Abstract
Over recent years, the interest in bio-adhesives, including soy-based adhesives, has increased rapidly. Among natural renewable resources suitable for industrial use, soy is a reasonable choice due to its high production volume and the small use of soy meal-based products for human food consumption. Soy flour can be an ideal raw material for the manufacturing of wood adhesives due to its low cost, high protein content and easy processing. There are also more concentrated forms of soy proteins, i.e. concentrates and isolates, which are also suitable raw materials for adhesive production except that their prices are higher. Extensive research has been carried out on improving the cohesive properties, especially water resistance, of soy-based adhesives. However, there is insufficient experimental data available for understanding the influences of modification methods on the structure of soy proteins and therefore for understanding the influences of structural changes on the adhesion. In this paper, some experimental techniques are proposed to be used for analysing soy-based adhesives to enable better understanding of those factors and improve future development. This review of soy-based adhesives is made with the focus on soy proteins’ chemical composition, soy protein product types (raw materials for adhesive production), modification methods for improving the adhesive properties of soy-based adhesives, and commercial soy-based adhesives.
1. Introduction
Soybeans are an industrial crop, mainly used for the production of oil and for human and animal feed. When processed, 27 kg of soybeans yield 5 kg of crude soybean oil and 21 kg of soybean meal.[Citation1] The production of soybeans is increasing rapidly, from 115 Mt in 1993 to 276 Mt in 2013,[Citation2] due to increased demand for soybean oil for human consumption and industrial chemicals. The production of soybean oil increased from 17 Mt in 1993 to 43 Mt in 2013.[Citation3] The main producers of soybeans are the United States of America, Brazil, Argentina and China.[Citation2] Most soybean meals are used for animal feedstock.[Citation1] Small percentages of proteins are processed for human consumption in the forms of soy milk, soy flours (SF), soy protein concentrates (SPC) and isolates (SPI), tofu and many retail food products. The soy proteins are also suitable for use in industrial products. Currently, soybeans are already used in some industrial products, such as biodiesel, bio-composites, candles, ink and wood adhesives in some particleboards, interior plywood and engineered wood flooring.[Citation1]
Soy products and other materials obtained from natural resources can be used as materials for the production of wood adhesives due to their ability to bond with different materials. The use of adhesives in wood products dates back to Egyptian, Greek and Babylonian civilizations. Back then, adhesives were made of blood, bones, hides, vegetables, eggs and milk, which contain starch or proteins as a binding agent. The early soy-based adhesives were made from defatted SF, which was dispersed in alkaline solutions.[Citation4] Those adhesives had to be used within eight hours, before the adhesive started to become less effective. Although SF contains carbohydrates as well as proteins, proteins are most likely the key adhesive components, especially in damp conditions.[Citation5] Soy-based adhesives were used extensively for wood composite manufacturing from the 1930s until the 1960s, when petroleum-based adhesives replaced them on the market due to their low price, better adhesive properties and water resistance.[Citation4,6] Although synthetic adhesives have very good properties, one of their major weaknesses is that they are derived from non-renewable and limited fossil resources. In addition, petroleum-based adhesives may not be completely biodegradable, which can lead to waste accumulation.[Citation7] As society recognises the use of non-renewable limited resources and non-biodegradable materials as major environmental problems, and encourages the use of natural biodegradable resources, the re-introduction of natural resources is also reasonable in the production of wood adhesives. Furthermore, very serious interest for bio-based adhesives exists in the industry.[Citation8] Adhesives based on natural materials are biodegradable, and their environmental pollution and volatile organic compounds (VOCs) levels are often low, which further reduces the environmental impact compared to synthetic adhesives. A Life Cycle Assessment (LCA) comparative analysis of petrochemical (urea-formaldehyde, UF) and bio (protein-lignin composite) adhesives with ‘cradle to grave’ perspective showed that petrochemical adhesive had a 22% higher environmental impact than the bio-adhesive.[Citation9] And for the bio-adhesive different recycling options, including composting for food production, are available, which could further reduce the overall environmental impact.[Citation9]
However, bio-adhesives have had inferior performance compared to synthetic ones, especially in terms of water resistance. Therefore, the development of adhesives based on natural materials, including soy, is focused on improving the mechanical properties and water resistance of the adhesive bonds using different processes such as thermal treatment, adding chemical or enzymatic modifiers and extraction of materials in a form containing a high proportion of protein.[Citation10–13] The thermal, chemical or enzymatic modification of soy proteins is necessary due to the highly ordered global structure of native soy proteins with some hydrophobic groups on the surface of the proteins, which provide the attraction between protein molecules. The modification of soy proteins breaks the internal bonds and may make more functional groups available for reaction with wood components, and for protein aggregation and cross-linking. This modification can alter the secondary, tertiary and quaternary structure levels of protein molecules. Structural changes in soy proteins caused by modification affect their adhesion performance.[Citation7,10,12–21] However, the structural and functional properties of modified soy proteins have been investigated in only a limited degree. Therefore, some analytical methods are proposed for using in the studies of soy-based adhesives. This paper aims at reviewing the adhesives based on soy proteins with emphasis on chemical composition and the properties of soy proteins, their product types, influence of the type of modification on the adhesion properties and commercial utility of soy-based adhesives.
2. Adhesive bonding of wood
In the human environment, wood-based composites have long been used as structural and decorative components. In these products wood is used in different forms, ranging from lumber and boards, to veneers, strands, particles and fibres. The diversity of wood forms enables efficient and nearly complete utilisation of raw materials and provides materials for producing structural and decorative products. These materials contain only the best properties of wood because the processing eliminates or minimises the defects present in solid wood. Combining them with other materials (adhesives, plastics, etc.) creates a wide variety of new products that meet market demands. In Europe, the most commonly produced wood-based panels are particleboard and medium density fibreboard (MDF).[Citation22] However, some other products are also important, namely oriented strand board (OSB), interior and exterior plywood, insulation board and hardboard. There are also more recent products that include laminated veneer lumber (LVL), oriented strand lumber (OSL), parallel strand lumber (PSL), laminated strand lumber (LSL), glued laminated timber (also called glulam), wooden I-beams, light MDF (LDF), high density fibreboard (HDF),cross-laminated timber (CLT), etc. The technological innovations over the years have spawned progress within the field of wood-based panels. Most notably, hot pressing and the concurrent adaptability of thermosetting resins have improved composites produced from particles and strands (particleboard, OSB, OSL, PSL, LSL), fibres (as MDF, HDF) and veneers (plywood, LVL).[Citation22]
Wood product manufacturers are the largest adhesive users. Wood adhesives present more than 65% by volume of the adhesives used in the world.[Citation23] Estimates suggest adhesive use for particleboards in Europe is split between UF (approximately 92%), melamine-urea-formaldehyde (MUF, approximately 7%) and isocyanates (e.g., polymeric diphenylmethane diisocyanate (pMDI), around 1%).[Citation22] UF is the primary adhesive, perhaps the only type of adhesive, used for MDF. On the other hand, OSB is primarily made with pMDI (75%), while MUF and UF are also used (approximately 15 and 10%, respectively).[Citation22] In the United States, large amount of phenol-formaldehyde (PF) is used for OSB production. The worldwide annual consumption of UF adhesives is approximately 11 Mt of resin solids.[Citation23] The resin is used in the production of an adhesive for bonding particleboard (61%), MDF (27%), plywood (5%) and a laminating adhesive for bonding (7%), e.g., furniture cased goods, overlays of panels and interior flush doors.[Citation24] The major disadvantage of UF adhesives is that they are formaldehyde emitters. Formaldehyde emissions are released during the production and use of bonded products. Environmental regulations and legislation regarding VOC emissions, particularly formaldehyde, within the wood products industry are important driving forces for technological changes. Over recent decades, panel products’ emissions have been significantly reduced. However, the reclassification of formaldehyde by the International Agency for Research and Cancer (IARC) as ‘carcinogenic to humans’ is forcing panel manufacturers, adhesive suppliers and researchers to develop systems, which further decrease formaldehyde emissions to levels as low as those present in natural wood.[Citation25] One group of these new developing systems is adhesives based on natural resources, including soy.
3. Chemical composition of soy proteins and their properties
Soy proteins consist of albumins and globulins. Albumins can be extracted by water and represent 10% of soy proteins, while globulins can be extracted by dilute salt solutions and represent 90% of proteins.[Citation26] Soy globulins consist of four major water-extractable fractions, 11S, 7S, 2S and 15S, where the S is in Svedberg units as determined by sedimentation chromatography; they can be isolated based on their sedimentation coefficients. The 11S (glycinin) and 7S (β-conglycinin) fractions are storage proteins and constitute 65–80% of the total protein content.[Citation27] The glycinin constitutes about 52% and β-conglycinin around 35% of the total seed proteins. The glycinin is a hexamer with six acidic and six basic subunits, and β-conglycinin is a trimeric protein with three different subunits.[Citation28,29] The detailed compositions of glycinin and β-conglycinin are described in the literature [Citation28,29].
