ABSTRACT
Background: Cerebral amyloid angiopathy (CAA) is a disorder characterized by amyloid deposition in the wall of cerebral blood vessels. The deposits of amyloid occur frequently in the blood vessels of the frontal, parietal and occipital cortex.
Objective: To examine the characteristics of CAA classified according to the Vonsattel scale in elderly dogs histologically and immunohistochemically as well as the semi-quantitative evaluation of the amyloid deposits in the different segments of the brain.
Animals and methods: The brains of 36 dogs of different breeds and sexes, which had been routinely necropsied, were used and divided into two groups: dogs from 1 to 5 and 10 to 18 years old. The tissue sections were stained by hematoxylin–eosin, Congo red and immunohistochemically.
Results: Amyloid was accumulated in the wall of cerebral blood vessels in 70% of dogs over the age of 10 years predominantly in the frontal cortex. CAA was demonstrated in elderly dogs as follows: in the frontal cortex (n = 19 or 63%), the parietal cortex (n = 12 or 40%), the hippocampus (40%) and the cerebellum (n = 5 or 17%). The deposits of amyloid in the wall of blood vessels detected by Congo red staining were also Aβ1-14 and Aβ1-42 immunohistochemically positive. Most commonly, the amyloid deposits affected a moderate number of blood vessels. The accumulation of amyloid was immunohistochemically revealed in the blood vessel walls as well as in the senile plaques and neurons.
Conclusion: The amount of amyloid in the arterial walls increased with age in dogs, whereas the amyloid accumulated in plaques was Congo red negative.
KEYWORDS:
1. Introduction
Amyloidoses are diseases with a heterogenic range and are characterized by interstitial deposition and accumulation of amyloid. Potentially, deposits of amyloid can be found in all tissues. Cerebral amyloid angiopathy (CAA) is characterized by the deposition and accumulation of beta amyloid (Aβ) in cerebral vasculature (Gahr et al. Citation2013). The term CAA refers to a heterogenic group of illnesses of the central nervous system, which are characterized histopathologically by the presence of amyloid in the wall of cerebral blood vessels (Biffi & Greenberg Citation2011; Gahr et al. Citation2013). CAA in humans can be divided into two forms: hereditary and sporadic. The occurrence of the hereditary (familiar) form is related to various forms of mutations (Thanvi & Robinson Citation2006; Yamada & Naiki Citation2012; Gahr et al. Citation2013). The sporadic form occurs much more frequently and mostly as a sporadic condition in the elderly, with an increasing incidence associated with age. This form is a very common finding in the brain of patients with Alzheimer's disease (AD) (Vinters et al. Citation1996; Revesz et al. Citation2002; Kumar-Singh Citation2008; Mendel et al. Citation2013). CAA in the animal brain is also related to aging (Walker Citation1997; Borras et al. Citation1999). The presence of amyloid in the blood vessel walls of senile dementia patients was first described by Scholtz in 1938 (Sholtz, Citation1938). Although amyloid in peripheral organs has been studied in experimental animals since the early part of the twentieth century, CAA was not described in a non-human species until the 1950s, when Von Braunmühl (Citation1956) reported senile plaques and congophilic angiopathy in aged dogs. CAA was described in various animal species such as a transgenic mouse, bear, cat, dog, monkey, camel, etc. (Walker Citation1997; Nakayama et al. Citation1999; Papaioannou et al. Citation2001; Rofina et al. Citation2006; Elfenbein et al. Citation2007).
Amyloid deposits most often occur in the frontal, temporal and occipital cortex. This abnormal protein accumulates in the wall of all blood vessel types of the cerebrum and cerebellum gray matter. Deposits of amyloid are rarely present in the blood vessels of white matter (Vinters et al. Citation1996; Revesz et al. Citation2002; Kumar-Singh Citation2008). It has been widely accepted that Aβ in the wall of cerebral vasculature is toxic to the vascular elements and causes structural and functional alterations of the blood vessels. Several studies have shown that endothelial cells and pericytes undergo degeneration in amyloid-laden vessels (Farkas & Luiten Citation2001; Kumar-Singh et al. Citation2002). Other structural changes such as microangiopathy, microaneurysmal dilatation, necrosis, etc. can also be observed in the cerebral blood vessel walls (Vinters et al. Citation1996; Revesz et al. Citation2002; Kumar-Singh Citation2008). Furthermore, CAA independently contributes to dementia and it is a risk factor for hemorrhagic stroke (Kumar-Singh Citation2008). Some studies have shown that CAA in the brain of a dog has a positive correlation with the frequency of hemorrhage (Uchida et al. Citation1991; Walker Citation1997) and cognitive decline (Cummings et al. Citation1996; Head et al. Citation1998; Head et al. Citation2000).
