ABSTRACT
Background: Canine visceral leishmaniasis (CVL) is a worldwide parasitic zoonosis caused by Leishmania (Leishmania) infantum around the world. Canids are the definitive hosts and sand flies the intermediate hosts.
Objective: To test the hypothesis that a new species-specific primers (Lch14:Lch15, targeting a multiple alignment for L. infantum kDNA minicircle) is an efficient diagnostic tool for L. infantum.
Methods: The presence of L. infantum DNA was assessed in blood samples of 69 stray dogs using the conventional PCR (cPCR) and quantitative PCR (qPCR). Additional 50 lymph nodes and 50 bone marrow samples (positive and negative samples for parasitological tests) from dogs from endemic and nonendemic areas for CVL were also used.
Results: L. infantum strains, and all positive lymph node and bone marrow samples for parasitological test gave positive results for cPCR and qPCR, presenting analytical sensitivity of ∼100 parasite mL−1. For the blood samples, 40/69 (58%; CI 95%; 46%–69%) resulted positive for L. infantum in both tests. All positive samples were confirmed by sequencing.
Conclusion: This study showed the importance of the specific detection of L. infantum based on species-specific primers by molecular techniques, highlighting the application as a confirmation method in epidemiological studies and to adopt the best control measures.
1. Introduction
Leishmaniasis is an anthropozoonotic disease caused by parasite protozoa of the genus Leishmania. It is endemic in tropical and subtropical areas of the world. It has an estimated incidence of 0.2–0.4 million of visceral leishmaniasis (VL) cases with a total of 12 million people worldwide affected (Alvar et al. Citation2012). VL is caused by the species Leishmania (Leishmania) infantum (syn. chagasi) (New World) (Mauricio et al. Citation2000), and L. (L.) infantum and L. (L.) donovani (Old World) (Acha & Szyfres Citation2003; Shaw Citation2006; Marcili et al. Citation2014). L. infantum is widely distributed in the Americas, and is endemic for both humans and dogs in many regions of Brazil (Acha & Szyfres Citation2003). The parasite is transmitted to vertebrate hosts by infected blood-sucking Phlebotominae sand flies of the Lutzomyia longipalpis (Lu. longipalpis) species, which is the main vector of VL in Brazil (Missawa et al. Citation2010; Michalsky et al. Citation2011). VL is also endemic in dogs from Mediterranean countries, i.e. Cyprus, Greece, Albania, Croatia, Italy, Malta, France, Spain, and Portugal. It is estimated that about 700 autochthonous VL human cases due to L. infantum are reported yearly and the average seroprevalence in domestic dogs is up to 25%, which has exhibited an expansion toward new locations in Europe by the increased mobility of dogs in association with other determinants of global change, such as climatic alterations and international tourism (Maia & Cardoso Citation2015). Also, the major focal areas of human VL exhibit a high prevalence of seropositive dogs (Athanasiou et al. Citation2012; Agallou et al. Citation2016). In urban areas, the dog is considered the main reservoir, whereas wild carnivores are the most important reservoirs in rural areas (Acha & Szyfres Citation2003). Since owned dogs can be infected by Lu. longipalpis or other potential vectors in endemic areas, and can move among cities and countries with the owner, infected stray dogs may be also a risk for the nonendemic surrounding areas.
The accurate and fast detection of the parasite is extremely important for the VL control, but depends on the available diagnostic methods. The low sensitivity and/or specificity of conventional diagnostic techniques are significant problems when used to differentiate infected from noninfected animals. Serological tests, the most used diagnostic method for VL, present some limitations and can provide false-positive or false-negative results, which can suggest a mistake induction of euthanasia in those false-positive animals, and keep dogs as reservoirs in areas of transmission (false-negative results). So, the serological status of dogs does not distinguish dogs infected with L. infantum from uninfected animals.
This problem can be improved with molecular techniques such as polymerase chain reaction (PCR). In canine VL (CVL), PCR assays constitute useful tools in cases of clinically healthy dogs, which may harbor the infection but may never develop clinical disease, preventing the importation of infected clinically healthy dogs to nonendemic areas (Tsokana et al. Citation2014). Seemingly healthy dogs, with antibody negative and PCR positive results can contribute to the infection by infecting sand flies, and spreading infection (Pennisi Citation2015).
Recently, PCR has significantly advanced with the use of quantitative real-time PCR (qPCR) that promotes a real-time and accurate quantification (Ramos et al. Citation2012; Solcà et al. Citation2012), and helps in confirmation of inconclusive cases of CVL, i.e. dogs not yet seroconverted, accurate detection of potential reservoirs, and determination of a decrease of the parasite load in these animals as a result of successful therapy.
