ABSTRACT
Three dogs were investigated for chronic unilateral nasal discharge. In all cases CT imaging showed an intranasal mass causing turbinate lysis and no evidence of metastasis. Cytology in cases 1 (a 14-year-old neutered male crossbreed dog) and 2 (a five-year-old neutered male German Shepherd dog) demonstrated a pleomorphic cell population with variable intracellular pigment suspicious of melanocytic neoplasia. Histopathology with immunohistochemistry (Melan-A and vimentin, plus PNL-2 in one case) confirmed the diagnosis of melanoma in all dogs. All dogs were treated with megavoltage radiotherapy using linear accelerators. Cases 1 and 3 (a nine-year-old neutered female beagle dog) received a hypofractionated (4 × 8 Gy) protocol and case 2 received a definitive (12 × 4 Gy) protocol. Complete remission was demonstrated on repeat CT scan five months after diagnosis in case 1 and seven months in case 2. Stable disease was documented on CT at four months for case 3; however, clinical signs in this dog remained controlled for 10 months in total. Case 1 died of unrelated causes five months after diagnosis, case 2 was euthanased due to the development of seizures 13 months after diagnosis, and case 3 was lost to follow-up 12 months after diagnosis. Melanoma should be considered as a rare differential diagnosis for primary nasal neoplasia in the dog and radiation therapy can be used as effective local therapy.
1. Introduction
Most intranasal neoplasms in the dog are carcinomas and sarcomas (Madewell et al. Citation1976; Kubicek et al. Citation2016) and rarely other tumours, including lymphoma (Patnaik Citation1989), mast cell tumours (Naganobu et al. Citation2000), transmissible venereal tumours (Papazoglou et al. Citation2001), haemangiosarcoma (Fujita et al. Citation2008), neuroblastoma (Ueno et al. Citation2007) and multilobular osteochondrosarcoma (Patnaik Citation1989) have been reported. The primary treatment of most canine intranasal neoplasms is radiotherapy (Lana et al. Citation2004; Lawrence et al. Citation2010) often with adjuvant chemotherapy (Lana et al. Citation2004; Adams et al. Citation2005). Neoadjuvant surgical debulking has also been described, but has not been shown to improve clinical outcome (Morris et al. Citation1994; Adams et al. Citation2005).
Most intranasal cancers in humans are squamous cell carcinomas (Peryaga et al. Citation2016). Primary intranasal melanoma is uncommon, accounting for approximately 3.6% of all nasal tumours in people (Gouldesbrough et al. Citation1992). Treatment typically involves radical surgery and adjunctive radiotherapy (Lin et al. Citation2003). Intranasal melanoma in the dog is considered exceptionally rare, with one case report in the literature describing a dog treated with surgical debulking (via rhinotomy) and adjunctive radiotherapy (Hicks & Fidel Citation2006).
Canine melanoma typically occurs in the oral cavity, lip, haired skin and digits, with other sites constituting 2% of the total (Smith et al. Citation2002). Melanomas originating from mucous membranes, specifically oral, are usually associated with invasive behaviour and a high metastatic rate (Millanta et al. Citation2002). Local control is achieved with radical surgery or hypofractionated radiotherapy. However, most dogs will die or be euthanased as a result of metastatic disease (Tuohy et al. Citation2014).
This report describes three cases of canine intranasal melanoma treated with radiotherapy.
2. Case series
2.1. Case 1
Case 1 was a 14-year-old neutered male, 23.5 kg crossbreed dog, presented for evaluation of a five-month history of sneezing and muco-haemorrhagic, right-sided nasal discharge. Physical examination revealed reduced airflow through the right naris but was otherwise unremarkable. A complete blood count, serum biochemistry panel and cytology of both mandibular lymph nodes were unremarkable.