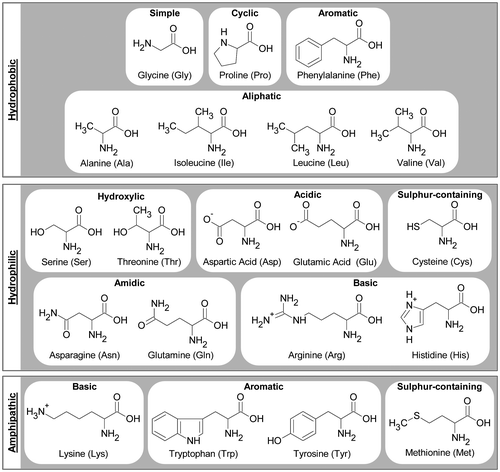
Soy protein molecules are complex macromolecules. They are composed of 20 different amino acids, each with a common backbone and a different side-chain (R),[Citation30] as shown in Figure . The backbone consists of the amino group (NH2), the α-carbon and the carboxylic group (COOH).[Citation31] The neutral form of an amino acid is shown in Figure . In solution at pH 7 the amino and carboxylic groups ionise to and COO−. Each amino acid has specific characteristics defined by the side-chain, R, which provides it with a unique role in a protein structure. Based on the tendencies of the side-chains to participate in interactions with each other and with water, the amino acid can be hydrophilic (polar), hydrophobic (non-polar) or amphipathic (residues have both polar and non-polar characteristics).[Citation31] According to the characteristics of the side-chains, amino acids can be divided into groups: aliphatic, cyclic, hydroxylic, acidic, amidic, basic, aromatic, sulphur-containing, and unique amino acids,[Citation30] as shown in Figure . Glycine is the simplest amino acid, because the side-chain is only a hydrogen atom. The aliphatic amino acids are alanine, isoleucine, leucine, valine, all hydrophobic. The cyclic amino acid is proline (hydrophobic) and it is unique because its aliphatic side-chain is bonded covalently to the nitrogen atom of peptide backbone to give the imino acid, which causes bends in the backbone disrupting crystallinity. The hydroxylic amino acids are serine and threonine, both hydrophilic. The acidic amino acids are aspartic acid and glutamic acid, both hydrophilic. The amidic amino acids are asparagine and glutamine, both hydrophilic. The basic amino acids are arginine, histidine (both hydrophilic) and lysine (amphipathic). The aromatic amino acids are phenylalanine (hydrophobic), tryptophan and tyrosine (both amphipathic). The sulphur-containing amino acids are methionine (amphipathic) and cysteine (hydrophilic).
Figure 1. The chemical structure of an amino acid. Backbones are the same for all amino acids, while side-chains (R) are different for each amino acid.
Figure 2. Amino acids grouped as hydrophobic, hydrophilic and amphipathic, and divided into groups according to the characteristics of the side-chains.
Amino acids are connected through peptide bonds to form the polypeptide chain, which is the primary structure of proteins and determines how the protein folds into higher level structures.[Citation31] Specific amino sequences form α-helices and β-sheets (crystallites), which are intra-chain structures. These are local structures stabilised by hydrogen bonds (secondary structure). The individual protein chains, elements of either α-helix or β-sheet or both, as well as loops and links that have no secondary structures, fold up within an aqueous environment forming the tertiary structure.[Citation31] The tertiary structure is generally stabilised by non-local interaction, most commonly the formation of a hydrophobic core due to the propensities of the hydrophobic side-chains to minimise their interactions with aqueous environment (hydrophobic collapse).[Citation31] The tertiary structure is also stabilised by intra-chain interactions such as salt bridges, covalent disulphide bonds and hydrogen bonds. Several protein molecules (polypeptide chains) interact with one another through inter-chain hydrophobic interactions, salt bridges, covalent disulphide bonds and hydrogen bonds thereby forming a quaternary structure. It is usually called a protein subunit and functions as a single protein complex.
Side-chains of the amino acids are able to bond with different materials, including cellulose and lingo-cellulosic material. However, some potentially reactive side-chains are not on the protein’s surface. They form different bonds within the protein, namely disulphide bridges between thiol groups, hydrogen bonds between polar groups, and salt bridges between acids and bases. Furthermore, protein adhesives usually possess low moisture- and water-resistance, and the failure of the adhesive bond in wet conditions occurs in the adhesive (cohesive failure) and not between adhesive and wood. In order to expose the functional groups buried inside and enable stronger bonding to wood, and aggregation and cross-linking of protein molecules, proteins have to be modified/denatured. During the process of denaturation, various intermolecular and intramolecular bonds, which stabilise the native structure of globular proteins, are interrupted. This results in reorganisation of both secondary and tertiary configuration, and usually in exposure of previously inward-oriented hydrophobic amino acids to the surface. Denaturation can also cause dissociation of protein subunits i.e. disruption of quaternary structure, which results in aggregation. The aggregation of the denatured proteins is usually caused by protein–protein hydrophobic interaction.[Citation32] The denatured proteins therefore exist as aggregated colloids, with even more complex structure as the native proteins. They retain much of their folded structure, although in a non-native state.[Citation33] While the native state of a protein is mainly one biologically active structure, denaturation of a protein can create multiple different structures depending on the type and extent of the denaturation.[Citation34] Proteins can also exist in a stable intermediate state, i.e. molten globule state, which is partly denatured state characterised by a relatively compact globule with native-like secondary structure and a disrupted tertiary structure.[Citation33] Native and denatured protein structures have small energy differences.[Citation31] This means that changing an aqueous environment by the addition of salts, organic compounds or heating can cause significant changes in the tertiary structures of proteins.[Citation5]
4. Methods for analysing structural changes in proteins induced by modification
For the development of the soy-based adhesives, it is essential to understand the influence of the modification method on the structure of proteins. However, adhesive literature does not provide sufficient experimental data to understand these changes in protein structure and function. With better analysis, the influences of the soy protein structural and functional properties on the adhesion and cohesion could be explained more accurately. Therefore, some methods for analysing the influence of modification on the structural and functional properties of proteins are described in this section.
4.1. Solubility
Proteins are soluble in water when electrostatic repulsive forces are greater than attractive hydrophobic interactions. Protein modification influences the structure of proteins hence their solubility.[Citation32,35,36] It is expressed as Nitrogen Solubility Index (NSI) or Protein Dispersibility Index (PDI). Different methods have been used for determination of protein solubility.[Citation18,35–37]
4.2. Surface hydrophobicity
Surface hydrophobicity is dependent on the size and shape of protein molecule, amino acid composition and sequence, and any intramolecular or intermolecular cross-links.[Citation38–40] The treatment conditions influence the degree of denaturation and hence the protein surface hydrophobicity,[Citation37,41] and water resistance.[Citation42] There are many methods of potential use for routine analysis reported in the literature, intended for measuring protein hydrophobicity.[Citation43]
4.3. Differential scanning calorimetry
Differential scanning calorimetry (DSC) is a technique for studying the thermal transition of proteins. The conversion of a protein from native to denatured state is accompanied by a heat uptake, seen as an endothermic peak in the DSC thermogram. The parameters obtained by the DSC thermogram are enthalpy of denaturation (ΔHd) and temperature of denaturation (Td). Exposure of proteins to chemical, enzymatic or thermal treatment changes their ΔHd and Td. They are also dependent on the concentrations of proteins and modifying agent.[Citation10,21,36,44–46]
4.4. Gel electrophoresis
Gel electrophoresis is a method for analysing proteins based on their size and charge. Molecules with different molecular masses migrate through the separation gel used with different rates. Differences between the electrophoretic patterns of proteins before and after modification can be used to determine changes in the molecular size of proteins, and to monitor denaturation and aggregation.[Citation18,36]
4.5. Fourier transform infrared spectroscopy
The particular changes in protein secondary structure induced by modification and the manner in which the aggregates of denatured proteins are formed can be detected by FTIR. Structural changes of proteins can be studied by Fourier transform infrared spectroscopy (FTIR) spectra from wavenumbers 4000–400 cm−1.[Citation47] Each functional group vibrates (stretch or bend) at a certain wavenumber giving a peak in the certain frequency range. The heights of certain peaks change and the peaks can also shift after denaturation, indicating the differences in the protein structure caused by modification.[Citation11,48–50]
4.6. Adhesive viscosity
The bonding strength of a protein adhesive depends on the adhesive viscosity. Viscosity affects the application of the adhesive, especially the spreading and penetration of adhesive into the wood.[Citation51] The unmodified soy proteins were unable to penetrate into the wood, and certain modifications of soy proteins have enabled adhesive penetration.[Citation13,52] The viscosity of soy protein adhesives is affected by protein content and concentration of modifying agent.[Citation13,37,53,54]
5. Soy protein product types
Soy-based adhesives can be made from SF, SPC or SPI. They differ in their contents of proteins, SPI having the highest percentage and SF the lowest. On the other hand, SF has the highest content of carbohydrates (approximately 38% on dry basis [Citation55]). During SPC production the soluble carbohydrates (approximately 17% on dry basis [Citation55]) are removed, leaving the insoluble ones (approximately 21% on dry basis [Citation55]), while during SPI production both soluble and insoluble carbohydrates are removed. The typical compositions of different types of soy products are given in Table . It is believed that the insoluble carbohydrates play a minor role in the adhesive properties of SF, while the soluble carbohydrates decrease the water resistance of SF-based adhesives because they increase water absorption.[Citation5] Interfacial shear strength of ramie fibre with soy protein resins increased with the protein content in soy resins (SF < SPC < SPI).[Citation56] Furthermore, soluble, lower molecular weight carbohydrates found in SF (which are removed in SPC and SPI) are more susceptible to thermal decomposition than are the insoluble, presumably higher molecular weight carbohydrates in SPC.[Citation57] SPI is more heat stable than either SF or SPC.