Several target sequences and different PCR protocols have been described for the detection of leishmania DNA (Tsokana et al. Citation2014). Most target the kDNA minicircle, which has been shown to be a good target for the detection and identification of leishmania parasites (Mary et al. Citation2004). LINR4 and LIN19 primers detect Leishmania spp. by the conventional PCR (cPCR) (Aransay et al. Citation2000), but do not distinguish species. On the other hand, Lachaud et al. (Citation2002) tested several sets of primers, targeting Leishmania spp., L. donovani sensu lato, L. donovani sensu lato, Leishmania spp., Leishmania spp., and L. donovani sensu lato, for the detection of just one specimen of L. infantum (MHOM/FR/78/LEM75), but just RV1-RV2 and K13A-K13B primers detected the parasite in all sampled symptomatic and seropositive dogs.
In order to test the proposed hypothesis that a new set of primers (Lch14:Lch15, targeting a multiple alignment for L. infantum kDNA minicircle) is an efficient diagnostic tool for the routine and confirmation detection for epidemiological studies, this study aimed to design specific primers for L. infantum and validate them, using the selected primers to test lymph node and bone marrow samples in order to assess sensitivity and specificity, and analyze the blood samples from 69 stray dogs to determine the prevalence of parasite DNA by cPCR and qPCR.
2. Materials and methods
2.1. Primer design
The species-specific primers () were designed using Primer3 (http://biotools.umassmed.edu/bioapps/primer3_www.cgi), PrimerBLAST (http://www.ncbi.nlm.nih.gov/tools/primer-blast/), and BioEdit (Hall Citation1999) programs, directed to multiple alignments of L. infantum kDNA minicircle sequences (GenBank accession n.AF308682.1, AF103738.1, AF169138.1, AF169132.1, AF103739.1, AF169139.1, AF169137.1). Priming sites were selected based on the melting temperature, GC content, internal stability, self- and heterodimers, and hairpins (). All primers and amplicons for all combinations were blasted to identify the maximum identity and query coverage with other microorganisms. The amplicons of all combinations presented 100% homology with at least one of the previously listed GenBank accession numbers for L. infantum, presenting query coverage of 100%.
Table 1. Characterization of oligonucleotide primers used for species-specific amplification of Leishmania infantum (GenBank accession n.AF308682.1). The position is based on the numbering in the original sequence where the first base of the inset at the 5’ end is the number 1.
For qPCR, the sense and antisense primers selected were those with a final amplicon ranging from 60 to 200 bp or, preferentially, 70–170 bp, for better sensitivity and specificity of the reaction. Shorter amplicons acted as a buffer against variations in template integrity. Primers designed to amplify larger regions are less likely to anneal with the same fragment in a slightly degraded nucleic acid sample. Sets of species-specific oligonucleotide primers have been paired and aligned to the target using in silica (computational) tests (BioEdit, Carlsbad, CA, USA). The best five sets (Lch7:Lch17, Lch9:Lch19, Lch11:Lch19, Lch14:Lch15, and Lch14:Lch17), considering the percentage matched and product size, were preselected, but just the best one (Lch14 (5′-CGCACGTTATATCTACAGGTTGAG-3′) and Lch15 (5′-TGTTTGGGATTGAGGTAATAGTGA-3′)) was selected for the in gel (molecular) tests. This set targets 167 bp of the L. infantum kDNA.
For the parasite strains, all Leishmania strains were kindly provided by Leishmania Collection of the Oswaldo Cruz Institute (CLIOC) and Service of Donation of Leishmania Samples type culture collection – LTCC-WDCM 731, and the Trypanosoma strain by Trypanosoma Collection of Wild and Domestic Mammals, and Vectors (ColTryp), all of them from Oswaldo Cruz Foundation. All strains were kept in culture media (Novy-MacNeal-Nicolle, NNN; and Liver Infusion Tryptose, LIT) in a chamber with controlled temperature of 27 °C.