The amyloid protein/peptide present in the vascular deposits of CAA is identical to the beta amyloid found in the senile plaques in human patients with AD. Many amyloid isoforms accumulate in the brain such as Aβ 1-40/1-42/1-43/3pE/pN3 (Schilling et al. Citation2008; Chambers et al. Citation2011; Saito et al. Citation2011; Mendel et al. Citation2013). In tissue, beta amyloid can be detected by Congo red staining as well as by immunohistochemical staining (Papaioannou et al. Citation2001).
The aim of the work was to examine the characteristics of CAA in the elderly dog population histologically and immunohistochemically as well as the semi-quantitative evaluation of the amyloid deposits in the different segments of the brain of elderly dogs.
2. Materials and methods
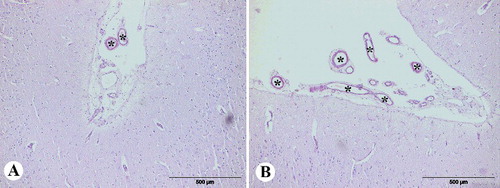
In this study, we examined the brains of 36 dogs of different breeds and sexes, which had been routinely necropsied at the Department of Veterinary Pathology of the Faculty of Veterinary Medicine, University of Belgrade (). The animals without clinical neurological signs or mental or behavioral dysfunctions during life, died or were euthanized for a variety of reasons. The examined dogs had been divided in two groups: group of young dogs (group A) consisted of six dogs (from 1 to 5 years old) and group of elderly dogs (group B) composed of 30 dogs (10–18 years old). After necropsy, the brains were removed, macroscopically examined and fixed in 10% neutral buffered formalin for six days. Coronal sections of the frontal cerebral cortex, parietal cerebral cortex, hippocampus, cerebellum and medulla oblongata were routinely processed, embedded in paraffin, cut into 4–6 µm thick sections and stained using the hematoxylin and eosin, and the Congo red method. The stained tissues were analyzed by the light microscope and some of the sections with characteristic findings were captured by a digital camera. The additional analyses of the sections stained by Congo red were done under polarized light. The frequency of CAA observed in each Congo red positive section was recorded according to a semi-quantitative grading scheme based on the number of Congo red positive vessels in the total number of vessels observed in 10 fields using 10x objective (100x magnification). After counting, the percentage of positive blood vessels were calculated and compared to the total number of vessels in each sample (number of positive/total number of blood vessels × 100). Based on the obtained results, the percentage of blood vessels with amyloid deposited was determined 0% (−), 1%–29% (+ low number of positive blood vessels), 30%–69% (++, moderate number of positive blood vessels) and >70% (+++, numerous positive blood vessels) ().
Table 1 Age, sex and breed of examined dogs (nos. 1–6 group of young dogs and nos. 7–36 group of elderly dogs).
Figure 1 Congo red staining (semi-quantitative evaluation), magnification 100x: (A) grade + (low number of the positive blood vessels of Congo red positive vessels in the total number of vessels in field), (B) grade ++ (moderate number of Congo red positive vessels in the total number of vessels in field).
In humans, the severity of CAA has been classified according to the Vonsattel scale as follows: mild, moderate and severe forms. The mild form is characterized by deposits of Aβ restricted to the congophilic rim around normal or atrophic smooth muscle fibers. In the moderate form, the tunica media is thicker than normal and is replaced by deposits of Aβ amyloid, with no evidence of blood leakage. The massive deposits of Aβ with focal wall fragmentation and at least one focus of perivascular leakage as evidenced by the presence of erythrocytes or hemosiderin, or both, are characteristics of the severe form (Vonsattel et al. Citation1991; Vinters et al. Citation1996; Revesz et al. Citation2002; Kumar-Singh Citation2008; Mendel et al. Citation2013). In this study, CAA has been classified according to the Vonsattel scale.