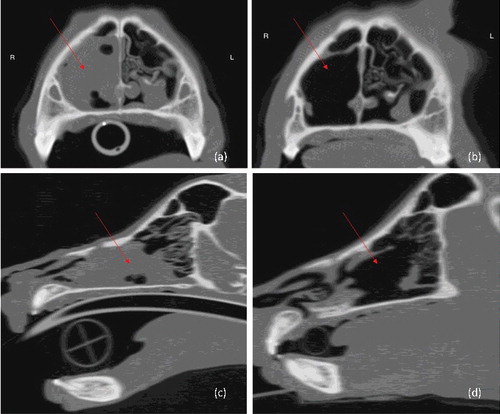
Contrast computed tomography (CT) of the head, neck, thorax and abdomen was performed under general anaesthesia. A soft tissue attenuating mass was present in the right nasal cavity (measuring approximately 4.8 cm rostrocaudally, 1.7 cm dorsoventrally and 1.9 cm lateromedially), associated with significant turbinate lysis and focal destruction of the right nasomaxillary suture (). No metastatic lesions were identified. Rhinoscopy was used to obtain biopsy and cytology samples.
Figure 1. Pre-treatment and post-treatment CT images of case 1: (a) pre-treatment transverse; (b) post-treatment transverse; (c) pre-treatment longitudinal; (d) post-treatment longitudinal. Arrows mark the location of the tumour.
Cytology of the nasal mass demonstrated a population of pleomorphic polyhedral to spindloid cells ((a,b)). Nuclei were centrally placed and round with stippled chromatin and one or multiple prominent nucleoli; occasional macronuclei or multinucleate cells were observed. The cellular pleomorphism and presence of green pigment in some cells were suggestive of a melanoma. Histopathology described pleomorphic, round to polygonal cells containing varying numbers of nuclei, abundant eosinophilic cytoplasm with light-brown intracellular granules, and fewer than one mitosis in 10 high-power (× 400) fields. The histogenesis of the tumour was unclear; melanoma, olfactory neuroblastoma, neuroendocrine tumour and undifferentiated carcinoma were considered differential diagnoses. Fontana–Masson stain for melanin granules was weakly positive. Immunohistochemical labelling () showed the neoplastic cells to stain strongly positive for melanocytic antigen recognized by cytotoxic T lymphocytes (Melan-A), vimentin and anti-melanoma antibody (PNL-2), and negative for S100 protein, smooth muscle actin (SMA) and multiple myeloma-1 protein (MUM-1), consistent with the diagnosis of melanoma (Busam & Jungbluth Citation1999; Ramos-Vara & Miller Citation2011).
Figure 2. Cytology from case 1: (a) multinucleate cells (black arrows) with variable pigmentation (red arrow); (b) a high degree of cellular pigment (red arrow) and the marked pleomorphism (white arrows).
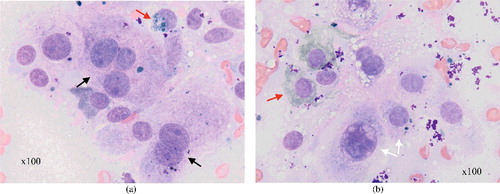
Table 1. Immunohistochemical markers used in the diagnosis of Cases 1 and 2 in relation to published levels of expression in canine melanoma. Primary and secondary antibodies used in the immunhositochemical labelling are given in brackets (1Ramos-Vara et al. Citation2000; 2Giudice et al. Citation2010; 3Koenig et al. Citation2001; 4Rabanal et al. Citation1989).
The dog was treated with a hypofractionated radiotherapy protocol using a Varian 6MV ‘Clinac’ linear accelerator and CT-based computer planning was performed with CadPlan 3.0 software (version 1.3, Varian-Dosetek, Palo Alto, CA, USA). The dog received general anaesthesia for each fraction (premedicated with 0.2 mg/kg BW butorphanol intravenously, induced with a total dose of 2.4 mg/kg BW propofol, given intravenously ‘to effect’, maintained with isofluorane in 100% oxygen via endotracheal tube). Positioning was facilitated using a bespoke mouth-gag, and a combination of polystyrene blocks and tape. A total dose of 32 Gray (Gy; 1 joule of radiation energy absorbed per kilogram of BW) was delivered to the tumour as four, once-weekly 8 Gy fractions. A single radiation beam was used; field depth was 98.5 cm, clinical target volume (CTV) was 0.6 cm around the gross tumour volume (GTV), and the planning target volume (PTV) was 1.0 cm around CTV; eyes and brain were not included in the treatment field. Lymph nodes were not treated. This treatment was tolerated with no apparent adverse effects and resulted in rapid and complete resolution of clinical signs. Repeat CT 4 months after treatment demonstrated complete response of the nasal tumour () and no evidence of metastatic disease. No medical therapy was given. The dog was euthanased due to discomfort associated with a soft tissue sarcoma in its hind leg five months after diagnosis; CT scan at this time revealed no metastatic disease from either neoplasm, and the diagnosis of soft tissue sarcoma was confirmed on immunohistochemistry (negative labelling for Melan-A, positive for vimentin). Necropsy was not performed.