A panel of 10 DNA samples of Leishmania strains and one Trypanosoma strain was used. The identification numbers of the strains are as follows: Leishmania major (MHOM/SU/1973/5-ASKH), L. amazonensis (IFLA/BR/1967/PH8), L. guyanensis (MHOM/BR/1975/M4147), L. infantum (MHOM/TN/1993/LV10), L. donovani (MHOM/ET/1967/HU3), L. braziliensis (MHOM/BR/1975/M2903), L. tropica (MHOM/SU/1958/STRAINOD), and Trypanosoma cruzi (ColTryp 0032/MCAN/BR/2008/CAO) were used in the present study. L. infantum was used as positive control, whereas the other Leishmania strains, T. cruzi, and other protozoa, bacteria, and fungi, i.e. Toxoplasma gondii, Sarcocystis hominis, Neospora caninum, Klebsiella pneumoniae, Staphylococcus aureus, Streptococcus pneumoniae, Paracoccidioides brasiliensis, and Aspergillus spp. were used as negative internal control.
2.2. Sampling
Parasite strains and 50 lymph node (25 positive and 25 negative for L. (L.) infantum each) and 50 bone marrow aspirate (25 positive and 25 negative for L. (L.) infantum each) samples were used to test the primers. Positive and negative (lymph node and bone marrow) samples for L. (L.) infantum were obtained from dogs from endemic (Bauru, 22°18′53″S, 49°03′38 ″W) and nonendemic (Botucatu, 22°53′09″S; 48°26′42″W) areas, respectively, in São Paulo State, Brazil. The infection was confirmed by the indirect fluorescent antibody test (IFAT ≥ 40) in serum samples from the animals, and parasitological test (GIEMSA stain and culturing) of the lymph node and bone marrow samples which is considered the gold standard to identify the parasite (Srivastava et al. Citation2011). Both lymph nodes and bone marrow were obtained from the same animal.
In addition, blood samples of 69 randomly selected stray dogs from an endemic area for VL, Bauru, were tested to determine the frequency of L. (L.) infantum DNA. The samples were collected from animals captured by the Zoonosis Control Center.
The study was approved by the Committee of Ethics of Use of Animals (CEUA), São Paulo State University (UNESP), and registered by the protocol #191/2010-CEUA.
2.3. Conventional polymerase chain reaction (cPCR) and restriction length fragment polymorphism PCR (RFLP-PCR)
The DNA extraction of the blood samples was performed by using Illustra™ blood genomicPrep Mini Spin Kit (GE Healthcare, Pittsburgh, PA, USA), while Illustra™ tissue and cells genomicPrep Mini Spin Kit (GE Healthcare) was used for tissue samples. The DNA concentration was measured using NanoVue™ (GE Healthcare). Each 0.2 μL microtube received the cPCR mixture composed of 1X PCR buffer (10 mM Tris HCl pH 8.0, 50 mM KCl), 1.5 mM MgCl2, 0.2 mM dNTP (Life Technologies, Carlsbad, CA, USA), 10 μM of each primer (IDTDNA, Coralville, IA, USA), 0.5 U Platinum Taq DNA polymerase (Life Technologies, 10 ng.μL DNA template, and ultrapure distilled water qs (Gibco, Waltham, MA, USA), to a total volume of 25 μL.
Genus-specific primers LINR4 (5′-GGGGTTGGTGTAAAATAGGG-3′) and LIN19 (5′-CAGAACGCCCCTACCCG-3′) were used according to Aransay et al. (Citation2000), with a final product of 720 bp. The amplification protocol for these primers consisted of preheating at 95 ºC for 3 min, followed by 30 cycles at 95 ºC for 30 s, 63 ºC for 30 s, and 72 ºC for 1 min, and a final extension at 72 ºC for 7 min. If the sample tested positive for LINR4-LIN19 (Leishmania spp.-specific primers), but negative for Lch14--Lch15 (L. infantum-specific primers), the characterization of the Leishmania spp. was determined using the restriction fragment length polymorphism PCR (RFLP-PCR) (Schönian et al. Citation2001, Citation2003), targeting the ITS1 gene using the primers LITSR (5′-CTGGATCATTTTCCGATG-3′) and L5.8S (5′-ACACTCAGGTCTGTAAAC-3′) (El Tai et al. Citation2000, Citation2001), with a final product of 300--350 bp. The restriction enzyme HaeIII (New England Biolabs, Ipswich, MA, USA) was used to digest the amplicon.
The new set of primers (Lch14--Lch15) was compared to RV1 (5′-CTTTTCTGGTCCCGCGGGTAGG-3′) and RV2 (5′-CCACCTGGCCTATTTTACACCA-3′) (Ravel et al. Citation1995; Le Fichoux et al. Citation1999), which targets a sequence in the LT1 fragment, located in the kinetoplast DNA minicircle of the L. donovani complex, and amplifies 145 bp. The cycling profile consisted in an initial denaturation at 94 ºC for 4 min, 40 cycles at 94 ºC for 30 s, 59 ºC for 30 s, and 72 ºC for 30 s, and a final extension at 70 ºC for 10 min (Lachaud et al. Citation2002). The cPCR protocol for the designed primers was optimized with the same cycling profile of RV1 and RV2.