The estimated proportion of beta amyloid deposit involvement in each blood vessel was recorded on the Mountjoy scale from 0 to 4. A score of 0 indicated the absence of amyloid. The finding of Aβ deposits of up to one-quarter of the vessel circumference was score 1. A score 2 marked Aβ deposits in up to one-half of the vessel circumference. The involvement of amyloid of up to three-quarters of the vessel circumference was score 3. Finally, score 4 indicated the total involvement of the vessel circumference (Mountjoy et al. Citation1982; Mendel et al. Citation2013).
Immunohistochemical staining was performed by the commercial kit LSAB2 (Labeled streptavidin-biotin2, DAKO, Glostrup, Denmark). As a negative control we used brain samples of young dogs that should not have been positive. In addition to the brain of young dogs, the brain of dogs that had not been treated with primary antibody was used as a negative control for immunohistochemical staining. The control slides were treated with a buffer (phosphate buffer solution (PBS)) instead of primary antibody. Polyclonal antibody against Aβ 1-14 (AbD serotec, Langford, UK, working dilutions 1:400) and monoclonal antibody against Aβ 1-42 (Invitrogen, Camarillo, CA, USA, working dilutions 1:500) were used as primary antibodies. The sections were incubated in 85% formic acid for 10 minutes to enhance the beta amyloid immunoreaction. PBS (pH 7.2–7.4) was used for rinsing. Both antibodies were incubated in a moist chamber at a temperature of 18–20 °C for 60 minutes. After incubation with the primary antibody, the sections were treated by commercial kit for detection (Dako, Cytomation, Glostrup, Denmark, LSAB2 System-HRP, K0675). The chromogen 3.3'-diaminobenzidine tetrahydrochloride (DAB+, DAKO, Glostrup, Denmark, K3468) was used for staining the antibody–antigen complex.
3. Results
No amyloid deposits were found in the brain of dogs from group A. The accumulation of amyloid in the brain of dogs from group B was proven by Congo red and immunohistochemical staining. The amyloid stained by the Congo red method was characterized as a red homogeneous structure under the light microscope, while it was fluorescent yellow–green under the polarized light (). Deposits were found in the meningeal and parenchymal blood vessels in all examined regions of the brain, except in the medulla oblongata. CAA was demonstrated by Congo red staining in the frontal cortex of 19 dogs (63%), the parietal cortex of 12 dogs (40%), hippocampus of 12 dogs (40%) and the cerebellum of 5 dogs (17%).
Figure 2 Congo red staining of the brain of aged dogs: (A) amyloid in the wall of meningeal blood vessel (arrow), frontal section; (B) amyloid in the wall of meningeal blood vessel (arrow), frontal section, under the polarized light; (C) amyloid in the wall of meningeal blood vessels of cerebellum (arrow), under polarized light.
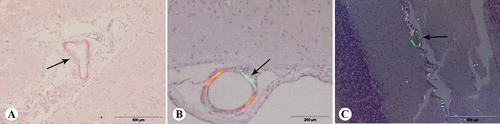
In all Congo red positive cases, amyloid deposits were always present in the wall of meningeal blood vessels. Moreover, this pathological protein was found in the wall of parenchymal blood vessels of the frontal cortex in 13 dogs (43%), of the parietal cortex in 9 dogs (30%), of the hippocampus in 7 dogs (23%) and in the blood vessel walls of the cerebellum in 4 dogs (13%). Congo red positive blood vessels were never observed in subcortical white matter. Semi-quantitive evaluation of Congo-red-stained sections () showed that CAA, including meningeal and parenchymal blood vessel, was present in frontal sections of 63% of dogs, in parietal sections of 40% of dogs, in the hippocampus of 40% of dogs and in the cerebellum of 17% of dogs. In this study, a moderate number of congophilic blood vessels in the frontal section were observed in 27% of dogs and numerous congophilic blood vessels in the frontal section were present in 13% of dogs. The lowest number of positive blood vessels was observed in the cerebellum (17%). In this study, of the total number of the examined dogs, 13% had a moderate number of positive blood vessels in the cerebellum.
Table 2 Semi-quantitive evaluation of the cerebral amyloid angiopathy in different tissue sections (Congo red staining).
The amyloid deposits were observed immunohistochemically in the wall of blood vessels, diffuse plaques and in some cases intraneuronally. The amyloid deposits were Aβ1–14 immunohistochemically positive in all of the three locations, while the deposits in the vasculature and in the plaques were only Aβ1–42 immunohistochemically positive.