2.2. Case 2
Case 2 was a five-year-old neutered male, 44 kg German Shepherd dog, presented for a three-week history of intermittent left-sided muco-haemorrhagic, nasal discharge and sneezing. Physical examination revealed reduction in left-sided nasal airflow and mild left mandibular lymphadenomegaly. Complete blood count, serum biochemistry, urinalysis, activated thromboplastin and prothrombin times, and blood pressure were within normal limits. Cytology of both mandibular lymph nodes was unremarkable.
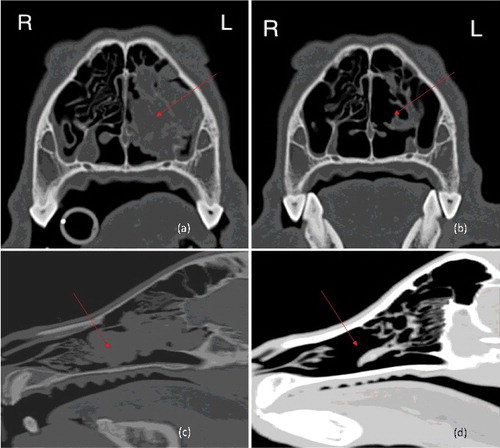
CT scan of the head, thorax and abdomen demonstrated a large, contrast-enhancing soft tissue lesion in the left nasal cavity (measuring approximately 6.4 cm rostrocaudally, 2.7 cm dorsoventrally and 2.4 cm lateromedially), causing significant turbinate lysis and focal destruction of the left nasomaxilliary bones (). No evidence of detectable metastasis was identified. Cytology and biopsy samples were obtained via rhinoscopy.
Figure 3. Pre-treatment and post-treatment CT images of case 2: (a) pre-treatment transverse; (b) post-treatment transverse; (c) pre-treatment longitudinal; (d) post-treatment longitudinal. Arrows mark the location of the tumour.
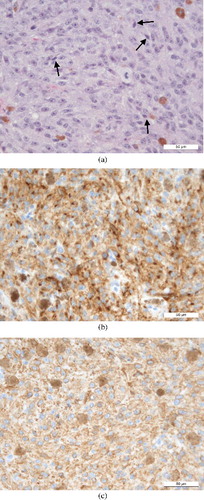
Cytology showed round to spindle-shaped cells with moderate anisocytosis and anisokaryosis, and variable nucleolar number and morphology. Dark pigment was occasionally seen within these cells and melanoma was deemed the most likely diagnosis. Histopathology () was consistent with a poorly differentiated, non-pigmented spindle cell malignancy. Mitotic activity was 0–2 per ×400 field. Immunohistochemical labelling with vimentin, SMA, desmin, neuron-specific enolase, glial fibrillary acidic protein and melan-A was performed. Neoplastic cells labelled with vimentin and melan A, supporting the diagnosis of amelanotic melanoma ((b,c), ).
Figure 4. Histology of the nasal mass in case 2: (a) H&E section (×40) demonstrating poor cellular differentiation, which in rare places tended towards spindle cell morphology (black arrows); (b) positive immunohistochemical labelling for Melan-A (×40); (c) positive immunohistochemical labelling for vimentin (×40).