Table 1. Typical compositions of different soy protein products (on a moisture free basis). [Citation29,58]
5.1. Soy flour
SFs are obtained by finely grinding defatted flakes or meals to pass through a 100-mesh or smaller standard screen.[Citation58] In order to make low-fat defatted flakes, soybeans are first cleaned, dried, de-hulled and cracked.[Citation59] Cracked beans are then heated to about 74 °C and flaked. One of the following processes is then used to produce defatted flakes or meals. Most widely used method for extracting oil is solvent extraction using hexane. This method reduces the level of oil in the flakes to 1% or less. The solvent solubilises the soybean lipid material, and the hexane-oil mixture is separated from the flakes. To remove the solvent resident from soybean flakes, one of the two main desolventising systems is used. The desolventiser-toaster system is used for the production of animal feed, and the flash desolventising system is used for production of edible and industrial proteins.[Citation60] During desolventising/toasting, the flakes undergo high-temperature thermal processing, which denatures and insolubilises soy protein.[Citation61] On the other hand, flash desolventising provides mild heating conditions, low moisture and minimum retention time to minimise protein denaturation.[Citation60] Defatted soy flakes with high solubility are obtained, which are also known as white flakes. Defatted flakes or meals can also be alternatively produced by mechanically extracting the oil and by extruding-expelling.[Citation59] Small extraction plants often prefer mechanical extraction, which squeezes out oil of the heated or cooked soybeans (screw-pressing).[Citation59,62] The extruding-expelling process is similar to the screw-pressing except the cooking step is replaced with extruding. Both, screw-pressing and extruding-expelling, cause extensive thermal denaturation of the proteins.
The SFs are available in three forms characterised by the PDI, i.e. the highly dispersible native state SF (90 PDI), the low dispersible, denatured SF (20 PDI) and the in-between SF (70 PDI).[Citation34] The SF intended for adhesive production should be processed at temperatures below 70 °C to prevent protein denaturation.[Citation4]
5.2. Soy protein concentrate
SPCs contain around 70% of proteins.[Citation58] They are prepared by removing most of the soluble non-protein constituents from de-hulled and defatted soy flakes with high protein solubility (usually white flakes). These constituents are primarily soluble carbohydrates (mono-, di- and oligosaccharides), and also some low molecular weight nitrogenous substances and minerals. Some low molecular weight proteins are also extracted along with the soluble carbohydrates. Therefore, the amino acid composition of the SPCs may differ slightly from that of the original SF. Three common processes exist for manufacturing SPC.[Citation58] Each process produces a different type of concentrate with different characteristics and particular uses. These processes are the aqueous alcohol wash process, the water extraction process with thermal denaturation and the acid wash process. The aqueous alcohol wash process and the water extraction process with thermal denaturation, both denature soy proteins to make them insoluble (PDI of 10–15%). The acid wash process does not denature soy proteins (PDI of 60–70%). The pH of water dispersion is adjusted to (4.2–4.5) with HCl. At this pH, soluble sugars are dissolved without using special solvents, and the proteins have low solubility since they are at their isoelectric point. The concentrate of solids is usually neutralised and then spray dried. Prior to drying, the SPCs can be jet cooked to increase functionality for food applications.[Citation63] In jet-cooking the SPC slurry is subjected to the high temperature (120–150 °C) for short time (10–60 s) with steam injection. Normally, 750 kg of SPCs are obtained from 1 t of defatted soybean flakes.[Citation58]
5.3. Soy protein isolate
SPIs or isolated soy proteins are the most concentrated form of commercially available soy protein products. They contain over 90% of protein, on a moisture free basis.[Citation58] The more common method of production, used by commercial manufacturers, uses the isoelectric point of the soy proteins. Defatted soy flakes (white flakes) are stirred into warm water (60 °C) and pH is adjusted to (7.5–9.0), sometimes even higher (9.0–11.0),[Citation64] by adding an alkali such as NaOH. The soluble carbohydrates and most of the proteins are separated from the insoluble fractions by centrifugation. The pH of the solution is lowered to 4.2–4.5 (isoelectric region) by adding HCl to precipitate most of the proteins. The soluble sugars are washed away from the precipitated proteins. The precipitated proteins are suspended and then neutralised with the addition of NaOH and freeze dried or spray dried to yield a PDI in the range of 80–95%. However, prior to spray or freeze drying SPIs are often jet-cooked to increase functionality for food applications.[Citation63] These functionalised proteins are commonly what have been used in the literature for studying adhesive properties.
6. Modification methods of soy proteins and their effects on the properties of soy-based adhesives
In order to enable protein adhesion, protein molecules have to disperse and denature in solution. The adhesion strength of a protein adhesive strongly depends on its structure and components, and consequently on the protein’s ability to disperse in water. In the case of SPI, the preparation time of dispersion in water influenced the properties of bonded particleboard.[Citation65] Longer dispersion preparation time enhanced the boards’ properties. The adhesion strength also depends on dispersion viscosity and solid content, as well as the ability of hydrophilic and hydrophobic side-chain groups in the proteins to interact with the wooden substrate.[Citation66,67] However, numerous reactive groups within native soy proteins are unable to interact with the wooden substrate in adhesion applications due to their highly ordered globular structures. Therefore, these structures have to be broken to enable good adhesion. Proteins’ hydrophilic and hydrophobic side-chains can be manipulated to modify surface reactivity and accessibility.[Citation11] Chemical, thermal and enzymatic modifications are known. These treatments cause the denaturation of protein molecules, and the degree of denaturation depends on the process parameters. The degree of denaturation increases with increased temperature of thermal treatment and with increased concentration of chemical modifier. Therefore, the concentrations of chemicals and temperatures of the thermal treatments have significant effects on the performances of modified soy protein-based adhesives.[Citation13,15,19] Denatured proteins form higher molecular weight complexes or aggregates, which then cross-link either with covalent interactions of their functional groups or with reactions of aggregates and added cross-linkers. The resulting cross-linked structures can significantly decrease cohesive failure of adhesive bonds compared to non-modified soy proteins.[Citation11,68]
The particular modification method and the resulting denatured structures of soy proteins influence the adhesive viscosity.[Citation13,18] The operating viscosity limits of soy adhesives depend on the application and the materials to be bonded.[Citation66] The spray application for particleboard adhesives requires lower viscosity than the roller application for plywood adhesives.[Citation4] Since soy adhesives are shear-thinning, the measured viscosity at low shear does not relate well to the high shear in application process. The viscosity of the shear-thinning fluid is a function of shear rate, and as the shear rate increases, viscosity decreases. Generally, the viscosity of protein dispersions increases with increased protein concentration. This is attributable to increased interaction between the hydrated protein molecules.[Citation33] Partial denaturation and/or polymerisation, which causes an increase in the hydrodynamic size of proteins, increases the viscosity.[Citation33]
The adhesive viscosity and the surface roughness influence the adhesive penetration and consequently the adhesion strength of soy adhesives.[Citation69,70] The adhesion strength of the protein adhesive is established with reformation of the chemical bonds between the protein chains, which were interrupted during denaturation.[Citation51] Curing of soy-based adhesive can be promoted with either hot pressing or cold pressing.[Citation4] Protein adhesives, including soy adhesives, have some backbone flexibility and are not highly cross-linked, which allow them to distribute strain in the adhesive interphase, which decreases interfacial stress.[Citation71] These properties are important in moisture-related tests due to differences in swelling and shrinking between wood and the adhesive, which can cause a large interfacial strain. Wood swelling often initiates bond failure.[Citation71] Soy adhesives have limited water resistance, but they recover their strength after drying.[Citation72]
Improvements of the functional properties and consequently adhesive properties of soy proteins by altering their molecular conformations through chemical modification have been well documented in the literature.[Citation11,19,48] On the other hand, to the authors’ knowledge, only a few articles have been published on the topic of thermal [Citation7,13,73] and enzymatic modification [Citation12,74] of soy proteins for developing wood adhesives.
6.1. Chemically modified soy proteins
Chemical modification methods can be divided into four categories. The first one is denaturation of soy proteins by breaking their internal structure.[Citation10,15,18,19,42,48] The second category is soy protein molecular modification, which focuses on grafting reactive groups of chemical reagents onto protein molecules. These groups can react with protein’s polar groups and form a cross-linked network after curing.[Citation11,50,68,75–77] The third category is the mixing of soy protein products with other natural materials such as lignin and tannin.[Citation78,79] The fourth category is the mixing of soy protein products with synthetic resins, such as PF, melamine-formaldehyde (MF), MUF and epoxy resin (EPR).[Citation80–83]
6.1.1. Soy protein denaturation
The chemicals normally used to denature soy proteins are acids, alkalis, anionic and cationic detergents, salts, and chaotropic agents.[Citation10,14,18–20,84] The mechanisms of denaturation are different for different groups of chemicals, and the resulting denatured protein structures probably also differ.[Citation85] Therefore, the bonding strength and water resistance of soy protein-based adhesives, modified with different denaturants, also differ.