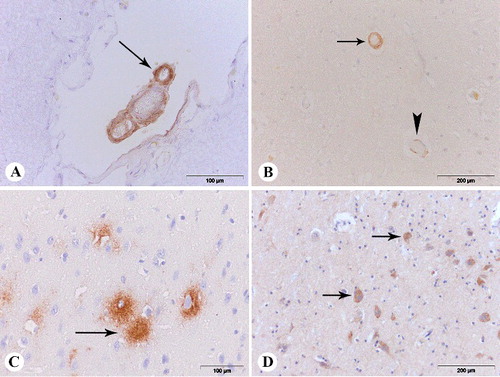
In almost all dogs (90%) with cerebrovascular amyloidosis, amyloid was accumulated in the meningeal and parenchymal blood vessel walls ((A,B)). A small number of dogs (10%) had deposits of amyloid only in the meningeal blood vessels, while none of the dogs had amyloid only in the parenchymal blood vessels.
Figure 3 (A) β-amyloid deposits in the wall of meningeal blood vessels (arrow), anti-Aβ1-42, frontal section; (B) β-amyloid deposits in the wall of parenchymal blood vessels (arrow – severe type of the Vonsattel scale and score 4 of the Mountjoy scale, arrowhead – mild type of the Vonsattel scale and score 1 of the Mountjoy scale), anti-Aβ 1-14, frontal section; (C) diffuse plaque (arrow), anti-Aβ 1-42 parietal section; (D) intracellular β-amyloid deposits (arrow), anti-Aβ 1-14, parietal section.
Using immunohistochemical staining, the amyloid deposits were detected in the blood vessel walls of all examined segments of the brain except in the medulla oblongata. The amyloid deposits were most frequently present in the wall of blood vessels of the frontal cortex, and were the least common in the cerebellum. In the meningeal blood vessels, the presence of amyloid deposits has been shown in the frontal cortex of 21 dogs (70%), in the parietal cortex of 16 dogs (53%), in the hippocampus of 15 dogs (50%) and in the cerebellum of 5 dogs (17%). The presence of amyloid was detected in the parenchymal blood vessels of the brain in the frontal cortex in 70% of dogs, in the parietal cortex in 50% of dogs, hippocampus in 50% of dogs and in the parenchymal blood vessels of the cerebellum in 13.33% of dogs from the group B.
Amyloid deposits were observed in the various layers of the wall of the blood vessel. In this study, three forms of CAA were identified according to the Vonsattel scale (mild, moderate and severe ()). All forms of amyloid deposits according to the Mountjoy scale were observed (). In some cases, they formed a ring around the entire blood vessel, while in other cases, their presence was intermittent and limited to one-quarter, one-half or three-quarters of the vessel circumference. There was no cellular response around the blood vessels caused by the amyloid deposits in any case. Diffuse plaques ((C)) were present in the brain of 19 dogs (63%) from the group B. Beta amyloid positive plaques were observed most frequently in the frontal cortex. The presence of plaques was less frequently (43%–53%) immunohistochemically proven in other examined tissue sections, while they were not observed at all in the cerebellum and the medulla oblongata. In this study, the amyloid accumulated in plaques was Congo red negative. Intraneuronal accumulation of amyloid was demonstrated only by immunohistochemical staining. The intraneuronal deposits in the frontal and parietal cortex as well as in the hippocampus were Aβ1–14 immunohistochemically positive in seven dogs (23%, (D)). All brains of the dogs from the group A were Aβ1–14 immunohistochemically negative.
4. Discussion
The changes in the blood vessels of the brain tissue may occur during the aging process and significantly affect the viability of neurons and glial cells and thus can damage the structure and function of the central nervous system. The lesions in the blood vessels can occur in the perivascular space, the wall or in the lumen. The most important and the most common changes during the aging process are the changes in the structure of the cerebral blood vessel walls, which may lead to changes in permeability, which in turn disrupts the normal flow of glucose and oxygen to neurons and glial cells, so that hypoxia and lack of nutrients lead to cell damage (Dimakopoulos & Mayer Citation2002).