All amplifications were performed in a MasterCycler EP gradient thermal cycler (Eppendorf, Hauppauge, NY, USA). DNA-extracted ultrapure water and DNA of other protozoa and bacteria listed above were used as negative controls. The cPCR products were analyzed using electrophoresis in 1.5% agarose, stained with SYBR® safe DNA gel stain (Invitrogen, Waltham, MA, USA), and visualized in an image analyzer (GelDoc-IT™ Imaging System – UVP, Upland, CA, USA) by using VisonWorks®LS Software (UVP).
The analytical sensitivity of cPCR was determined using a suspension of 106 promastigotes mL−1 of L. infantum (MHOM/TN/1993/LV10). Clinical samples (canine blood, lymph node, and bone marrow) were diluted in ultrapure water and the parasites were counted in a hemocytometer. Each clinical sample was collected from dogs proved to be healthy by serology, parasitological tests, and cPCR. The sample volumes were adjusted to the same volume used in the experiment. Serial dilutions for each type of sample and parasite were obtained, as follows: 105, 104, 103, 102, 101, and 100 parasites mL−1. cPCR reactions were performed with Lch14 and Lch15 primers to determine the minimum amount of DNA that could be detected. The canine housekeeping gene β-globin (Quaresma et al. Citation2009) was also used to assure the quality of the DNA, check for PCR inhibitors, contamination, and normalize the reaction. The positive amplification of the housekeeping gene was considered successful.
2.4. Quantitative PCR (qPCR)
qPCR was carried out using a StepOne™ Plus Real-Time PCR System (Life Technologies). The master mix was composed of 12.5 μL Power SYBR®Green PCR Master Mix (Life Technologies), 10 μM of each primer (Lch14 and Lch15, 5 μM), 100 ng.μL−1 DNA template, and sterile ultrapure distilled water q.s. (Gibco), to a final volume of 25 μL. A standard curve was generated using serial dilutions of DNA of L. infantum (MHOM/TN/1993/LV10), with dilutions from 105 to 100 parasites mL−1. Cycling parameters were 95 ºC for 10 min, 50 cycles at 95 °C for 15 s, 60 °C for 1 min, and followed by a dissociation curve. All the samples and negative controls were analyzed in triplicate. The same canine housekeeping gene β-globin was used to normalize the reactions. The efficiency of amplification of the gene was determined in the exponential phase of the amplification curve provided by the software. The fluorescence intensity of each sample, which is proportional to the amount of DNA present, was expressed in terms of the PCR threshold cycle (CT) defined as the number of PCR cycles required for the fluorescence signal to exceed a preset threshold (background noise) (Quaresma et al. Citation2009). Data were collected and analyzed with the Step One™ Software v.2.1 (Life Technologies).
2.5. Sequencing
All amplicons were purified by employing ExoSAP (USB-Affymetrix, Cleveland, OH, USA), and the sequencing reactions were carried out on both strands in a 3500 Genetic Analyzer (Applied Biosystems by Life Technologies), according to DYEnamicTM ET Dye Terminator Cycle Sequencing kit (GE Healthcare). The obtained sense and antisense sequences were visualized (Chromas 2.3 software, Technelysium Pty Ltd., South Brisbane, Australia), aligned by BioEdit Sequence Alignment Editor 7.0.9.0 software (Hall Citation1999), and compared with the National Center for Biotechnology Information (NCBI) GenBank database using the Basic Local Alignment Search Tool (BLASTn) (http://www.ncbi.nlm.nih.gov/BLAST) to confirm each parasite.
2.6. Statistics
The concordance between cPCR and qPCR results for L. infantum, and detection was measured using the Cohen's kappa coefficient (κ) (Mackinnon Citation2000; Ayres et al. Citation2007). Mann–Whitney (U) test was used to analyze the distribution of the parasite load of L. infantum detected by qPCR, according to the sex of the dogs. All analyses considered a significance level (α) of 5%. To do so, GraphPad Prism v.5.01 (GraphPad Software Inc., La Jolla, CA, USA) was used.