The bonding strengths and water resistances have been shown to improve with addition of any of the previously listed denaturants. The most widely used alkali is NaOH. It improves the bonding strengths and water resistances or wet shear strengths of SPC,[Citation67] SPI,[Citation7,13,42] and also isolated 7S and 11S globulins.[Citation17] Among chaotropic agents urea and guanidine hydrochloride (GuHCl) have been used to improve adhesion properties.[Citation7,15,17,21] The anionic detergents that improve bonding performances of soy-based adhesives are sodium dodecyl sulphate (SDS) and sodium dodecyl benzene sulphonate (SDBS),[Citation10,45,86,87] and the cationic detergents tested are hexadecyltrimethyl ammonium bromide, ethylhexadecyldimethyl ammonium bromide or benzyldimethylhexadecyl ammonium chloride.[Citation19] The salts that can be used for denaturing soy proteins and improving their bonding strength, especially water resistance, are NaHSO3 and Na2SO3.[Citation18,36,37,88] Modification of soy proteins with NaHSO3 (MSP) have also other advantages such as higher solid content, good flow-ability and longer shelf life.[Citation36,37] Therefore, the MSP adhesives have been studied in combination with cross-linkers and are described in the next section. On the other hand, NaCl have been shown to have a negative effect on the adhesive performance.[Citation89] Furthermore, a combination of acid (HCl), salt (CaCl2) and alkali (NaOH) yielded an adhesive that significantly improved water resistance and strength of bonded plywood.[Citation48]
A lot of this adhesive literature lack in investigating the structural changes of soy proteins induced by addition of certain modifier. The influences of pH values and addition of salts on the protein structure have been investigated in food literature.
The addition of an alkali increases the pH value of dispersion, which allows the electrostatic repulsion to overcome hydrophobic attraction. The repulsive electrostatic forces are induced by the large net charge of the proteins, resulting in denaturation.[Citation32] At higher temperatures, increased pH can also cause the chain scission.
The effect of the salt addition on the denaturation of proteins is strongly dependent on the salt concentration.[Citation32] Depending also on the individual properties of the ion, either the ions can just affect the ionic strength, or at higher concentration they can act as structure stabilisers or as destructing ions.[Citation90] The hydration of these ions results in a changed water structure around the protein, hence changed hydrophobic interactions.
The influence of modification of defatted SF by a combination of acid (HCl), salt (CaCl2) and alkali (NaOH) on the protein structure has also been investigated.[Citation48] The modification with the combination of acid and salt caused amide links hydrolysis and decarboxylation, and the concentrations of the active groups, –NH2, –COOH, and –OH, increased. Alkali modification caused some aminolysis and further increased the concentrations of the active groups. Adhesive curing generated new amide linkages, resulting in the improved cross-linking of soy protein and consequently improved water resistance.[Citation48]
6.1.2. Soy protein molecular modifications
Different cross-linkers have been used to modify the molecular structure of soy proteins and consequently improve the water resistances of soy protein-based adhesives.[Citation68,75,76] These cross-linkers react with polar groups, –NH2, –COOH, –SH and –OH, of soy protein during the hot press process. The native proteins have commonly been denatured (NaHSO3 or NaOH modification) prior to the molecular modification to expose functional groups and make them available for reactions with cross-linkers. The influence of the added cross-linkers on the structure of soy proteins has been investigated in greater extent than the influence of denaturants.
Modification of MSP with 2-octen-1-ylsuccinic anhydride (OSA) significantly improved the wet shear strength of the resulting plywood.[Citation68] OSA was grafted onto soy protein molecules through a reaction between the amine and hydroxyl groups of protein, and anhydride groups of OSA. When using the MSP with inorganic calcium silicate hydrate and cross-linker 3-aminopropyltriethoxysilane (APTES), CHS formed covalent linkages with soy proteins, which improved the adhesion strengths of resulting composites.[Citation11] The partly denatured soy proteins reacted with APTES, which facilitated the formation of a cross-linked interface between soy protein and CHS. These promoted attachment to the solid surface and consequently led to improved bonding strength.
Modification of NaOH modified SPI with undecylenic acid (UA) in the presence of catalyst EDC (1-ethyl-3-[3-dimethylaminopropyl] carbodiimide hydrochloride) significantly improved the wet strengths of the bonded wood specimens.[Citation91] UA was grafted onto the proteins through the reactions of amine groups from protein and carboxyl groups from UA. The shear strengths and water resistances of the resulting wood composites were significantly improved also with modification of SPI with NaOH, maleic anhydride (MA) and polyethylenimine (PEI).[Citation75] MA was first grafted onto the SPI via amide and ester linkages. Then the amino groups of PEI reacted with maleyl ester to form maleyl amides and also with the C=C bonds of maleyl groups via the Michael addition reaction during the curing of adhesives. Furthermore, NaOH, MA and PEI were used for modifying SF and plywood panels bonded with modified SF-based adhesive exceeded the requirements for interior applications.[Citation92] However, a different procedure of adhesive preparation was needed to improve the adhesive strengths and water resistances of the resulting plywood panels. First PEI, MA and NaOH were mixed and then SF was added. Particleboard bonded with SF modified with NaOH, MA and PEI met the industrial requirement of M-2 grade particleboard.[Citation77]
The adhesion strengths of soy-based adhesives had also been improved with the addition of dopamine, cysteamine, CaCO3, MgO and POCl3. Modifications of soy protein using dopamine,[Citation93] or cysteamine [Citation94] in alkaline solution have effectively converted soy proteins to a strong and water resistant wood adhesive. Dopamine or cysteamine was grafted onto a SPI via amide linkages. The adhesive strengths and water resistances of the wood composites bonded with modified SPIs depended on the amount of phenolic,[Citation93] or –SH [Citation94] groups. Furthermore, bio-mimetic soy protein/CaCO3 hybrid adhesive, prepared by co-precipitating calcium carbonate in SPI alkaline solution, greatly improved water resistances and bonding strengths of soy protein-based adhesives by the formation of compact rivets or interlocking links, and ion cross-linking of calcium, carbonate and hydroxyl ions in the adhesive.[Citation16] The water resistance of SF-based adhesive was improved also with the addition of MgO due to the formations of water resistant complexes with interactions between MgO and soy proteins and not soy carbohydrates.[Citation35] The resulting plywood panels met the industrial requirements for interior plywood. Chemical phosphorylation of SF in alkaline solution using POCl3 significantly increased the wet bond strength because the phosphate groups were incorporated into the proteins and carbohydrates, and acted as a cross-linker.[Citation50] The phosphate groups reacted via covalent esterification with hydroxyl groups of the wood, and via ionic and hydrogen bonding with functional groups of the wood.
Different cross-linkers, such as glyoxal, glutaraldehyde, renewable glycerol polyglycidyl ether (GPE), and mixture of ethylene glycol diglycidyl ether (EGDE) and diethylenetriamine (DETA), have also been used to improve bonding strengths of soy-based adhesives. Glyoxal reacted with the amide groups of soy proteins and formed –N–CHR–OH groups that further reacted with tannin and pMDI.[Citation76] The resulting glyoxalated SF adhesives with total content of natural material of 70 or 80% met standard specifications for interior particleboard. Wet shear strength of SPI adhesive was improved with cross-linking of SPI with glutaraldehyde, which decreased the number of amino groups and increased the number of hydrophobic groups in proteins.[Citation95] Furthermore, renewable GPE has been used as cross-linking agent and low-cost attapulgite (ATP) as an enhancer for SF-based adhesive.[Citation96] SF was modified with polyvinyl alcohol and GPE. The modified SF did not react with the ATP; however, ATP was inserted into the modified SF and acted like a physical bond increasing the shear strength of the adhesive.[Citation96] Moreover, cross-linking of SDS modified soy proteins with the mixture of EGDE and DETA improved wet shear strength of the plywood bonded with soy meal-based adhesive, which met the interior use plywood requirement.[Citation97] DETA reacted with EGDE to form a long chain structure with epoxy groups, which cross-linked the soy protein molecules to form a denser cured adhesive layer. In addition, the long chain structure formed an interpenetrating network with the soy protein molecules.
