Abstract
Background: Increased density and distribution of wild boar populations are likely to promote interactions and transmission of certain pathogens, not only among wild boar but also from wild boar to livestock or humans and vice versa.
Objective: The purpose of this study was to determine seroprevalence against seven selected pathogens in wild boar living in four different areas in Greece.
Animals and methods: In total, 359 serum samples were collected from extensively farmed wild boar (Sus scrofa scrofa) originating from four distinct geographical areas throughout Greece from April 2012 to August 2013. Samples were tested for antibodies to Actinobacillus pleuropneumoniae, African swine fever virus (ASFV), Aujeszky's disease virus (ADV), classical swine fever virus (CSFV), Erysipelothrix rhusiopathiae, Mycoplasma hyopneumoniae and porcine reproductive and respiratory syndrome virus (PRRSV). Prevalence was compared among the four regions using Fisher's exact test.
Results: Low overall seropositivities of 2.4% and 5.6% were detected for E. rhusiopathiae and PRRSV, respectively, higher ones for ADV (32.0%) and the highest (72.5% and 90.5%) for M. hyopneumoniae and A. pleuropneumoniae, respectively. All sera tested were found negative for antibodies directed against CSFV and ASFV.
Conclusions: This is the first report of exposure of wild boars to selected pig pathogens in Greece. These results are indicative of the circulation of these pathogens in Greece with the exception of CSFV and ASFV and suggestive of the potential role of wild boars on their maintenance and transmission to their domestic counterparts and vice versa.
1. Background
Increased density and distribution of wild boar populations are likely to promote interactions and transmission of certain pathogens, not only among wild boar but also from wild boar to livestock or humans and vice versa (Gortazar Citation2007; Meng et al. Citation2009). The European wild boar (Sus scrofa scrofa) is an important and very popular game species in Europe including Greece (Acevedo et al. Citation2007). Although hunting would be expected to reduce wild boar populations, a number of circumstances have favored wild boar. Night hunting was banned in Greece and the entrance of wild boar from neighboring Turkey, due to excessive hunting pressure, has led to an increase in wild boar population at least in Northern Greece (Beskardes et al. Citation2010). Furthermore, wild boars share habitats with free-ranging pigs, which promotes hybridization and the so-called feral pigs, feral swine or feral hogs (Ruiz-Fons et al. 2008). The cross-breeding between wild boar and free-ranging pigs or local domestic breeds (mainly Greek black pig) is a common practice in many wild boar farms in Greece (Papatsiros et al. Citation2012) and hybrids between wild boar (Sus scrofa scrofa) and domestic pigs (Sus scrofa f. domestica) have been genetically identified (Koutsogiannouli et al. Citation2010).
Many viruses as well as bacteria have been reported to be shared among wild and domestic suids. Furthermore, Actinobacillus pleuropneumoniae, Mycoplasma hyopneumoniae and Erysipelothrix rhusiopathiae are three common bacterial pathogens limiting pig production (Gortazar Citation2007; Halli et al. Citation2012) while the latter is also easily transmitted to humans by direct contact with infected hosts (Vicente et al. Citation2002; Boadella et al. Citation2012b).
There is paucity of data regarding exposure of wild boar to these pathogens in Greece. This is more important for eradicated and notifiable diseases of domestic pigs since exposure of wild boar is an indication of pathogen circulation in the area.
The purpose of this study was to determine seroprevalence against seven selected pathogens in wild boar living in four different areas in Greece.
2. Animals and methods
The wild boars of the current study are referred to as either extensively farmed or free-ranging animals. Free range denotes a method of farming husbandry where the animals can roam freely outdoors, rather than being confined in an enclosure (Miao et al. Citation2004). The free-ranged (extensive) farming is preferred because wild boars are kept under conditions which simulate their natural environment. The wild boar farms were located in semi-mountainous or mountainous areas of 30–100 hectares. In the borderlines of these farms with crop fields, fences were placed in order to avoid possible damages caused by wild boar entrance. Since the outdoors ranging area was fenced and the animals were kept in big enclosures they can better be defined as extensively farmed wild boars.