The accumulation of amyloid in the blood vessel walls is a significant lesion in old patients, patients with familiar CAA and in almost all patients with AD (Vinters et al. Citation1996; Revesz et al. Citation2002; Kumar-Singh Citation2008). In this study, it has been found that amyloid was accumulated in the wall of cerebral blood vessels in 70% of dogs over the age of 10 years. These results confirm that CAA is an age-dependent change. The amount of amyloid in the arterial walls increased with age which correlated with findings of Rofina et al. Citation2006, but in our study amyloid plaques in cortex were Congo red negative which is not in accordance with the results of these authors (Rofina et al. Citation2006). Recent data show that there are three types of amyloid plaques: diffuse, primitive and neuritic (Dimakopoulos & Mayer Citation2002; Brellou et al. Citation2005). The diffuse plaques are believed to further ‘mature’ into dense core plaques such as primitive or neuritic plaques. The dense core plaques often contain dystrophic neurites and higher burden of Aβ 1–40, whereas the diffuse plaques are composed predominantly Aβ 1–42 and occur mostly perineuronally and occasionally as large ‘clouds’. Only diffuse plaques are not detectable by Congo red staining. These plaques are the predominant subtype of Aβ in the aging dog brain (Cummings et al. Citation1996; Head et al. Citation1998; Head et al. Citation2000; Brellou et al. Citation2005), but in several studies, the primitive plaques have been found in the brain of elderly dog (Papaioannou et al. Citation2001; Rofina et al. Citation2006).
Amyloid accumulates in the wall of the leptomeningeal and parenchymal blood vessels. It is believed that Aβ accumulated in the wall of a cerebral blood vessel has a toxic effect on vascular elements. Consequently, endothelial cells and pericytes are the subject of degeneration (Farkas & Luiten Citation2001; Kumar-Singh et al. Citation2002). Considering that amyloid affects vascular permeability, it causes poor perfusion of the brain and indirectly leads to neuronal damage. The results obtained in this study showed that the majority of the dogs in the experimental group had CAA that affected the leptomeningeal and parenchymal blood vessels at the same time, while a small number of dogs revealed the presence of amyloid only in the wall of the leptomeningeal blood vessels.
It is believed that the appearance of CAA in humans is associated with the occurrence of dementia. In addition to the CAA as a factor that affects the occurrence of dementia, it is also a risk factor for the occurrence of hemorrhage in the brain (Kumar-Singh Citation2008). Hemorrhage was not found in any of the examined brains in our study. The absence of hemorrhage might be due to the fact that dogs from the experimental group were of different breeds and therefore had different life spans.
Age-related CAA has been noted in a variety of vertebrate species (Walker Citation1997; Kumar-Singh et al. Citation2000; Brellou et al. Citation2005; Elfenbein et al. Citation2007). The lack of appropriate animal models is an obstacle to understanding CAA (Revesz et al. Citation2003; Elfenbein et al. Citation2007), but presently there are a few well-established, natural models of sporadic CAA. Aged dogs and non-human primates have been used often as natural models of CAA (Walker & Cork Citation1999; Walker & Durham Citation1999; Walker Citation2000; Elfenbein et al. Citation2007). Dogs are particularly suitable for modeling the human process of aging and sporadic CAA, since they share a number of biological similarities and living space with humans, and because CAA is primarily a form of cerebral β-amyloidosis in this species. In this study, we confirmed that dogs are predisposed to age-related CAA, and showed that (1) amyloidotic blood vessels in the brain of dogs are congophilic; (2)Aβ accumulated in all types of cerebral blood vessels as well as deposits involve different layers of the blood vessel walls; (3) deposits of amyloid can affect from one-quarter to the entire circumference of the wall of cerebral vessels; (4) Aβ1–42 exists in most microvascular and parenchymal lesions in dogs over 10 years; (5) the density and anatomical distribution of CAA and parenchymal plaques overlap; (6)Tte most frequently affected region is the frontal cortex, with Aβ deposits primarily in the parenchyma and blood vessel walls; (7) deposits of amyloid in plaques and intraneuronally are Congo red negative.
So far, there are no data about the semi-quantitative evaluation of CAA neither in dogs nor other animals. This study contains first semi-quantitative evaluation of the CAA in dogs that presents the percentage of affected blood vessels depending on the brain segment of the Congo red positive cases. The obtained results may indicate a connection between changes in the brain's blood vessels and functional disorders that can manifest as behavioral disorders in dogs which was shown in the study of Rofina and coworkers (Rofina et al. Citation2006) previously.