3. Results
The analytical sensitivity for Lch14 and Lch15 in all tested clinical samples was ∼100 parasite mL−1 (10−5 copies of the target). qPCR presented an excellent amplification plot (Eff% = 100.71%; R2 = 0.991; slope = −3.305), without dimmers or hairpins. kDNA and β-globin PCR amplicons showed a single peak of fluorescence for the melting temperatures (Tm) to the dissociation curve (74.3 °C for kDNA; 53.3 °C for β-globin). In this way, a single PCR product was generated in each assay.
The concordance of cPCR and qPCR was perfect for all lymph node and bone marrow aspirate samples from positive and negative animals for CVL. All 50 positive samples (lymph node and bone marrow) by the parasitological test gave positive results for genus-specific (Leishmania spp.) and species-specific (L. infantum) cPCR and qPCR, while the 50 negative lymph node and bone marrow samples were negative for both tests. The highest quantities of L. infatum detected by qPCR were 10,108.04 parasites mL−1 (lymph node) and 80,051.25 (bone marrow). On the other hand, RV1--RV2 amplified DNA from L. infantum and other Leishmania species, with 96%–100% homology to L. major, L. infantum, and L. donovani (GenBank accession n.J04654.1, Z35276.1, AB678348.1, FR799614.1, EU437403.1, EU437405.1, EU437406.1, EU437407.1, FJ416603.1, AF027578.1, Z35270.1, Z35271.1, Z35272.1, Z35273.1, Z35274.1, Z35275.1, Z35500.1, Z35501.1, AJ223724.1).
For the detection in blood samples, cPCR resulted in 50/69 positive (73%; CI 95%: 61%–82%) samples for Leishmania spp., and in 40 out of that 69 (58%; CI 95%: 46%–69%) for L. infantum. No amplification was observed to the new set of primers concerning the negative blood samples for LINR4 and LIN19 primers. As the sequencing of LINR4--LIN19 amplicons did not characterize the species, ITS1 gene was chosen to characterize the species of the other 10/50 positive samples for Leishmania spp. but negative for L. infantum by Lch14–Lch15 primers. All 10 samples were identified as L. braziliensis by RFLP-PCR and sequencing (GenBank accession n. EU370877.1).
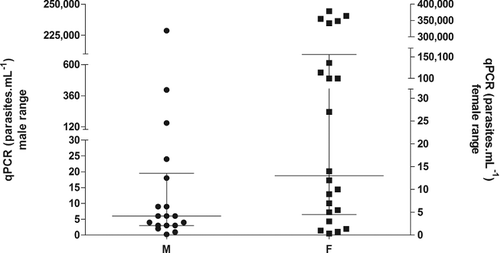
The concordance of cPCR and qPCR for the blood samples to L. infantum was also perfect. shows that 18/40 (45%; CI 95%: 31%–60%) positive blood samples for L. infantum were from male dogs (median = 6 parasites mL−1; P25 = 3.28 parasites mL−1, and P75 = 16.1 parasites mL−1; highest value = 228,712 parasites mL−1) and 22/40 (55%; CI 95%; 40%–69%) from females (median = 13 parasites mL−1; P25 = 4.50 parasites mL−1, and P75 = 166,527 parasites mL−1; highest value = 378,843 parasites mL−1) (U = 136.00; p-value = 0.09).
4. Discussion
The frequency of positive stray dogs observed in this study (73% Leishmania spp. and 58% L. infantum) is almost similar to other studies also using peripheral blood, i.e. Bigeli et al. (Citation2012) (59%, 121/204), higher than Lazari et al. (Citation2016) (45%, 45/101), but lower than Lachaud et al. (Citation2002) (93%, 27/29).
The new set of primers presented specificity and positive predictive values of 100% for both molecular techniques, considering the stray dog population studied. Ceccarelli et al. (Citation2014) observed a similar sensitivity in qPCR, and detected a range of 0.1–26.91 parasites mL−1 (symptomatic group), 4.263–22.273 parasites mL−1 (asymptomatic group), and 0.21–26.21 parasites mL−1 (monitored after therapy group) in canine blood samples, compared to the present results with a range of 0.21–378,843 parasites mL−1 of blood. Leishmania parasites have a high number of kDNA minicircles copies (10,000 copies) per parasite, which increases the sensitivity of the assay (Quaresma et al. Citation2009; Solcà et al. Citation2012), as observed in this study with ∼100 parasite mL−1.
In the study described here, RV1 and RV2 were not specific for L. infantum. Solcà et al. (Citation2012) demonstrated that these primers are capable to amplify the DNA from other leishmanias, not just L. infantum, but with a very different sensitivity and with the specificity dependent on the concentration of the parasite. As observed in the present study, RV1 and RV2 were not capable to discriminate L. infantum, L. amazonensis, and L. major by cPCR. On the other hand, Lima-Junior et al. (Citation2009) detected no amplification of L. amazonensis and L. braziliensis DNA strains using the same primers by cPCR.