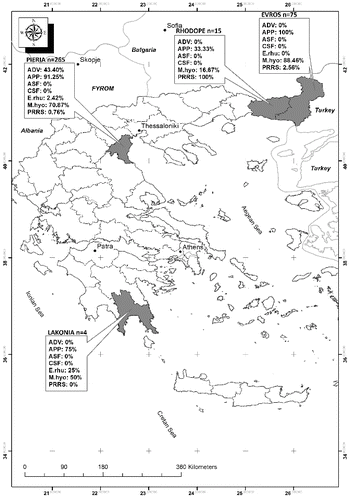
Blood samples were taken from the anterior vena cava of wild boar living in Greece from April 2012 to August 2013. A total of 75 samples was collected from the regional unit of Evros (41°10′N 26°05′E) and 15 samples from Rhodope (41°05′N 25°25′E), both parts of the region of East Macedonia and Thrace, 265 from Pieria (40°15′N 22°25′E) located in the southern part of Macedonia, in the Region of Central Macedonia and 4 from Lakonia (36°55′N 22°40′E) situated in the south-eastern part of the Peloponnese peninsula for a period of 15 months.
Samples were collected by the local veterinary authorities in these regions for the implementation of the annual serosurveillance program for classical swine fever virus (CSFV) that was implemented by the Central Veterinary Services of the Greek Ministry of Rural Development and Food. The sample size from each area was determined to cover the needs of this serosurveillance program. The exact distribution of total samples collected from each area is presented in and in . The final number of samples tested for each pathogen in cases where testing for all pathogens was not possible due to inadequate quantity of sample is also presented in .
Table 1. Frequency (%) of seropositivity against seven pathogens (Actinobacillus pleuropneumoniae (App), African swine fever virus (ASFV), Aujeszky's disease virus (ADV), classical swine fever virus (CSFV), Erysipelothrix rhusiopathiae (E. rhu), Mycoplasma hyopneumoniae (M. hyo) and porcine reproductive and respiratory syndrome virus (PRRSV)) tested according to the regional unit in Greece from April 2012 to August 2013.
Figure 1. Map of Greece presenting the total number of samples collected from each regional unit and the seropositivity against each pathogen tested per regional unit from April 2012 to August 2013.
For the detection of antibodies commercially available ELISA test kits were used.
Prevalence of antibodies directed against each pathogen was compared among the four regions of sampling using Fisher's exact test. Statistical significance was accepted at P values < 0.05. Data were analyzed using SPSS software version 20.0 (SPSS Inc, Chicago, IL, USA).
3. Results
Frequency of seropositivity among different pathogens and different geographical areas is presented in and . Seroprevalence of porcine reproductive and respiratory syndrome virus (PRRSV), M. hyopneumoniae, A. pleuropneumoniae, Aujeszky's disease virus (ADV) and E. rhusiopathiae differed significantly among the four regional units (P = 0.000, P = 0.001, P = 0.000, P = 0.000 and P = 0.008, respectively).
Seropositivity against only one pathogen was detected in 75 animals; 8 animals (2.5%) for PRRSV, 12 animals (3.8%) for M. hyopneumoniae, 54 (15.1%) for A. pleuropneumoniae and 1 (0.6%) animal for E. rhusiopathiae. Double and triple pathogen seropositivity that was detected in seven different combinations and the statistically significant differences among them are presented in .
Table 2. Frequency (%) of double and triple pathogen seropositivity in nine different combinations based on seven different pathogens (Actinobacillus pleuropneumoniae (App), African swine fever virus (ASFV), Aujesky's disease virus (ADV), classical swine fever virus (CSFV), Erysipelothrix rhusiopathiae (E. rhu), Mycoplasma hyopneumoniae (M. hyo) and porcine reproductive and respiratory syndrome virus (PRRSV)).
4. Discussion
Data presented here regarding CSFV and ASFV support the disease-free status of Greece in accordance with the reports of the World Organization for Animal Health (OIE Citation2014). The risk of introduction of ASFV by wild boar into the European Union as estimated after the outbreaks in the Caucasus region and Russian Federation concerns mostly Finland, Romania, Latvia and Poland and not Greece for the moment (De la Torre et al. Citation2013). Although ASF is not a zoonotic disease, surveillance to timely identify isolates and prevent spreading of the disease is of great importance given the absence of an effective vaccine available or any effective treatment.