6.1.3. Combination of soy-based adhesives and other natural materials
Adding some natural materials to the soy-based adhesives has been shown to improve their water resistance. Blending of sorghum lignin or extruded sorghum lignin with SPI or MSP improved the shear strength and water resistance of wood veneer joints.[Citation79] Furthermore, the addition of sucrose and glucose fractions to mixtures of defatted SF and SPI improved the water resistance and bonding strength of soy-based adhesives.[Citation78] Reduced carbohydrate content or increased sucrose and glucose fractions in the carbohydrate, increased the hydrophobicity and therefore increased the bonding strengths of the cured soy-based adhesives. Sucrose and glucose reacted with proteins through the Maillard reaction and enhanced the cross-linking of the cured soy-based adhesive.[Citation78] The carbohydrates in defatted SF are mainly polysaccharides, which were enzymolysed by Viscozyme L to produce reducing sugars such as galactose, glucose and arabinose.[Citation98,99] Reducing sugars were cross-linked with proteins using the Maillard reaction during the curing process of SF-based adhesives, hence enhancing the water resistance and bonding strength of bonded plywood.[Citation99]
6.1.4. Combination of soy-based products and synthetic resins
Mixing soy-protein products with synthetic resins can improve water resistance compared to soy-based adhesives. Co-polymerisation of SF with resole PF resin converted (55–86%) of previously water-soluble SF into water-insoluble material and bonded random strand-boards had performance comparable to those bonded with commercial PF resin.[Citation83] Plywood bonded with soy/PF resin-based adhesive containing 70% of soy-based adhesive and 30% of PF resin met the type I plywood requirement and the emission level E0 of formaldehyde emission.[Citation100] The addition of 20% of MUF resin to SF-based adhesive increased the water resistances and wet shear strengths of the bonded plywood panels due to the formation of methylene bridges through the reaction of the methylol group of MUF resin with soy units during hot pressing.[Citation101] Furthermore, the wet adhesion strength of the mixture of 40% of MSP and 60% of PBG (press bond glue, UF-based resin) was significantly improved.[Citation82] The carboxylic, hydroxyl and amino groups of MSP cross-linked with the hydroxymethyl group of PBG, and at the same time acted as an acidic catalyst for self-polymerisation of UF-based resin. EPR, MF and their mixture EPR + MF were used as cross-linkers of SF-based adhesive.[Citation81] All three cross-linkers improved the water resistance of the SF-based adhesive and when the hybrid EPR + MF was used type I plywood panels were prepared. The aqueous emulsions consisting of chemically modified thermosetting aliphatic polyketones and soy proteins (with mass fraction of up to 40% with respect to the non-modified polyketone) fulfilled wood adhesive requirements according to European Standard EN-314.[Citation102]
When a new type of curing agent (CA), polyamidoamine resin, was combined with MA they formed a cross-linking network and improved the water resistance of the SF-based adhesives, meeting the requirement for bonding type-II plywood.[Citation103] Furthermore, the new CAs were derived from the reaction of epichlorohydrin and ammonium hydroxide for curing SF adhesive.[Citation104,105] The CAs were effective with SF for making interior plywood panels. The particleboards bonded with adhesive based on SF and CA derived from ammonia and epichlorohydrin met the industrial requirements of M-2 particleboards.[Citation106] Moreover, a new bio-based CA itaconic acid-based polyamidoamine-epichlorohydrin (PAE) was synthesised from renewable itaconic acid.[Citation107] The resulting adhesive met the requirements for bonding type-II plywood.
A novel scheme that could contribute to the development of water resistant soy protein wood adhesives was proposed.[Citation108] The SPI was caustic degraded (DSP) and then nano-modified by montmorillonite (MMT) and chemically cross-linked with polymethylene polyphenyl polyisocyanate (MDI).[Citation108,109] Caustic degradation improved the technical applicability of DSP adhesives by reducing the viscosity at a high solid content of 36.7–38.2%. The MMT nano-modification and MDI-modification of DSP adhesives improved water resistance but slightly reduced bond strength.
6.2. Thermally modified soy proteins
Several methods of thermal modification of soy proteins have been developed over the years, i.e. thermal treatment of soy proteins’ dispersion,[Citation7,73,110] thermal treatment of the dry soy proteins’ powder [Citation13] and thermal treatment of the bonded specimens.[Citation111]
The thermal treatment of proteins is the most common process of denaturing proteins. The rate of protein denaturation increases with increased temperature.[Citation32] As a result of denaturation, side-chains buried inside the molecule are exposed to the surface.[Citation112] The exposed -SH or hydrophobic side-chains combine the protein molecules through –SH, –SS– interchange reaction or hydrophobic interaction, resulting in protein aggregation. One of the major consequences of thermal denaturation is decreased protein solubility.[Citation32] The thermal modification of SPI dispersion in water at 50 °C improved adhesive strength compared to non-modified SPI.[Citation7] Preheating of SPI suspension in water for 20 min had significant effect on protein structure and adhesion performance.[Citation110] Adhesion strength reached maximum at 80 °C preheating temperature, while severe preheating (over 110 °C) caused complete denaturation of proteins and decreased adhesion strength. Esterified SPI (modified with ethanol) also showed the maximum adhesion strength at preheating temperature of 80 °C, while no effect of moderate preheating was observed on the cross-linked SPI (modified with glutaraldehyde).[Citation110] The thermal modification of SPI dispersion at 50 °C combined with alkali pre-treatment with NaOH to pH 11 improved the adhesive strength compared to SPI dispersion thermally modified at 50 °C.[Citation7] Alkali thermally modified SPI slurry had lower viscosity and was thermally more stable compared to thermally modified and non-modified SPI. Thermal treatment of SPI at 120 °C combined with acid treatment with HCl (thermal acid treatment) also improved the adhesion strength of SPI dispersion.[Citation73] The mechanical properties and water resistances of soy protein-wood flour composites were improved due to partial protein denaturation, re-polymerisation of soy protein via –SH and S–S interchange reactions and the rearrangement of hydrophobic bonds during thermal acid treatment.[Citation73] The mechanical strengths and water resistances of composites based on thermally acid-treated SPI were further improved by cross-linking with synthetic resins, namely polyisocyanate, water-borne epoxy latex and modified polyamide, due to the formation of strong chemical bonds in the protein-wood interfaces. Modified polyamide was the more preferable cross-linker based on tensile strength and water resistance.[Citation73]
A new method of thermally modifying SPI powder within the vacuum chamber was developed recently.[Citation13] The thermal modification within a vacuum chamber at 50 °C in combination with pH adjustment of dispersion to 10 with NaOH, and dispersion preparation temperature of 50 °C, significantly improved adhesive wet shear strength compared to the adhesive prepared under the same conditions with non-modified SPI powder. The bond shear strengths of either natural or thermo-hydro-mechanical compressed bamboo bonded with thermally modified SPI adhesives within the vacuum chamber were similar to other strengths of bamboo strips bonded with PF resin.[Citation113] For bonding bamboo, the best performing adhesive dispersion was prepared at 24 °C with SPI thermally modified within the vacuum chamber at 50 °C and pH adjusted dispersion to 10.0 with NaOH.
Thermal treatment of the bonded specimens also influenced the adhesion performance of soy meal-based adhesive.[Citation111] The thermal treatment of bonded plywood after hot pressing improved the water resistance of adhesive by improving the cross-linking density of the adhesive layer in plywood.