5. Conclusion
Sporadic CAA is an age-related change in the brain of the elderly. This study showed that amyloid deposit was always present in the walls of meningeal blood vessels, but in the brain parenchyma, the amyloid deposits were most frequently present in the wall of blood vessels of the frontal cortex. There is no definitive premortem diagnosis or treatment for CAA. Animal models are a critical link in the chain of discovery leading to the development of diagnostic and therapeutic tools for CAA. In the future, the further successful incorporation of animal models into examinations may be helpful in understanding the development of CAA in AD and other disorders.
Disclosure statement
Authors declare that they have no competing financial, professional or any non-financial competing interests that might be perceived to influence the interpretation of data or presentation of information described in this manuscript.
References
- Biffi A, Greenberg SM. 2011. Cerebral amyloid angiopathy: a systematic review. J Clin Neurol. 7:1–9.
- Borras D, Ferrer I, Pumarola M. 1999. Age-related changes in the brain of the dog. Vet Pathol. 36:202–211.
- Brellou G, Vlemmas I, Lekkas S, Papaioannou N. 2005. Immunohistochemical investigation of amyloid beta-protein (Abeta) in the brain of aged cats. Histol Histopathol. 20:725–731.
- Chambers JK, Mutsuga M, Uchida K, Nakayama H. 2011. Characterization of AbetapN3 deposition in the brains of dogs of various ages and other animal species. Amyloid. 18:63–71.
- Cummings BJ, Head E, Afagh AJ, Milgram NW, Cotman CW. 1996. Beta-amyloid accumulation correlates with cognitive dysfunction in the aged canine. Neurobiol Learn Mem. 66:11–23.
- Dimakopoulos AC, Mayer RJ. 2002. Aspects of neurodegeneration in the canine brain. J Nutr. 132:1579S–1582S.
- Elfenbein HA, Rosen RF, Stephens SL, Switzer RC, Smith Y, Pare J, Mehta PD, Warzok R, Walker LC. 2007. Cerebral beta-amyloid angiopathy in aged squirrel monkeys. Histol Histopathol. 22:155–167.
- Farkas E, Luiten PG. 2001. Cerebral microvascular pathology in aging and Alzheimer's disease. Prog Neurobiol. 64:575–611.
- Gahr M, Nowak DA, Connemann BJ, Schonfeldt-Lecuona C. 2013. Cerebral amyloidal angiopathy – a disease with implications for neurology and psychiatry. Brain Res. 1519:19–30.
- Head E, Callahan H, Muggenburg BA, Cotman CW, Milgram NW. 1998. Visual-discrimination learning ability and beta-amyloid accumulation in the dog. Neurobiol Aging. 19:415–425.
- Head E, McCleary R, Hahn FF, Milgram NW, Cotman CW. 2000. Region-specific age at onset of beta-amyloid in dogs. Neurobiol Aging. 21:89–96.
- Kumar-Singh S. 2008. Cerebral amyloid angiopathy: pathogenetic mechanisms and link to dense amyloid plaques. Genes Brain Behav. 7:67–82.
- Kumar-Singh S, Cras P, Wang R, Kros JM, van Swieten J, Lubke U, Ceuterick C, Serneels S, Vennekens K, Timmermans JP, et al. 2002. Dense-core senile plaques in the Flemish variant of Alzheimer's disease are vasocentric. Am J Pathol. 161:507–520.
- Kumar-Singh S, De Jonghe C, Cruts M, Kleinert R, Wang R, Mercken M, De Strooper B, Vanderstichele H, Lofgren A, Vanderhoeven I, et al. 2000. Nonfibrillar diffuse amyloid deposition due to a gamma(42)-secretase site mutation points to an essential role for N-truncated A beta(42) in Alzheimer's disease. Hum Mol Genet. 9:2589–2598.
- Mendel TA, Wierzba-Bobrowicz T, Lewandowska E, Stepien T, Szpak GM. 2013. The development of cerebral amyloid angiopathy in cerebral vessels. A review with illustrations based upon own investigated post mortem cases. Pol J Pathol. 64:260–267.
- Mountjoy CQ, Tomlinson BE, Gibson PH. 1982. Amyloid and senile plaques and cerebral blood vessels. A semi-quantitative investigation of a possible relationship. J Neurol Sci. 57:89–103.