All positive ADV samples of our study originated from a region with wealthy wildlife and mountainous areas, where the positive free-ranging wild boar were likely to have had contact with ADV-positive wild boar sometime in the past. In previous studies (Gortazar et al. Citation2002; Boadella et al. Citation2012a), ADV has been reported to remain almost stable in wild boar populations.
Based on our results, A. pleuropneumoniae is the most prevalent pathogen in Greek wild boar farms which could be attributed to the outdoor housing of farmed wild boar in mountainous or semi-mountainous areas. On the contrary, the seroprevalence against E. rhusiopathiae is low in Greece whereas vaccinations for E. rhusiopathiae are not commonly performed in wild boar farms. In the present study, a high prevalence of antibodies directed against M. hyopneumoniae was detected which could be perceived as a potential risk for wild boar and pigs.
Previous studies based on serological testing for the detection of antibodies to PRRS indicate that wild boar may constitute a reservoir worldwide including hybrid wild boar in Korea (Choi et al. Citation2012). The PRRSV seropositivity may be attributed to possible contact with positive domestic pigs, introduction of vaccinated breeding animals or even mechanical transmission of PRRSV (e.g., human, tracks, fomites, etc.) (Reiner et al. CitationCitation2009). Based on the epidemiology of PRRSV and its high prevalence in Greek swine industry, the vaccination of female-farmed wild boar against PRRSV is suggested to prevent losses.
Mixed respiratory infections are common in swine practice; the porcine respiratory disease complex (PRDC) refers to a combination of one or two viruses as well as M. hyopneumoniae and Pasteurella multocida. Based on the results of the present study ( and ), PRDC seems to be an important respiratory problem for Greek wild boar farming. PRRSV is the most common virus isolated from cases of PRDC (Thacker Citation2001), while M. hyopneumoniae along with PRRSV and P. multocida is the most common combination of pathogens in PRDC (Brockmeier & Lager Citation2002). In contrast, the seroprevalence of PRRSV ( and ) in our study did not reveal PRRSV as the major pathogen of PRDC in farmed wild boar. The high seroprevalence of co-infections with M. hyopneumoniae and A. pleuropneumoniae is in agreement with previous studies where M. hyopneumoniae was indicated as a predisposing factor for A. pleuropneumoniae infection (Yagihashi et al. Citation1984). Infection with M. hyopneumoniae or A. pleuropneumoniae cause stimulation of alveolar macrophage functions, and M. hyopneumoniae infections may suppress phagocytic responses when pigs are challenge-exposed to a secondary pathogen (A. pleuropneumoniae). This potential suppression may represent a predisposition of the host by M. hyopneumoniae to secondary bacterial infections (Caruso & Ross Citation1990). In our study, the high seroprevalence of M. hyopneumoniae and A. pleuropneumoniae could be associated with the introduction of breeding animals (including local domestic pigs or free-ranging pigs) in wild boar farms. Transmission of M. hyopneumoniae by carrier pigs is considered to be the main source of infection under field conditions as infected pigs may harbour the microbe in the respiratory tract for a long period (Maes et al. Citation2001; Pieters et al. Citation2009).
Co-infections of bacteria and viruses have a synergic role in producing more severe disease than those induced by each pathogen alone (Yagihashi et al. Citation1984; Thacker et al. Citation1999). In our study, most prevalent co-infections were M. hyopneumoniae and A. pleuropneumoniae and A. pleuropneumoniae + ADV and less frequently PRRSV + M. hyopneumoniae and PRRSV + A. pleuropneumoniae. It seems that viral infections due to ADV or PRRSV predisposed to secondary infections by M. hyopneumoniae and A. pleuropneumoniae.
Wild boar exposure is a well-grounded indication of circulation of pathogens in an area since extensive wild boar farming increases the risk of transmission between wild boar and their domestic counterparts (Papatsiros et al. Citation2012; Boutsini et al. Citation2014).