6.3. Enzymatically modified soy proteins
Papain, urease [Citation12] and trypsin [Citation74] modified SPIs were reported to have better adhesive strengths compared to non-modified SPI adhesive, while chymotrypsin modified SPI showed zero adhesive strength.[Citation12] Furthermore, some research has been done on the physicochemical properties of soy protein dispersions. Enzymatic hydrolysis of SPI with trypsin, chymotrypsin, papain and urease resulted in significant reductions in the molecular masses of soy proteins.[Citation114] However, the thermal stability of hydrolysed SPI was similar to native SPI indicating that it was independent of molecular mass. Papain modified SPI was reported to have significantly higher solubility and emulsifying properties than non-modified proteins.[Citation115]
7. Commercial soy-based adhesives
Soy protein-Kymene® adhesive system developed by Li [Citation116,117] has become widely used for interior plywood and engineered wood flooring with some limited acceptance in particleboard.[Citation118,119] This adhesive consists of SF and a petrochemical-based CA PAE resin.[Citation116,117] Bonded wood composites have shear strengths comparable to or higher than those bonded with commercial UF resins. The original studies on the soy-PAE adhesives used purified SPI, but the SF with much lower cost is now used commercially. The level of dispersible protein (PDI) and particle size of SF do not affect the basic bond strength of SF-PAE adhesive.[Citation120] SOYBABY® and OZERO® are used for the productions of MDF, HDF and laminated floor products on a large scale in China.[Citation103,Citation121] SOYBABY® has much greater water resistance than that of UF resins. However, SOYBABY® has a disadvantage of high hot pressing temperature (higher than 170 °C), while OZERO® has the disadvantage of high adhesive viscosity (more than 50,000 mPas).[Citation103,Citation121] Soyad™ adhesives (developed from soy-PAE system) are water-based systems formulated with natural SF and a proprietary cross-linking resin.[Citation122] When blended together the resin reacts with the protein in the SF to form a durable and water resistant thermosetting adhesive that is comparable in strength and performance to that of UF-based adhesives. Soyad™ adhesives are used for hardwood plywood, particleboard, MDF and engineered wood flooring.[Citation122] Cargill’s Prolia™ is used for plywood, panelling and particleboard.[Citation123]
8. Future prospects
The main reasons for increasing interest in soy-based adhesives are the legislation and increasing interest in environmentally friendly products. Most of the thermosetting adhesive resins used today in the wood industry for composite panels depend on petrochemicals. UF resins are widely used in the forest product industry and are formaldehyde emitters. Formaldehyde was declared as a carcinogen by IARC in 2004. Therefore, the restrictions on formaldehyde emissions for wood-based adhesives became stricter. It can be assumed that further reduction of the current legal formaldehyde emission limit will be specified with legislations for all wood-based materials. Furthermore, society recognises the protection of health and the environment, and consequently the use of synthetic products as major environmental problems, thus encourages the sustainable use of renewable natural resources. The disposal of inert petrochemical products, water and air pollution have increased awareness about environmental problems and forced industry to look for replacements in eco-friendly materials. The soy is appropriate for application in industrial products due to extensive soybean production. It is used for the production of soybean oil and the by-product, soy meal, is mainly used for animal feedstock. The human consumption of soy food, based on soy meal is small. Soy meal can be an ideal raw material for the manufacturing of wood adhesives due to its low cost, high protein content and easy processing. Soy-based adhesives have a limited water resistance but recover in strength after drying. They also have the disadvantage of high viscosity at high solid content. There are some commercial soy-based adhesives available on the market. However, to achieve good bonding strength, they mostly require different manufacturing processes of wood-based composites than that with commercial synthetic adhesives, i.e. higher hot pressing temperature and/or longer hot pressing time. Although soy-based adhesives have a big advantage of cold curing, the optimal pressing temperature for bonding wood composites of high water resistance is mostly much higher (higher than 120 °C). Therefore, replacement of synthetic resins with soy-based adhesives presents high cost of equipment replacement and consequently low market share of soy-based adhesives. Over recent years, new adhesive systems have been developed with low viscosity at high solid content, and also new systems with significantly improved water resistance. However, the challenge remains to produce the soy-based adhesive with low viscosity at high protein content, which will yield high water resistant wood-based composites at similar process parameters to those of synthetic resins. Such an adhesive system might capture a larger market share. A lot of research has already been done on the modification and cross-linking of soy proteins with synthetic materials and there are still opportunities remaining for the developments of purely natural soy-based adhesives. However, the structural changes induced by different modifiers should be investigated in detail, since that would enable better understanding of denatured protein structures hence enable a selection of new potential protein modifiers. Furthermore, natural fibre development, novel composite materials based on renewable sources and the use of fast-growing woody species such as bamboo in bonded products (flooring) offer significant opportunities for the development of soy-based adhesives.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- NCSPA: uses of soybeans [Internet]. Raleigh: North Carolina Soybean Producers Association; [cited 2015 Nov 4]. Available from: http://ncsoy.org/media-resources/uses-of-soybeans/
- FAOSTAT: production/crops [Internet]. Rome: Food and Agriculture Organization of the United Nations; [cited 2015 Nov 4]. Available from: http://faostat3.fao.org/browse/Q/QC/E
- FAOSTAT: production/crops processed [Internet]. Rome: Food and Agriculture Organization of the United Nations; [cited 2015 Nov 4]. Available from: http://faostat3.fao.org/browse/Q/QD/E
- Lambuth AL. Protein adhesives for wood. In: Pizzi A, Mittal KL, editors. Handbook of adhesive technology. 2nd ed. Abingdon: Taylor & Francis; 2003. p. 457–478.
- Frihart CR, Birkeland MJ, Allen AJ, et al. Soy adhesives that can form durable bonds for plywood, laminated wood flooring, and particleboard. In: SWST, editor. International Convention of Society of Wood Science and Technology and United Nations Economic Commission for Europe – Timber Committee. Proceedings; 2010 Oct 11–15; Geneva, Switzerland. Manona (WI): Society of Wood Science and Technology and United Nations Economic Commission for Europe; 2010. Paper WS-20.
- Zhong Z, Sun XS, Fang X, et al. Adhesion properties of soy protein with fiber cardboard. J. Am. Oil. Chem. Soc. 2001;78:37–41.10.1007/s11746-001-0216-0
- Sun X, Bian K. Shear strength and water resistance of modified soy protein adhesives. J. Am. Oil Chem. Soc. 1999;76:977–980.10.1007/s11746-999-0115-2
- Pizzi A. Recent developments in eco-efficient bio-based adhesives for wood bonding: opportunities and issues. J. Adhes. Sci. Technol. 2006;20:829–846.10.1163/156856106777638635
- McDevitt JE, Grigsby WJ. Life cycle assessment of bio- and petro-chemical adhesives used in fiberboard production. J. Polym. Environ. 2014;22:537–544.10.1007/s10924-014-0677-4
- Huang W, Sun X. Adhesive properties of soy proteins modified by sodium dodecyl sulfate and sodium dodecylbenzene sulfonate. J. Am. Oil Chem. Soc. 2000;77:705–708.10.1007/s11746-000-0113-6
- Kim MJ, Sun XS. Adhesion properties of soy protein cross linked with organic calcium silicate hydrate hybrids. J. Appl. Polym. Sci. 2014;131:8615–8623.
- Kumar R, Choudhary V, Mishra S, et al. Enzymatically-modifed soy protein part 2: adhesion behaviour. J. Adhes. Sci. Technol. 2004;18:261–273.10.1163/156856104772759458
- Vnučec D, Goršek A, Kutnar A, et al. Thermal modification of soy proteins in the vacuum chamber and wood adhesion. Wood Sci. Technol. 2015;49:225–239.10.1007/s00226-014-0685-5
- Ciannamea EM, Martucci JF, Stefani PM, et al. Bonding quality of chemically-modified soybean protein concentrate-based adhesives in particleboards from rice husks. J. Am. Oil Chem. Soc. 2012;89:1733–1741.10.1007/s11746-012-2058-2
- Huang W, Sun X. Adhesive properties of soy proteins modified by urea and guanidine hydrochloride. J. Am. Oil Chem. Soc. 2000;77:101–104.10.1007/s11746-000-0016-6
- Liu D, Chen H, Chang PR, et al. Biomimetic soy protein nanocomposites with calcium carbonate crystalline arrays for use as wood adhesive. Bioresour. Technol. 2010;101:6235–6241.10.1016/j.biortech.2010.02.107
- Mo X, Sun X, Wang D. Thermal properties and adhesion strength of modified soybean storage proteins. J. Am. Oil Chem. Soc. 2004;81:395–400.10.1007/s11746-004-0912-9
- Qi G, Li N, Wang D, et al. Physicochemical properties of soy protein adhesives obtained by in situ sodium bisulfite modification during acid precipitation. J. Am. Oil Chem. Soc. 2012;89:301–312.10.1007/s11746-011-1909-6
- Wang Y, Wang D, Sun XS. Thermal properties and adhesiveness of soy protein modified with cationic detergent. J. Am. Oil Chem. Soc. 2005;82:357–363.10.1007/s11746-005-1078-1
- Zhang Z, Hua Y. Urea-modified soy globulin proteins (7S and 11S): effect of wettability and secondary structure on adhesion. J. Am. Oil Chem. Soc. 2007;84:853–857.10.1007/s11746-007-1108-7
- Zhong Z, Sun XS, Fang X, et al. Adhesive strength of guanidine hydrochloride-modified soy protein for fiberboard application. Int. J. Adhes. Adhes. 2002;22:267–272.10.1016/S0143-7496(02)00003-9
- Kutnar A, Burnard MD. The past, present, and future of EU wood adhesive research and market. In: FPS, editor. International Conference on wood adhesives. Proceedings; 2013 Oct 9–11; Toronto, Canada. Madison (WI): Forest Products Society; 2014. p. 22–35.
- Pizzi A. Wood products and green chemistry. Ann. For. Sci. [Internet]. 2015 [cited 2015 Nov 15]. Available from: http://link.springer.com/10.1007/s13595-014-0448-3
- Conner AH. Urea-formaldehyde adhesive resins. In: Salomone JC, editor. Polymeric matererials encyclopedia. Vol. 11. Boca Raton (FL): CRC Press; 1996. p. 8496–8501.
- Athanassiadou E, Tsiantzi S, Markessini C. Towards composites with formaldehyde emission at natural wood levels. In: COST, editor. COST Action E49 Conference ‘Measurement and Control of VOC Emissions from Wood-Based Panels’. Proceedings; 2007 Nov 28–29; Braunschweig, Germany. Brussels (BE): COST Association; 2007.
- Fukushima D. Recent progress of soybean protein foods: chemistry, technology, and nutrition. Food Rev. Int. 1991;7:323–351.10.1080/87559129109540915
- García MC, Torre M, Marina ML, et al. Composition and characterization of soyabean and related products. Crit. Rev. Food Sci. Nutr. 1997;37:361–391.10.1080/10408399709527779
- Sun XS. Isolation and processing of plant materials. In: Wool RP, Sun XS, editors. Bio-based polymers and composites. Burlington (MA): Elsevier-Academic Press; 2005. p. 33–55.10.1016/B978-012763952-9/50004-6
- Utsumi S, Matsumura Y, Mori T. Structure-function relationships of soy proteins. In: Damodaran S, Paraf A, editors. Food proteins and their applications. New York (NY): Marcel Dekker; 1997. p. 257–291.