The dog was treated with a definitive radiotherapy protocol directed to the primary tumour and mandibular lymph nodes. For planning CT and treatment delivery, the patient was placed under general anaesthesia, (premedicated with intravenous medetomidine [5 ug/kg BW] and butorphanol [0.015–0.03 mg/kg BW], or medetomidine [7–10 ug/kg BW] alone for later fractions, induced with propofol, maintained with sevoflurane in 100% oxygen via endotracheal tube) and positioned in sternal recumbency. Immobilization was achieved using a thermoplastic mask and a customized head support, secured to a plastic head-frame with four points of fixation. Radiotherapy consisted of 12 fractions of 4 Gy on a Monday, Wednesday, Friday basis (48 Gy total), delivered using a Dual Energy Linear Accelerator (Clinac 2100 C, Varian, Palo Alto, CA, USA) with 0.5-cm multi-leaf collimator (MLC). All treatments were carried out at 6 MV, and were 3D planned from CT using Pinnacle (version 9), with beam collimation using MLC and beam modification using dynamic wedges. Planning was carried out with the intent to include 95% of the PTV (GTV plus 1–2 cm normal tissue for CTV, planning treatment volume based on clinical target volume plus 5 mm, adjusting final planned treatment volume margins manually to spare left eye and reduce dose to brain tissue) in the 95%–105% iso-dose, i.e. scheduled dose heterogeneity less than 15%. The primary tumour was treated with three coplanar beams (left lateral oblique 254°; dorsoventral 356° with dynamic wedge; ventrodorsal oblique 160° with dynamic wedge). The submandibular lymph nodes were treated with a separate two field plan (left lateral 273°; right lateral 83°). Portal imaging was carried out twice during the treatment protocol to verify position: no adjustments were required. The lymph nodes were irradiated as per the institutional policy for malignant melanoma. In terms of organs at risk, the maximum dose to the frontal lobes was 4740 centiGray (cGy): these were not contoured separately from the brain. For the whole brain, the minimum dose was 64 cGy, and the mean 871 cGy. The mean dose to proximal cord was 93 cGy, minimum 14 cGy, maximum 368 cGy. The mean dose for the right eye was 4724 cGy, minimum 3476 cGy, maximum 4878 cGy. The mean dose for the left eye was 2076 cGy, minimum 1334 cGy, maximum 4413 cGy.
Concurrent prednisolone at 0.5 mg/kg BW orally, once daily was administered for anti-inflammatory effect. During the third week of radiation therapy conjunctivitis in the left eye was noted; facial nerve function was normal and initially fluorescein uptake of the left cornea was negative. Two weeks later a Schirmer tear test demonstrated low tear production in the left eye (10 mm) however fluorescein uptake remained negative; oral prednisolone was reduced, topical ketorolac (Allergan Pharmaceuticals Ireland, Westport, County Mayo, Ireland) and lubrication were introduced, and a buster collar was placed to stop the dog traumatizing the eye. Two weeks following, however, a deep ulcer was noted. Enucleation was elected over other treatments by the owners due to dog's acute discomfort.
Clinical signs of nasal discharge completely resolved within four weeks of starting radiotherapy. Seven months after diagnosis, a repeat CT scan of the head, thorax and abdomen showed complete remission of the nasal tumour; mucosal thickening and a small amount of fluid was present within the nasal cavity, but no contrast-uptake was seen, consistent with a complete response to therapy (). No evidence of metastatic disease was seen. At this point, firocoxib (Previcox, Merial, Lyon, France) at 5 mg/kg BW orally, once-daily, was prescribed to for potential anti-cancer effect as a COX-2 inhibitor (Proulx et al. Citation2003; Martinez et al. Citation2011). The firocoxib therapy was continued for five months and clinically no adverse effects of treatment were observed; supportive treatment (for example, gastroprotectants) was therefore not considered necessary. The dog developed seizure activity 13 months after diagnosis and was euthanized after further investigations were declined. Necropsy was not performed, and the aetiology of the seizures remains unclear.
2.3. Case 3
Case 3 was a nine year-old neutered female, 17.1 kg beagle dog with a four-week history of sneezing and left-sided haemorrhagic nasal discharge. Physical examination, a complete blood count and serum biochemistry panel were unremarkable. Blind nasal biopsies had been performed by the referring veterinary surgeon and histopathology demonstrated a diffuse cellular proliferation of pleomorphic neoplastic cells containing moderate amounts of pigment. The cells were arranged in haphazard bundles, had a polygonal to spindle-shaped morphology, mild anisocytosis and anisokaryosis, and contained a moderate amount of deep eosinophilic cytoplasm with variably distinct cellular borders. Nuclei varied between round and oval, and were mostly hyperchromatic with stippled chromatin and multiple amphophilic nucleoli. Approximately one mitotic figure was evident per high power field (400×). Malignant melanoma, plasma cell tumour or epithelioid haemangiosarcoma were considered likely diagnoses based on cellular morphology and the presence of intracellular pigment. The diagnosis of melanoma was confirmed with strong positive immunohistochemical labelling with PNL2, S100 and Melan A (MUM1 negative).