The domestic animal/wildlife interface is becoming a global issue of growing interest (Martin et al. Citation2011). Furthermore, diseases, when expressed in free-ranging animals, can have a significant effect on wildlife ecologies, in domestic animals and humans (Gortazar Citation2007). Housing condition of extensive farming, including wild boar farming, may predispose the animals to various infectious pathogens due to their proximity to wildlife and their exposure to environmental risks (Papatsiros et al. Citation2012;Boutsini et al. Citation2014). Most of the wild boar farms in Greece are located in mountainous or semi-mountainous areas, where wild boar populations also exist. Greek farmers of free-ranging pigs or farmed wild boar report usual visits of wild boar into their farms. The motives of wild boar are usually feed search or adult (sexual mature) females while human hunting pressure also results in population movements. Consequently, a direct contact between farmed wild boar and wild boar also occurs. These interactions between wild boar and outdoor pigs are not uncommon, thereby increasing the risk of pathogen spill-over (Wu et al. Citation2011).
This is the first report of exposure of wild boar in Greece to selected pig pathogens. Disease eradication programs should also take into consideration free-ranging farmed animals and their contacts with wildlife as well as possible sources of infection caused by animal cross-border movements. Spatial and temporal differences in population density and domestic animal vaccination protocols impose the importance of surveillance programs to prevent and effectively eliminate outbreaks.
Acknowledgements
Authors are grateful to Julia Moschou, National Reference Laboratory for CSF and ASF, Institute of Infectious and Parasitic Diseases, Centre of Veterinary Institutes, Greek Ministry of Rural Development and Food, for her expertise in technical assistance during the preparation of samples and ELISA testing.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Acevedo P, Vicente J, Hofle U, Cassinello J, Ruiz-Fons F, Gortazar C. 2007. Estimation of European wild boar relative abundance and aggregation: a novel method in epidemiological risk assessment. Epidemiol Infect. 135:519–527.
- Beskardes V, Yilmaz E, Oymen T. 2010. Evaluation on management of wild boar (Sus scrofa L.) population in Bolu-Sazakici hunting ground. J Environ Biol. 31:207–212.
- Boadella M, Gortazar C, Vicente J, Ruiz-Fons F. 2012a. Wild boar: an increasing concern for Aujeszky's disease control in pigs? BMC Vet Res. 8:7.
- Boadella M, Ruiz-Fons JF, Vicente J, Martin M, Segales J, Gortazar C. 2012b. Seroprevalence evolution of selected pathogens in Iberian wild boar. Transbound Emerg Dis. 59:395–404.
- Boutsini S, Papatsiros VG, Stougiou D, Marucci G, Liandris E, Athanasiou LV, Papadoudis A, Karagiozopoulos E, Bisias A, Pozio E. 2014. Emerging Trichinella britovi infections in free ranging pigs of Greece. Vet Parasitol. 199:278–282.
- Brockmeier SL, Lager KM. 2002. Experimental airborne transmission of porcine reproductive and respiratory syndrome virus and Bordetella bronchiseptica. Vet Microbiol. 89:267–275.
- Caruso JP, Ross RF. 1990. Effects of Mycoplasma hyopneumoniae and Actinobacillus (Haemophilus) pleuropneumoniae infections on alveolar macrophage functions in swine. Am J Vet Res. 51:227–231.
- Choi EJ, Lee CH, Hyun BH, Kim JJ, Lim SI, Song JY, Shin YK. 2012. A survey of porcine reproductive and respiratory syndrome among wild boar populations in Korea. J Vet Sci. 13:377–383.
- De la Torre A, Bosch J, Iglesias I, Munoz MJ, Mur L, Martinez-Lopez B, Martinez M, Sanchez-Vizcaino JM. 2013. Assessing the risk of African swine fever introduction into the European Union by wild boar. Transbound Emerg Dis. doi:10.1111/tbed.12129
- Gortazar ARF, Ferroglio E, Hofle U, Frolich K, Vicente J. 2007. Diseases shared between wildlife and livestock: a European perspective. Eur J Wild Res. 53:241–256.