- Creighton TE. Proteins: structures and molecular properties. 2nd ed. New York (NY): W.H. Freeman; 1996.
- Petsko GA, Ringe D. From sequence to structure. In: Lawrence E, Robertson M, editors. Protein structure and function. London: New Science Press; 2004. p. 2–47.
- Boye JI, Ma CY, Harwalkar VR. Thermal denaturation and coagulation of proteins. In: Damodaran S, Paraf A, editors. Food proteins and their applications. New York (NY): Marcel Dekker; 1997. p. 25–56.
- Damodaran S. Food proteins: an overview. In: Damodaran S, Paraf A, editors. Food proteins and their applications. New York (NY): Marcel Dekker; 1997. p. 1–24.
- Frihart CR, Birkeland MJ. Soy properties and soy wood adhesives. In: Brentin RP, editor. Soy-based chemicals and materials. Washington (DC): American Chemical Society; 2014. p. 167–192.
- Jang Y, Li K. An all-natural adhesive for bonding wood. J. Am. Oil Chem. Soc. 2015;92:431–438.10.1007/s11746-015-2610-y
- Qi G, Li N, Wang D, et al. Adhesion and physicochemical properties of soy protein modified by sodium bisulfite. J. Am. Oil Chem. Soc. 2013;90:1917–1926.10.1007/s11746-013-2343-8
- Kalapathy U, Hettiarachchy NS, Rhee KC. Effect of drying methods on molecular properties and functionalities of disulfide bond-cleaved soy proteins. J. Am. Oil Chem. Soc. 1997;74:195–199.10.1007/s11746-997-0123-z
- Aider M, Djenane D, Ounis WB. Amino acid composition, foaming, emulsifying properties and surface hydrophobicity of mustard protein isolate as affected by pH and NaCl. Int. J. Food Sci. Technol. 2012;47:1028–1036.10.1111/ifs.2012.47.issue-5
- Hu H, Wu JH, Li-Chan CYE, et al. Effects of ultrasound on structural and physical properties of soy protein isolate (SPI) dispersions. Food Hydrocolloid. 2012;30:647–655.
- Shen L, Tang CH. Microfluidization as a potential technique to modify surface properties of soy protein isolate. Food Res. Int. 2012;48:108–118.10.1016/j.foodres.2012.03.006
- Petruccelli S, Añón MC. Thermal aggregation of soy protein isolates. J. Agr. Food Chem. 1998;43:3035–3041.
- Hettiarachchy NS, Kalapathy U, Myers DJ. Alkali-modified soy protein with improved adhesive and hydrophobic properties. J. Am. Oil Chem. Soc. 1995;72:1461–1464.10.1007/BF02577838
- Wrolstad RE, Acree TE, Decker EA, et al. Handbook of food analytical chemistry. Vol. 1, Water, proteins, enzymes, lipids, and carbohydrates. (NJ): Wiley; 2005. p. 301–314.
- Renkema JMS, Lakemond CMM, de Jongh HHJ, et al. The effect of pH on heat denaturation and gel forming properties of soy proteins. J. Biotechnol. 2000;79:223–230.10.1016/S0168-1656(00)00239-X
- Zhong Z, Sun XS, Fang X, et al. Adhesion strength of sodium dodecyl sulfate-modified soy protein to fiberboard. J. Adhes. Sci. Technol. 2001;15:1417–1427.10.1163/156856101753213277
- Sorgentini DA, Wagner JR. Comparative study of structural characteristics and thermal behavior of whey and isolate soybean proteins. J. Food Biochem. 1999;23:489–507.10.1111/jfbc.1999.23.issue-5
- Wang C, Jiang L, Wei D, et al. Effect of secondary structure determined by FTIR spectra on surface hydrophobicity of soybean protein isolate. Procedia Eng. 2011;15:4819–4827.10.1016/j.proeng.2011.08.900
- Lin Q, Chen N, Bian L, et al. Development and mechanism characterization of high performance soy-based bio-adhesives. Int. J. Adhes. Adhes. 2012;34:11–16.10.1016/j.ijadhadh.2012.01.005
- Santoni I, Pizzo B. Evaluation of alternative vegetable proteins as wood adhesives. Ind. Crops Prod. 2013;45:148–154.10.1016/j.indcrop.2012.12.016
- Zhu D, Damodaran S. Chemical phosphorylation improves the moisture resistance of soy flour-based wood adhesive. J. Appl. Polym. Sci. [Internet]. 2014;131:40451. Available from: http://doi.wiley.com/10.1002/app.40451
- Frihart CR. Wood adhesion and adhesives. In: Rowell R, editor. Handbook of wood chemistry and wood composites. 2nd ed. Boca Raton (FL): CRC Press; 2013. p. 255–278.
- Nordqvist P, Nordgren N, Khabbaz F, et al. Plant proteins as wood adhesives: bonding performance at the macro- and nanoscale. Ind. Crops Prod. 2013;44:246–252.10.1016/j.indcrop.2012.11.021
- Vnučec D, Mikuljan M, Kutnar A, et al. Influence of process parameters on the bonding performance of wood adhesive based on thermally modified soy proteins. Eur. J. Wood Prod. [Internet]. 2016 [cited 2016 Apr 27]. Available from: http://link.springer.com/article/10.1007%2Fs00107-016-1018-1
- Xu HN, Shen QY, Ouyang XK, et al. Wetting of soy protein adhesives modified by urea on wood surfaces. Eur. J. Wood Wood Prod. 2012;70:11–16.10.1007/s00107-010-0502-2
- Smith AK, Circle SJ. Soybeans: chemistry and technology. Vol. 1, Proteins. Westport (CT): AVI Publishing Company; 1972.
- Kim JT, Netravali AN. Effect of protein content in soy protein resins on their interfacial shear strength with ramie fibers. J. Adhes. Sci. Technol. 2010;24:203–215.10.1163/016942409X12538812532159
- O’Dell JL, Hunt CG, Frihart CR. High temperature performance of soy-based adhesives. J. Adhes. Sci. Technol. 2013;27:2027–2042.10.1080/01694243.2012.696945
- Berk Z. Technology of production of edible flours and protein products from soybeans. FAO Agricultural Services Bulletin No. 97, Chapters 1, 4, 5, and 6. Rome: Food and Agriculture Organization of the United Nations [Internet]. 1992 [cited 2015 May 11]. Available from: http://www.fao.org/docrep/t0532e/t0532e02.htm
- Johnson L, Smith K. Fact sheet: soybean processing. United soybean board [Internet]. 2016 [cited 2016 Mar 31]. Available from: http://www.soymeal.org/FactSheets/processing3.pdf
- Vavlitis A, Milligan ED. Flash desolventizing. In: Applewhite TH, editor. Proceedings of the world conference on oilseed technology and utilization. Champaign (IL): AOCS press; 1993. p. 286–289.
- Refstie S, Sahlström S, Bråthen E, et al. Lactic acid fermentation eliminates indigestible carbohydrates and antinutritional factors in soybean meal for Atlantic salmon (Salmo salar). Aquaculture. 2005;246:331–345.10.1016/j.aquaculture.2005.01.001
- Woerfel JB. Extraction. In: Erickson DR, editor. Practical handbook of soybean processing and utilization. Champaign (IL): AOCS press; 1995. p. 65–92.10.1016/B978-0-935315-63-9.50010-3
- Egbert WR. Isolated soy protein. In: Liu K, editor. Soybeans as functional foods and ingredients. Champaign (IL): AOCS press; 2004. p. 134–162.