In this way, Lch14 and Lch15 primers were designed and validated focusing on a fast, sensitive, and specific detection of L. infantum in culture and in clinical samples of dogs and, probably, other hosts by cPCR and qPCR; therefore, being a useful tool for clinical diagnostics and epidemiological studies. Lch14 and Lch15 primers confirmed our hypothesis based on the observed specificity to detect L. infantum, confirmed by sequencing with 100% homology for L. infantum (MHOM/TN/1993/LV10). No false-positive amplification was observed in cPCR or qPCR for the other species of leishmanias.
Species-specific PCR for L. infantum targeting the kDNA minicircle (Le Fichoux et al. Citation1999) or 18S rRNA (Vides et al. Citation2011) has been widely used. In Brazil, many studies focusing on L. infantum increased the importance of epidemiological studies, e.g. the identification of L. infantum in dermatological and visceral lesions of cats (Vides et al. Citation2011) or dogs (Savani et al. Citation2005; Queiroz et al. Citation2011) from endemic areas, or nonendemic areas (Braga et al. Citation2014), and research in phlebotomines (Missawa et al. Citation2010; Michalsky et al. Citation2011).
The new set of primers, Lch14 and Lch15, presented useful results for the detection of the infection in the studied stray dog population, and may be helpful for screening in epidemiological studies and help in the clinical diagnosis of VL in the dog population as well as other hosts.
High concentrations of L. infantum DNA were observed in some female dogs by qPCR, but the median of both male and female groups was not significant (p-value = 0.09). This high parasite load in some female dogs may be a totally random finding, due to the place where the animal lives and frequent exposure to the source of infection or the vector. Athanasiou et al. (Citation2012) also observed a nonsignificant higher prevalence in female than male dogs, but Ciaramella et al. (Citation1997) and Miranda et al. (Citation2008) observed that males are more predisposed to the infection.
In this way, and to have a more precise and accurate diagnostic test to help control VL, molecular techniques can be applied in different types of samples, and combined with other strategies, i.e. serological tests. In this study, a set of species-specific primers is demonstrated to detect at least one parasite mL−1. The authors suggest the application of this tool in both diagnostic routine and epidemiological studies, combined to serological tests, to analyze the host immune response, which will be helpful to control programs in endemic areas.
5. Conclusions
This study presented high frequency of stray dogs infected with L. infantum, with considerable parasite load, which is expected for both stray and household dogs from endemic areas for CVL. Also, the present results suggest that this new set of primers could be used as diagnostic method to detect L. infantum DNA in infected clinical samples in dogs, as well as for epidemiological studies, which can help in surveillances and control measures for VL. Further studies should be conducted to evaluate their use as a confirmation assay for epidemiological studies in other hosts and vectors, as well as to analyze the risk factors related to the infection in stray and owned dogs from different endemic and nonendemic areas for CVL.
Acknowledgments
We thank Oswaldo Cruz Foundation, Leishmania Collection of the Oswaldo Cruz Institute (CLIOC), and Trypanosoma Collection of Wild and Domestic Mammals, and Vectors (ColTryp) for providing all 10 panels of Leishmania spp., and Trypanosoma cruzi strains, and São Paulo State University (UNESP) for the logistical support.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Acha P, Szyfres B. 2003. Zoonoses and communicable diseases common to man and animals. 3rd ed. Geneva: Pan American Health Organization.
- Agallou M, Athanasiou E, Samiotaki M, Panayotou G, Karagouni E. 2016. Identification of immunoreactive Leishmania infantum protein antigens to asymptomatic dog sera through combined immunoproteomics and bioinformatics analysis. PLoS One. 11:e0149894.
- Alvar J, Velez ID, Bern C, Herrero M, Desjeux P, Cano J, Jannin J, den Boer M, Team WHOLC. 2012. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 7:e35671.
- Aransay AM, Scoulica E, Tselentis Y. 2000. Detection and identification of Leishmania DNA within naturally infected sand flies by seminested PCR on minicircle kinetoplastic DNA. Appl Environ Microbiol. 66:1933–1938.
- Athanasiou LV, Kontos VI, Saridomichelakis MN, Rallis TS, Diakou A. 2012. A cross-sectional sero-epidemiological study of canine leishmaniasis in Greek mainland. Acta Trop. 122:291–295.