Contrast CT of the head and thorax was performed for radiotherapy planning and staging of disease under general anaesthesia. A relatively small soft tissue attenuating mass was present in the rostral half of the left nasal cavity (measuring approximately 2.0 cm rostrocaudally, 1.7 cm dorsoventrally and 1.1 cm lateromedially), with mild involvement of the nasal bone dorsally and the nasal septum medially (). No metastatic lesions were identified locoregionally or in lungs on the CT scan. Cytology of local lymph nodes was not assessed.
Figure 5. Pre-treatment and post-treatment CT images of case 3: (a) pre-treatment transverse; (b) post-treatment transverse. Arrows mark the tumour.
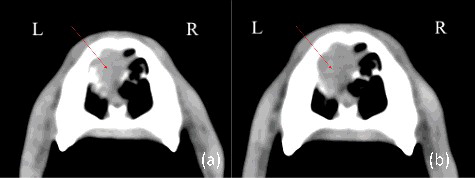
The dog was treated with a hypofractionated radiotherapy protocol. Planning, set-up and anaesthesia were as described in case 1. A total dose of 32 Gy was prescribed to the tumour as four, once-weekly 8 Gy fractions, using three fields, consisting of dorsal, right and left lateral beams; field depth was 1.6 cm dorsal, 1.8 cm right lateral and 2.4 cm left lateral, CTV was 0.2 cm around the GTV, and the planning target volume PTV was 0.4 cm around CTV; eyes and brain were not included in the treatment field. Lymph nodes were not treated. This treatment was tolerated with minimal adverse effects; sneezing episodes and minor epistaxis remained present throughout the treatment protocol. Repeat CT of the head only was performed 18 days after the treatment due to reports of persistent epistaxis. The CT revealed stable tumour size but with decreased contrast uptake. At this point, the dog started therapy with meloxicam (Metacam oral suspension for dogs, Boehringer Ingelheim, Ingelheim am Rhein, Germany) at a dose of 0.1 mg/kg BW orally, once-daily, after which clinical signs (sneezing and epistaxis episodes) resolved; the course of meloxicam continued for approximately six weeks. The dog remained asymptomatic and repeat CT scan (of the head only) four months after completion of the treatment revealed continued stability in tumour size (). Clinical signs recurred 10 months after completion of hypofractionated radiotherapy treatment. Further investigation and treatment were declined. The dog received no further treatment and was lost to follow-up 12 months after radiation therapy.
3. Discussion
Primary nasal melanoma is described in three dogs. In all cases, the variable histological appearance of melanoma precluded a definitive histomorphological diagnosis (Smith et al. Citation2002; Choi & Kusewitt Citation2003; Smedley et al. Citation2011). Immunohistochemistry was necessary to provide the final diagnosis in all cases after positive staining with Melan-A, PNL-2 and vimentin.
Canine intranasal melanoma has been successfully treated with surgery and radiotherapy once before (Hicks & Fidel Citation2006). Radiotherapy is a common primary treatment for intranasal neoplasia, producing clinical benefit in over 80% of dogs and a median survival time (MST) of over 500 days (Fujiwara et al. Citation2013). Radiotherapy is also effective in controlling oral melanomas, with an overall response rate of 83% (Bateman et al. Citation1994; Proulx et al. Citation2003) and a MST of 80–758 days, depending on factors such as tumour stage, sub-location in the oral cavity and the degree of osteolysis (Freeman et al. Citation2003; Proulx et al. Citation2003; Kawabe et al. Citation2015). Given its established efficacy, radiotherapy was performed first-line in the dogs in this report; surgery was considered unnecessary since all dogs experienced satisfactory control of disease with radiotherapy.
