that blood vessels lining the peritoneal cavity are hyperpermeable to macromolecules (such as fibrinogen) which extravasate into peritoneal lining tissues to form a cross-linked fibrin network (Nagy et al. Citation1995). Given the presumed exfoliation and transperitoneal dissemination of neoplastic cells, the ascitic fluid may contain isolated neoplastic cells or tumor acini. Although, previous reports of intra-abdominal adenocarcinomas in goats do not mention detection of neoplastic cells within the peritoneal fluid obtained antemortem. Similarly, no neoplastic cells were observed on cytologic evaluation of the abdominal fluid in the current case. Possible explanations for the inability to identify neoplastic cells within the peritoneal fluid may be a low number of exfoliating cells or sequestration of cells within fibrin.
In summary, the current report describes the clinical, pathological, and immunohistochemical findings of a metastatic signet-ring cell carcinoma in a Boer goat.
Disclosure statement
No potential conflict of interest was reported by the authors.
Note
Notes
1. Critical Care Xpress, Nova Biomedical, Waltham, MA.
References
- Bildfell R, Valentine B, Whitney K. 2002. Cutaneous vasoproliferative lesions in goats. Vet Pathol. 39:273–277.
- Brandly P, Migaki G. 1963. Types of tumors found by federal meat inspectors in an eight-year survey. Ann NY Acad Sci. 108:872–879.
- Carrasco V, Canfrán S, Rodriguez-Franco F, Benito A, Sáinz A, Rodriguez-Bertos A. 2011. Canine gastric carcinoma immunohistochemical expression of cell cycle proteins (p53, p21, and p16) and heat shock proteins (Hsp27 and Hsp70). Vet Pathol. 48:322–329.
- El-Safadi S, Stahl U, Tinneberg HR, Hackethal A, Muenstedt K. 2010. Primary signet ring cell ovarian carcinoma: a case review and literature review. Case Rep Oncol. 3:451–457.
- Haibel G. 1990. Intestinal adenocarcinoma in a goat. J Vet Med Assoc. 196:326–328.
- Head K, Else R, Dubielzig R. 2002. Tumors of the glandular stomach. In: Meuten DJ, editor. Tumors in domestic animals. 4th ed. Ames (IA): Blackwell; p. 451–457.
- Head KW, Cullen JM, Dubielzig, Else RW, Misdorp W, Patnaik AK, Tateyama S, van der Gaag I. 2003. Histological classification of tumors of the alimentary system of domestic animals. Washington (DC): Armed Forces Institute of Pathology.
- Hefnawy AE, Shousha S, Youssef S. 2011. Hematobiochemical profile of pregnant and experimentally pregnancy toxemic goats. J Basic Appl Chem. 1:65–69.
- Howerth E, Butler A. 2011. Survey of goat tumors, Department of Pathology and Athens Veterinary Diagnostic Laboratory, College of Veterinary Medicine, UGA, from 2007-2011. Vet Pathol. 48:E21. doi:10.1177/0300985811425342
- Leen S, Singh N. 2012. Pathology of primary and metastatic mucinous ovarian neoplasms. J Clin Pathol. 65:591–595.
- Löhr C. 2013. One hundred two tumors in 100 goats (1987-2011). Vet Pathol. 50:668–675.
- McCluggage W, Young R. 2008. Primary ovarian mucinous tumors with signet ring cells: report of 3 cases with discussion of so-called primary Krukenberg tumor. Am J Surg Pathol. 32:1373–1379.
- Memon M, Schelling S, Sherman D. 1995. Mucinous adenocarcinoma of the ovary as a cause of ascites in a goat. J Am Vet Med Assoc. 206:362–364.
- Nagy J, Meyes M, Masse E. 1995. Pathogenesis of ascites tumor growth: fibrinogen influx and fibrin accumulation in tissues lining the peritoneal cavity. Cancer Res. 55:369–375.
- Pearson G, McCaughey W. 1978. Two cases of intestinal adenocarcinoma in aged ewes. N Z Vet J. 26:123–124, 129.
- Pérez V, Corpa J, García Marín J. 1998. Adenocarcinoma of the small intestine in a goat. J Comp Pathol. 119:311–316.
- Pugh DG. 2002. Appendix III – normal values and conversions. In: Pugh DC, editor. Sheep and goat medicine. Philadelphia (PA): Saunders; p. 451–455.
- Sugihara H, Hattori Y, Imamura Y, Noriki S, Fukada M, Katsura K, Tsuchihashi Y, Fujita S. 1991. Morphology and modes of cell proliferation in earliest signet-ring-cell carcinomas induced in canine stomachs by N-ethyl-N'-nitro-N-nitrosoguanidine. J Cancer Res Clin Oncol. 117:197–204.
- Young R. 2006. From Krukenberg to today: the ever present problems posed by metastatic tumors in the ovary. Part I. Historical perspective, general principles, mucinous tumors including the Krukenberg tumor. Adv Anat Pathol. 3:205–227.
- Zubaidy A. 1976. Caprine neoplasms in Iraq: case reports and review of the literature. Vet Pathol. 13:460–461.