- Ayres M, Ayres Jr., M, Ayres DL, Santos AS. BioEstat 5.0. Aplicações estatísticas nas áreas das ciências biológicas e médicas [ Computer program]. 2007. Version v.5.0. Belém: Instituto de Desenvolvimento Sustentável Mamirauá.
- Bigeli JG, Oliveira Jr., WP, Teles NM. 2012. Diagnosis of Leishmania (Leishmania) chagasi infection in dogs and the relationship with environmental and sanitary aspects in the municipality of Palmas, State of Tocantins, Brazil. Rev Soc Bras Med Trop. 45:18–23.
- Braga AR, Langoni H, Lucheis SB. 2014. Evaluation of canine and feline leishmaniasis by the association of blood culture, immunofluorescent antibody test and polymerase chain reaction. J Venom Anim Toxins incl Trop Dis. 20:5
- Ceccarelli M, Galluzzi L, Migliazzo A, Magnani M. 2014. Detection and characterization of Leishmania (Leishmania) and Leishmania (Viannia) by SYBR green-based real-time PCR and high resolution melt analysis targeting kinetoplast minicircle DNA. PLoS One. 9:e88845.
- Ciaramella P, Oliva G, Luna RD, Gradoni L, Ambrosio R, Cortese L, Scalone A, Persechino A. 1997. A retrospective clinical study of canine leishmaniasis in 150 dogs naturally infected by Leishmania infantum. Vet Rec. 141:539–543.
- El Tai NO, El Fari M, Mauricio I, Miles MA, Oskam L, El Safi SH, Presber WH, Schonian G. 2001. Leishmania donovani: intraspecific polymorphisms of Sudanese isolates revealed by PCR-based analyses and DNA sequencing. Exp Parasitol. 97:35–44.
- El Tai NO, Osman OF, El Fari M, Presber W, Schonian G. 2000. Genetic heterogeneity of ribosomal internal transcribed spacer in clinical samples of Leishmania donovani spotted on filter paper as revealed by single-strand conformation polymorphisms and sequencing. Trans R Soc Trop Med Hyg. 94:575–579.
- Hall TA. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser. 41:95–98.
- Lachaud L, Marchergui-Hammami S, Chabbert E, Dereure J, Dedet JP, Bastien P. 2002. Comparison of six PCR methods using peripheral blood for detection of canine visceral leishmaniasis. J Clin Microbiol. 40:210–215.
- Lazari P, Almeida ABPF, Dutra V, Nakazato L, Dias AFLR, Brito VN, Oliveira CM, Sousa VRF. 2016. Leishmania chagasi in dogs from the city of Jaciara, Mato Grosso, Brazil. Ciênc Rural. 46:315–317.
- Le Fichoux Y, Quaranta JF, Aufeuvre JP, Lelievre A, Marty P, Suffia I, Rousseau D, Kubar J. 1999. Occurrence of Leishmania infantum parasitemia in asymptomatic blood donors living in an area of endemicity in southern France. J Clin Microbiol. 37:1953–1957.
- Lima-Junior MS, Andreotti R, Dorval ME, Oshiro ET, Oliveira AG, Matos MF. 2009. Identification of Leishmania species isolated in human cases in Mato Grosso do Sul, by means of the polymerase chain reaction. Rev Soc Bras Med Trop. 42:303–308.
- Mackinnon A. 2000. A spreadsheet for the calculation of comprehensive statistics for the assessment of diagnostic tests and inter-rater agreement. Comput Biol Med. 30:127–134.
- Maia C, Cardoso, L. 2015. Spread of Leishmania infantum in Europe with dog travelling. Vet Parasitol. 213:2–11.
- Marcili A, Speranca MA, da Costa AP, Madeira Mde F, Soares HS, Sanches Cde O, Acosta Ida C, Girotto A, Minervino AH, Horta MC, et al. 2014. Phylogenetic relationships of Leishmania species based on trypanosomatid barcode (SSU rDNA) and gGAPDH genes: taxonomic revision of Leishmania (L.) infantum chagasi in South America. Infect Genet Evol. 25:44–51.
- Mary C, Faraut F, Lascombe L, Dumon H. 2004. Quantification of Leishmania infantum DNA by a real-time PCR assay with high sensitivity. J Clin Microbiol. 42:5249–5255.
- Mauricio IL, Stothard JR, Miles MA. 2000. The strange case of Leishmania chagasi. Parasitol Today. 16:188–189.