Fifty-three per cent of oral melanomas have evidence of metastasis at diagnosis (Williams & Packer Citation2003). Even if local control of the primary tumour has been effective, most dogs will die of distant metastasis (Freeman et al. Citation2003; Proulx et al. Citation2003). Case 1 did not have metastasis at diagnosis or follow-up; the tumour's low mitotic index may be pertinent in this regard, since a low mitotic index has been shown to correlate with indolent behaviour of oral melanomas (Spangler & Kass Citation2006; Esplin Citation2008). The existing report of a canine intranasal melanoma also describes a low mitotic index and absence of metastasis (Hicks & Fidel Citation2006), nevertheless, the short survival of case 1 makes evaluation of the metastatic propensity of this tumour unreliable. The metastatic status of case 2 is unknown since the cause of the dog's seizures is unclear; metastasis, local extension of disease and delayed radiation toxicity are possible aetiologies. It is not possible to comment on the metastatic status of case 3 since thorough staging and restaging of disease was declined.
Cases 1 and 3 received hypofractionated radiotherapy, since no significant difference in response and survival between definitive and hypofractionated protocols for canine oral melanoma have been demonstrated (Proulx et al. Citation2003). Case 2 received definitive radiotherapy, which has been shown to be the most effective protocol for primary canine intranasal neoplasia (Théon et al. Citation1993; Adams et al. Citation2005), and was also judged to be a safer therapy for this dog given the proximity of the tumour to the brain. Definitive protocols are more expensive and more likely to cause acute adverse effects than hypofractionated protocols; case 2 developed a non-healing descemetocele, likely an acute adverse effect of treatment. Desmetocoele is an uncommon adverse effect of radiation therapy in dogs; however, ulcerative keratitis has been reported to occur in 26% dogs where the orbit is included in the radiation field (Pinard et al. Citation2012). Due to the location of this tumour, the dog's globe received a high dose of radiation, and since no pre-existing, or previous ocular disease was reported in this dog, it is considered plausible that ulcerative keratitis was exacerbated by the use of oral prednisolone and possibly self-trauma by the patient, resulting in a desmetocoele.
We are unable to comment on differences in the long-term tumour control between cases 1 and 2, since case 1 died from unrelated causes five months after treatment and the cause of seizures in case 2 remains undetermined. Nevertheless it is interesting that case 3 demonstrated good control of clinical signs for 10 months before clear signs of disease progression, despite the documentation of no more than stable disease on CT follow-up. The low mitotic activity of this tumour may signify a relatively indolent behaviour in this case.
Since there is no clear evidence that adjuvant immunotherapy or cytotoxic chemotherapy is beneficial in canine melanoma (Proulx et al. Citation2003; Murphy et al. Citation2005), none of the dogs in this report received medical therapy other than a COX-2 inhibitor (in cases 2 and 3). Although the effect of COX-2 inhibition has not been investigated in canine melanoma, in certain canine cancers, COX-2 expression correlates with malignancy and is negatively associated with survival (Proulx et al. Citation2003; Martinez et al. Citation2011).
Melanoma should be considered a rare differential diagnosis for primary intranasal neoplasia in dogs and immunohistochemistry is useful for definitive diagnosis. These three cases suggest that radiotherapy alone can be used to manage this disease.
Acknowledgements
The authors would like to thank Philippa McLaren, Michael Day, Henny Martineau and Sam Beck, the staff of the Royal Veterinary College Diagnostic Laboratory Services and Bridge Pathology Ltd for interpretation of clinical and anatomic pathology submissions.
Disclosure statement
The authors declare no conflicts of interest.
References
- Adams WM, Bjorling DE, McAnulty JE, Green EM, Forrest LJ, Vail DM. 2005. Outcome of accelerated radiotherapy alone or accelerated radiotherapy followed by exenteration of the nasal cavity in dogs with intranasal neoplasia: 53 cases (1990–2002). J Am Vet Med Assoc. 227(6):936–941.
- Bateman KE, Catton PA, Pennock PW, Kruth SA. 1994. 0-7-21 Radiation therapy for the treatment of canine oral melanoma. J Vet Intern Med. 8(4):267–272.
- Busam KJ, Jungbluth AA. 1999. Melan-A, a new melanocytic differentiation marker. Adv Anat Pathol. 6(1):12–18.