- Gortazar C, Vicente J, Fierro Y, Leon L, Cubero MJ, Gonzalez M. 2002. Natural Aujeszky's disease in a Spanish wild boar population. Ann N Y Acad Sci. 969:210–212.
- Halli O, Ala-Kurikka E, Nokireki T, Skrzypczak T, Raunio-Saarnisto M, Peltoniemi OA, Heinonen M. 2012. Prevalence of and risk factors associated with viral and bacterial pathogens in farmed European wild boar. Vet J. 194:98–101.
- Koutsogiannouli EA, Moutou KA, Sarafidou T, Stamatis C, Mamuris Z. 2010. Detection of hybrids between wild boars (Sus scrofa scrofa) and domestic pigs (Sus scrofa f.domestica) in Greece, using the PCR-RFLP method on melanocortin-1 receptor (MC1R) mutations. Mammalian Biol. 75:69–73.
- Maes D, Chiers K, Haesebrouck F, Laevens H, Verdonck M, de Kruif A. 2001. Herd factors associated with the seroprevalences of Actinobacillus pleuropneumoniae serovars 2, 3 and 9 in slaughter pigs from farrow-to-finish pig herds. Vet Res. 32:409–419.
- Martin C, Pastoret PP, Brochier B, Humblet MF, Saegerman C. 2011. A survey of the transmission of infectious diseases/infections between wild and domestic ungulates in Europe. Vet Res. 42:70.
- Meng XJ, Lindsay DS, Sriranganathan N. 2009. Wild boars as sources for infectious diseases in livestock and humans. Philos Trans R Soc London B. 364:2697–2707.
- Miao ZH, Glatz PC, Ru YJ. 2004. Review of production, husbandry and sustainability of free-range pig production systems. Asian-Aust J Anim Sci. 117:1615–1634.
- OIE, 2014. World Animal Health Information Database (WAHID) Interface. Country Animal Health Report. Available from: http://www.oie.int/wahis_2/public/wahid.php/Countryinformation/countryhome
- Papatsiros VG, Tassis PD, Christodoulopoulos G, Boutsini S, Tsirigotakis G, Tzika ED. 2012. Health and production of Greek organic pig farming: current situation and perspectives. JHVMS. 63:37–44.
- Pieters M, Pijoan C, Fano E, Dee S. 2009. An assessment of the duration of Mycoplasma hyopneumoniae infection in an experimentally infected population of pigs. Vet Microbiol. 134:261–266.
- Reiner G, Fresen C, Bronnert S, Willems H. 2009. Porcine reproductive and respiratory syndrome virus (PRRSV) infection in wild boars. Vet Microbiol. 136:250–258.
- Ruiz-Fons F, Segales J, Gortazar C. 2008. A review of viral diseases of the European wild boar: effects of population dynamics and reservoir role. Vet J. 176:158–169.
- Thacker EL. 2001. Immunology of the porcine respiratory disease complex. Vet Clin North Am Food Anim Pract. 17:551–565.
- Thacker EL, Halbur PG, Ross RF, Thanawongnuwech R, Thacker BJ. 1999. Mycoplasma hyopneumoniae potentiation of porcine reproductive and respiratory syndrome virus-induced pneumonia. J Clin Microbiol. 37:620–627.
- Vicente J, Leon-Vizcaino L, Gortazar C, Jose Cubero M, Gonzalez M, Martin-Atance P. 2002. Antibodies to selected viral and bacterial pathogens in European wild boars from southcentral Spain. J Wildl Dis. 38:649–652.
- Wu N, Abril C, Hinic V, Brodard I, Thur B, Fattebert J, Hussy D, Ryser-Degiorgis MP. 2011. Free-ranging wild boar: a disease threat to domestic pigs in Switzerland?. J Wildl Dis. 47:868–879.
- Yagihashi T, Nunoya T, Mitui T, Tajima M. 1984. Effect of Mycoplasma hyopneumoniae infection on the development of Haemophilus pleuropneumoniae pneumonia in pigs. Nihon Juigaku Zasshi. 46:705–713.