- Michalsky ÉM, Guedes KS, Silva FOL, França-Silva JC, Dias CLF, Barata RA, Dias ES. 2011. Natural infection with Leishmania infantum chagasi in Lutzomyia (Lutzomyia) longipalpis (Diptera: Psychodidae) sandflies captured in the municipality of Janaúba, State of Minas Gerais, Brazil. Rev Soc Bras Med Trop. 44:58–62.
- Miranda S, Roura X, Picado A, Ferrer L, Ramis A. 2008. Characterization of sex, age, and breed for a population of canine leishmaniosis diseased dogs. Res Vet Sci. 85:35–38.
- Missawa NA, Michalsky EM, Fortes-Dias CL, Santos Dias E. 2010. Lutzomyia longipalpis naturally infected by Leishmania (L.) chagasi in Varzea Grande, Mato Grosso State, Brazil, an area of intense transmission of visceral leishmaniasis. Cad Saude Publ. 26:2414–2419.
- Pennisi MG. 2015. Leishmaniosis of companion animals in Europe: an update. Vet Parasitol. 208:35–47.
- Quaresma PF, Murta SM, Ferreira Ede C, da Rocha-Lima AC, Xavier AA, Gontijo CM. 2009. Molecular diagnosis of canine visceral leishmaniasis: identification of Leishmania species by PCR-RFLP and quantification of parasite DNA by real-time PCR. Acta Trop. 111:289–294.
- Queiroz NM, Silveira RC, Noronha Jr., AC, Oliveira TM, Machado RZ, Starke-Buzetti WA. 2011. Detection of Leishmania (L.) chagasi in canine skin. Vet Parasitol. 178:1–8.
- Ramos RA, Ramos CA, Jusi MM, de Araujo FR, Machado RZ, Faustino MA, Alves LC. 2012. Polymerase chain reaction and real-time PCR for diagnosing of Leishmania infantum chagasi in dogs. Rev Bras Parasitol Vet. 21:192–195.
- Ravel S, Cuny G, Reynes J, Veas F. 1995. A highly sensitive and rapid procedure for direct PCR detection of Leishmania infantum within human peripheral blood mononuclear cells. Acta Trop. 59:187–196.
- Savani ES, Nunes VL, Galati EA, Castilho TM, Araujo FS, Ilha IM, Camargo MC, D'Auria SR, Floeter-Winter LM. 2005. Occurrence of co-infection by Leishmania (Leishmania) chagasi and Trypanosoma (Trypanozoon) evansi in a dog in the state of Mato Grosso do Sul, Brazil. Mem Inst Oswaldo Cruz. 100:739–741.
- Schönian G, Nasereddin A, Dinse N, Schweynoch C, Schallig HDFH, Presber W, Jaffe CL. 2003. PCR diagnosis and characterization of Leishmania in local and imported clinical samples. Diagn Microbiol Infect Dis. 47:349–358.
- Schönian G, Schnur L, El Fari M, Oskam L, Kolesnikov AA, Sokolowska-Kohler W, Presber W. 2001. Genetic heterogeneity in the species Leishmania tropica revealed by different PCR-based methods. Trans R Soc Trop Med Hyg. 95:217–224.
- Shaw JJ. 2006. Further thoughts on the use of the name Leishmania (Leishmania) infantum chagasi for the aetiological agent of American visceral leishmaniasis. Mem Inst Oswaldo Cruz. 101:577–579.
- Solcà MS, Guedes CE, Nascimento EG, Oliveira GG, Santos WL, Fraga DB, Veras PS. 2012. Qualitative and quantitative polymerase chain reaction (PCR) for detection of Leishmania in spleen samples from naturally infected dogs. Vet Parasitol. 184:133–140.
- Srivastava P, Dayama A, Mehrotra S, Sundar S. 2011. Diagnosis of visceral leishmaniasis. Trans R Soc Trop Med Hyg. 105:1–6.
- Tsokana CN, Athanasiou LV, Valiakos G, Spyrou V, Manolakou K, Billinis C. 2014. Molecular diagnosis of leishmaniasis, species identification and phylogenetic analysis. In: Claborn DM, editor. Leishmaniasis – trends in epidemiology, diagnosis and treatment. Rijeka: InTech; p. 161–193.
- Vides JP, Schwardt TF, Sobrinho LS, Marinho M, Laurenti MD, Biondo AW, Leutenegger C, Marcondes M. 2011. Leishmania chagasi infection in cats with dermatologic lesions from an endemic area of visceral leishmaniosis in Brazil. Vet Parasitol. 178:22–28.