- Choi C, Kusewitt DF. 2003. Comparison of tyrosinase-related protein-2, S-100, and Melan A immunoreactivity in canine amelanotic melanomas. Vet Pathol. 40(6):713–718.
- Esplin DG. 2008. Survival of dogs following surgical excision of histologically well-differentiated melanocytic neoplasms of the mucous membranes of the lips and oral cavity. Vet Pathol. 45:889–896.
- Freeman KP, Hahn KA, Harris FD, King GK. 2003. Treatment of dogs with oral melanoma by hypofractionated radiation therapy and platinum-based chemotherapy (1987–1997). J Vet Intern Med. 17(1):96–101.
- Fujita M, Takaishi Y, Yasuda D, Hasegawa D, Taniguchi A, Takahashi K, Orima H. 2008. Intranasal hemangiosarcoma in a dog. J Vet Med Sci. 70(5):525–528.
- Fujiwara A, Kobayashi T, Kazato Y, Yayoshi N, Fujita M. 2013. Efficacy of hypofractionated radiotherapy for nasal tumours in 38 dogs (2005–2008). J Small Anim Pract. 54(2):80–86.
- Giudice C, Ceciliani F, Rondena M, Stefanello D, Grieco V. 2010. Immunohistochemical investigation of PNL2 reactivity of canine melanocytic neoplasms and comparison with Melan A. J Vet Diagn Invest. 22:389–394.
- Gouldesbrough D, Martin-Hirsch D, Lannigan F. 1992. Intranasal malignant melanoma arising in an inverted papilloma. Histopathology. 20:523–526.
- Hicks DG, Fidel JL. 2006. Intranasal malignant melanoma in a dog. J Am Anim Hosp Assoc. 42(6):472–476.
- Kawabe M, Mori T, Ito Y, Murakami M, Sakai H, Yanai T, Maruo K. 2015. Outcomes of dogs undergoing radiotherapy for treatment of oral malignant melanoma : 111 cases (2006–2012). J Am Vet Med Assoc. 247(10):1146–1153.
- Koenig A, Wojcieszyn J, Weeks BR, Modiano JF. 2001. Expression of S100a, vimentin, NSE, and melan A/MART-1 in seven canine melanoma cells lines and twenty-nine retrospective cases of canine melanoma. Vet Pathol. 38:427–435.
- Kubicek L, Milner R, An Q, Kow K, Chang M, Cooke K, Fox L, Farese J, Bacon N, Lurie D. 2016. Outcomes and prognostic factors associated with canine sinonasal tumors treated with curative intent cone-based stereotactic radiosurgery (1999–2013). Vet Radiol Ultrasound. 57(3):331–340.
- Lana SE, Dernell WS, Lafferty MH, Withrow SJ, LaRue SM. 2004. Use of radiation and a slow-release cisplatin formulation for treatment of canine nasal tumors. Vet Radiol Ultrasound. 45(6):577–581.
- Lawrence JA, Forrest LJ, Turek MM, Miller PE, Mackie TR, Jaradat HA, Vail DM, Dubielzig RR, Chappell R, Mehta MP. 2010. Proof of principle of ocular sparing in dogs with sinonasal tumors treated with intensity-modulated radiation therapy. Vet Radiol Ultrasound. 51(5):561–570.
- Lin C, Yang S, Lai C. 2003. Primary malignant melanoma of the nasal cavity. Chang Gung Med J. 26(11):857–862.
- Madewell B, Priester W, Gillette E. 1976. Neoplasms of the nasal passages and paranasal sinuses in domesticated animals as reported by 13 veterinary colleges. Am J Vet Res. 37(7):851–856.
- Martinez CM, Penafiel-Verdu C, Vilafranca M, Ramirez G, Mendez-Gallego M, Buendia, AJ, Sanchez J. 2011. Cyclooxygenase-2 expression is related with localization, proliferation, and overall survival in canine melanocytic neoplasms. Vet Pathol. 48(6):1204–1211.
- Millanta F, Fratini F, Corazza M, Castagnaro M, Zappulli V, Poli A. 2002. Proliferation activity in oral and cutaneous canine melanocytic tumours: Correlation with histological parameters, location, and clinical behaviour. Res Vet Sci. 73(1):45–51.