- Nakayama H, Katayama K, Ikawa A, Miyawaki K, Shinozuka J, Uetsuka K, Nakamura S, Kimura N, Yoshikawa Y, Doi K. 1999. Cerebral amyloid angiopathy in an aged great spotted woodpecker (Picoides major). Neurobiol Aging. 20:53–56.
- Papaioannou N, Tooten PC, van Ederen AM, Bohl JR, Rofina J, Tsangaris T, Gruys E. 2001. Immunohistochemical investigation of the brain of aged dogs. I. Detection of neurofibrillary tangles and of 4-hydroxynonenal protein, an oxidative damage product, in senile plaques. Amyloid. 8:11–21.
- Revesz T, Ghiso J, Lashley T, Plant G, Rostagno A, Frangione B, Holton JL. 2003. Cerebral amyloid angiopathies: a pathologic, biochemical, and genetic view. J Neuropathol Exp Neurol. 62:885–898.
- Revesz T, Holton JL, Lashley T, Plant G, Rostagno A, Ghiso J, Frangione B. 2002. Sporadic and familial cerebral amyloid angiopathies. Brain Pathol. 12:343–357.
- Rofina JE, van Ederen AM, Toussaint MJ, Secrève M, van der Spek A, van der Meer I, Van Eerdenburg FJ, Gruys E. 2006. Cognitive disturbances in old dogs suffering from the canine counterpart of Alzheimer's disease. Brain Res. 1069:216–226.
- Saito T, Suemoto T, Brouwers N, Sleegers K, Funamoto S, Mihira N, Matsuba Y, Yamada K, Nilsson P, Takano J, et al. 2011. Potent amyloidogenicity and pathogenicity of Abeta43. Nat Neurosci. 14:1023–1032.
- Schilling S, Zeitschel U, Hoffmann T, Heiser U, Francke M, Kehlen A, Holzer M, Hutter-Paier B, Prokesch M, Windisch M, et al. 2008. Glutaminyl cyclase inhibition attenuates pyroglutamate Abeta and Alzheimer's disease-like pathology. Nat Med. 14:1106–1111.
- Sholtz W. 1938. Studien zur Pathologie der Hirngefässe. II Die drüsige Entartung der Hirnarterien und capillären [Studies on pathology of the brain vessels II: The glandular degeneration of brain arteries and capillaries]. Z Gesamte Neurol Psychiatr. 162:694–715.
- Thanvi B, Robinson T. 2006. Sporadic cerebral amyloid angiopathy – an important cause of cerebral haemorrhage in older people. Age Ageing. 35:565–571.
- Uchida K, Nakayama H, Goto N. 1991. Pathological studies on cerebral amyloid angiopathy, senile plaques and amyloid deposition in visceral organs in aged dogs. J Vet Med Sci. 53:1037–1042.
- Vinters HV, Wang ZZ, Secor DL. 1996. Brain parenchymal and microvascular amyloid in Alzheimer's disease. Brain Pathol. 6:179–195.
- Von Braunmühl A. 1956. Congophile angiopathy and senile plaques in aged dogs. Arch Psychiatr Nervenkr Z Gesamte Neurol Psychiatr. 194:396–414.
- Vonsattel JP, Myers RH, Hedley-Whyte ET, Ropper AH, Bird ED, Richardson EP, Jr. 1991. Cerebral amyloid angiopathy without and with cerebral hemorrhages: a comparative histological study. Ann Neurol. 30:637–649.
- Walker LC. 1997. Animal models of cerebral beta-amyloid angiopathy. Brain Res Brain Res Rev. 25:70–84.
- Walker LC. 2000. Cerebral amyloid angiopathy in aged dogs and nonhuman primates. In: Verbeek MM, Vinters HV, de Waal RM, editors. Cerebral amyloid angiopathy in Alzheimer's disease and related disorders. Dordrecht: Kluwer; p. 313–324.
- Walker LC, Cork LC. 1999. The neurobiology of aging in nonhuman primates. In: Terry RD, Katzman R, Bick KL, Sisodia SS, editors. Alzheimer disease. Philadelphia (PA): Lippincott Williams and Wilkins; p. 233–243.
- Walker LC, Durham RA. 1999. Cerebrovascular amyloidosis: experimental analysis in vitro and in vivo. Histol Histopathol. 14:827–837.
- Yamada M, Naiki H. 2012. Cerebral amyloid angiopathy. Prog Mol Biol Transl Sci. 107:41–78.