- Morris JS, Dunn JK, Dobson JM, White RAS. 1994. Effects of radiotherapy alone and surgery and radiotherapy on survival of dogs with nasal tumours. J Small Anim Pract. 35(11):567–573.
- Murphy S, Hayes AM, Blackwood L, Maglennon G, Pattinson H, Sparkes AH. 2005. Oral malignant melanoma – the effect of coarse fractionation radiotherapy alone or with adjuvant carboplatin therapy. Vet Comp Oncol. 3(4):222–229.
- Naganobu K, Ogawa H, Uchida K, Yamaguchi R, Ohashi F, Kubo K, Aoki M, Kuwamura M, Ogawa Y, Matsuyama K. 2000. Mast cell tumour in the nasal cavity of a dog. J Vet Med Sci. 62(9):1009–1011.
- Papazoglou LG, Koutinas AF, Plevraki AG, Tontis D. 2001. Primary intranasal transmissible venereal tumour in the dog: a retrospective study of six spontaneous cases. J Vet Med A. 48(7):391–400.
- Patnaik A. 1989. Canine sinonasal neoplasms: clinicopathological study of 285 cases. J Am Anim Hosp Assoc. 25:103–114.
- Peryaga G, Lafond C, Pointreau Y, Giraud P, Maingon P. 2016. Nasal cavity and paransal sinus cancer. Cancer Radiother. 20:S99–103.
- Pinard CL, Mutsaers AJ, Mayer MN, Paul Woods J. 2012. Retrospective study and review of ocular radiation side effects following external-beam Cobalt-60 radiation therapy in 37 dogs and 12 cats. Can Vet J. 53(12):1301–1307.
- Proulx DR, Ruslander DM, Dodge RK, Hauck ML. 2003. A retrospective analysis of 140 dogs with oral melanoma treated with external beam radiation. Vet Radiol Ultrasound. 44(3):352–359.
- Rabanal R, Fondevila DM, Montané V, Domingo M, Ferrer L. 1989. Immunocytochemical diagnosis of skin tumours of the dog with special reference to undifferentiated types. Res Vet Sci. 47(1):129–133.
- Ramos-Vara JA, Beissenherz ME, Miller MA, Johnson GC, Pace LW, Fard A, Kottler SJ. 2000. Retrospective study of 338 canine oral melanomas with clinical, histologic, and immunohistochemical review of 129 cases. Vet Pathol. 37(6):597–608.
- Ramos-Vara JA, Miller MA. 2011. Immunohistochemical identification of canine melanocytic neoplasms with antibodies to melanocytic antigen PNL2 and tyrosinase: comparison with Melan A. Vet Pathol. 48:443–450.
- Smedley RC, Spangler WL, Esplin DG, Kitchell BE, Bergman PJ, Ho HY, Bergin IL, Kiupel M. 2011. Prognostic markers for canine melanocytic neoplasms: a comparative review of the literature and goals for future investigation. Vet Pathol. 48(1):54–72.
- Smith SH, Goldschmidt MH, McManus PM. 2002. A comparative review of melanocytic neoplasms. Vet Pathol. 39(6):651–678.
- Spangler WL, Kass PH. 2006. The histologic and epidemiologic bases for prognostic considerations in canine melanocytic neoplasia. Vet Pathol. 43(2):136–149.
- Théon A, Madewell BR, Harb MF, Dungworth DL. 1993. Megavoltage irradiation of neoplasms of the nasal and paranasal cavities in 77 dogs. J Am Vet Med Assoc. 202(9):1469–1475.
- Tuohy JL, Selmic LE, Worley DR, Ehrhart NP, Withrow SJ. 2014. Outcome following curative-intent surgery for oral melanoma in dogs : 70 cases (1998–2011). J Am Vet Med Assoc. 245(11):1266–1273.
- Ueno H, Kobayashi Y, Yamada K. 2007. Olfactory esthesioneuroblastoma treated with orthovoltage radiotherapy in a dog. Aust Vet J. 85(7):271–275.
- Williams LE, Packer RA. 2003. Association between lymph node size and metastasis in dogs with oral malignant melanoma: 100 cases (1987–2001). J Am Vet Med Assoc. 222(9):1234–1236.