- Lusas EW, Rhee KC. Soybean protein processing and utilization. In: Erickson DR, editor. Practical handbook of soybean processing and utilization. Champaign (IL): AOCS press; 1995. p. 117–160.10.1016/B978-0-935315-63-9.50012-7
- Khosravi S, Khabbaz F, Nordqvist P, et al. Protein-based adhesives for particleboards. Ind. Crops Prod. 2010;32:275–283.10.1016/j.indcrop.2010.05.001
- Kumar R, Choudhary V, Mishra S, et al. Adhesives and plastics based on soy protein products. Ind. Crops Prod. 2002;16:155–172.10.1016/S0926-6690(02)00007-9
- Ciannamea EM, Stefani PM, Ruseckaite RA. Medium-density particleboards from modified rice husks and soybean protein concentrate-based adhesives. Bioresour. Technol. 2010;101:818–825.10.1016/j.biortech.2009.08.084
- Qi G, Li N, Wang D, et al. Physicochemical properties of soy protein adhesives modified by 2-octen-1-ylsuccinic anhydride. Ind. Crops Prod. 2013;46:165–172.10.1016/j.indcrop.2013.01.024
- Cheng E, Sun X. Effects of wood-surface roughness, adhesive viscosity and processing pressure on adhesion strength of protein adhesive. J. Adhes. Sci. Technol. 2006;20:997–1017.10.1163/156856106777657779
- Kim JT, Netravali AN. Performance of protein-based wood bioadhesives and development of small-scale test method for characterizing properties of adhesive-bonded wood specimens. J. Adhes. Sci. Technol. 2013;27:2083–2093.10.1080/01694243.2012.697658
- Frihart CR. Adhesive groups and how they relate to the durability of bonded wood. J. Adhes. Sci. Technol. 2009;23:601–617.10.1163/156856108X379137
- Suárez JC. Bioadhesives. In: da Silva LFM, Öchsner A, Adams RD, editors. Handbook of adhesion technology. Berlin Heidelberg: Springer-Verlag; 2011. p. 1385–1408.10.1007/978-3-642-01169-6
- Gao Z, Zhang Y, Fang B, et al. The effects of thermal-acid treatment and crosslinking on the water resistance of soybean protein. Ind. Crops Prod. 2015;74:122–131.10.1016/j.indcrop.2015.04.026
- Kalapathy U, Hettiarachchy NS, Myers D, et al. Modification of soy proteins and their adhesive properties on woods. J. Am. Oil Chem. Soc. 1995;72:507–510.10.1007/BF02638849
- Liu Y, Li K. Development and characterization of adhesives from soy protein for bonding wood. Int. J. Adhes. Adhes. 2007;27:59–67.10.1016/j.ijadhadh.2005.12.004
- Amaral-Labat GA, Pizzi A, Gonçalves AR, et al. Environment-friendly soy flour-based resins without formaldehyde. J. Appl. Polym. Sci. 2008;108:624–632.10.1002/(ISSN)1097-4628
- Gu K, Li K. Preparation and evaluation of particleboard with a soy flour-polyethylenimine-maleic anhydride adhesive. J. Am. Oil Chem. Soc. 2011;88:673–679.10.1007/s11746-010-1706-7
- Chen N, Lin Q, Rao J, et al. Water resistances and bonding strengths of soy-based adhesives containing different carbohydrates. Ind. Crops Prod. 2013;50:44–49.10.1016/j.indcrop.2013.06.038
- Xiao Z, Li Y, Wu X, et al. Utilization of sorghum lignin to improve adhesion strength of soy protein adhesives on wood veneer. Ind. Crops Prod. 2013;50:501–509.10.1016/j.indcrop.2013.07.057
- Chen N, Lin Q, Zeng Q, et al. Optimization of preparation conditions of soy flour adhesive for plywood by response surface methodology. Ind. Crops Prod. 2013;51:267–273.10.1016/j.indcrop.2013.09.031
- Lei H, Du G, Wu Z, et al. Cross-linked soy-based wood adhesives for plywood. Int. J. Adhes. Adhes. 2014;50:199–203.10.1016/j.ijadhadh.2014.01.026
- Qi G, Sun XS. Soy protein adhesive blends with synthetic latex on wood veneer. J. Am. Oil Chem. Soc. 2011;88:271–281.10.1007/s11746-010-1666-y
- Wescott JM, Frihart CR, Traska AE. High-soy-containing water-durable adhesives. J. Adhes. Sci. Technol. 2006;20:859–873.10.1163/156856106777638734
- Zhong ZK, Sun XS. Thermal and mechanical properties and water absorption of guanidine hydrochloride-modified soy protein (11S). J. Appl. Polym. Sci. 2000;78:1063–1070.10.1002/(ISSN)1097-4628
- Tanford C. Protein denaturation. Adv. Protein Chem. 1968;23:121–282.10.1016/S0065-3233(08)60401-5
- Li X, Li Y, Zhong Z, et al. Mechanical and water soaking properties of medium density fiberboard with wood fiber and soybean protein adhesive. Bioresour. Technol. 2009;100:3556–3562.10.1016/j.biortech.2009.02.048
- Li X, Wang D, Ratto JA, et al. Production and characterization of high strength, thin-layered, pulp fiberboard using soy protein adhesives. J. Adhes. Sci. Technol. 2013;27:2065–2074.10.1080/01694243.2012.696957
- Sun X, Xhu L, Wang D, et al. Latex adhesives derived from ionic strength induced soy protein complexes. United States patent US 20080287635A1. 2008 Nov 20.
- Mo X, Sun XS. Soy proteins as plywood adhesives: formulation and characterization. J. Adhes. Sci. Technol. 2013;27:2014–2026.10.1080/01694243.2012.696916
- Koning MMG, Visser H. Protein interactions. In: Visser H, editor. An overview, protein interactions. New York (NY): VCH; 1992. p. 1–24.
- Liu H, Li C, Sun XS. Improved water resistance in undecylenic acid (UA)-modified soy protein isolate (SPI)-based adhesives. Ind. Crops Prod. 2015;74:577–584.10.1016/j.indcrop.2015.05.043
- Huang J, Li K. A new soy flour-based adhesive for making interior type II plywood. J. Am. Oil Chem. Soc. 2008;85:63–70.10.1007/s11746-007-1162-1
- Liu Y, Li K. Chemical modification of soy protein for wood adhesives. Macromol. Rapid Commun. 2002;23:739–742.10.1002/1521-3927(20020901)23:13<739::AID-MARC739>3.0.CO;2-0
- Liu Y, Li K. Modification of soy protein for wood adhesives using mussel protein as a model: the influence of a mercapto group. Macromol. Rapid Commun. 2004;25:1835–1838.10.1002/(ISSN)1521-3927
- Wang Y, Mo X, Sun XS, et al. Soy protein adhesion enhanced by glutaraldehyde crosslink. J. Appl. Polym. Sci. 2007;104:130–136.10.1002/(ISSN)1097-4628
- Li H, Li C, Gao Q, et al. Properties of soybean-flour-based adhesives enhanced by attapulgite and glycerol polyglycidyl ether. Ind. Crops Prod. 2014;59:35–40.10.1016/j.indcrop.2014.04.041
- Li J, Luo J, Li X, et al. Soybean meal-based wood adhesive enhanced by ethylene glycol diglycidyl ether and diethylenetriamine. Ind. Crops Prod. 2015;74:613–618.10.1016/j.indcrop.2015.05.066
- Chen N, Zeng Q, Rao J, et al. Effect of preparation conditions on bonding strength of soy-based adhesives via Viscozyme L action on soy flour slurry. BioResources. 2014;9:7444–7453.
- Chen N, Zeng Q, Lin Q, et al. Development of defatted soy flour based bio-adhesives using Viscozyme L. Ind. Crops Prod. 2015;76:198–203.10.1016/j.indcrop.2015.04.008
- Gao Q, Dang R, Sang ZT, et al. Investigation of soybean/PF resin based adhesive applying on plywood. Adv. Mat. Res. 2010;113–116:2007–2011.10.4028/www.scientific.net/AMR.113-116
- Fan DB, Qin TF, Chu FX. A soy flour-based adhesive reinforced by low addition of muf resin. J. Adhes. Sci. Technol. 2011;25:323–333.10.1163/016942410X524147
- Hamarneh AI, Heeres HJ, Broekhuis AA, et al. Use of soy proteins in polyketone-based wood adhesives. Int. J. Adhes. Adhes. 2010;30:626–635.10.1016/j.ijadhadh.2010.06.002
- Gui C, Liu X, Wu D, et al. Preparation of a new type of polyamidoamine and its application for soy flour-based adhesives. J. Am. Oil Chem. Soc. 2013;90:265–272.10.1007/s11746-012-2160-5
- Huang J, Gu K, Li K. Development and evaluation of new curing agents derived from glycerol for formaldehyde-free soy-based adhesives in wood composites. Holzforschung. 2013;67:659–665.
- Jang Y, Huang J, Li K. A new formaldehyde-free wood adhesive from renewable materials. Int. J. Adhes. Adhes. 2011;31:754–759.10.1016/j.ijadhadh.2011.07.003
- Gu K, Huang J, Li K. Preparation and evaluation of particleboard bonded with a soy flour-based adhesive with a new curing agent. J. Adhes. Sci. Technol. 2013;27:2053–2064.10.1080/01694243.2012.696950
- Gui C, Wang G, Wu D, et al. Synthesis of a bio-based polyamidoamine-epichlorohydrin resin and its application for soy-based adhesives. Int. J. Adhes. Adhes. 2013;44:237–242.10.1016/j.ijadhadh.2013.03.011
- Zhang Y, Zhu W, Lu Y, et al. Water-resistant soybean adhesive for wood binder employing combinations of caustic degradation, nano-modification, and chemical crosslinking. BioResources. 2013;8:1283–1291.
- Zhang Y, Zhu W, Lu Y, et al. Nano-scale blocking mechanism of MMT and its effects on the properties of polyisocyanate-modified soybean protein adhesive. Ind. Crops Prod. 2014;57:35–42.10.1016/j.indcrop.2014.03.027
- Wang Y, Sun XS, Wang D. Effects of preheating treatment on thermal property and adhesion performance of soy protein isolates. J. Adhes. Sci. Technol. 2007;21:1469–1481.10.1163/156856107782844756
- Luo J, Luo J, Gao Q, et al. Effects of heat treatment on wet shear strength of plywood bonded with soybean meal-based adhesive. Ind. Crops Prod. 2015;63:281–286.10.1016/j.indcrop.2014.09.054
- Fukushima D. Soy proteins. In: Yada RY, editor. Proteins in food processing. New York (NY): Woodhead Publishing Limited and CRC Press LLC; 2004. p. 123–145.10.1533/9781855738379.1.123
- Semple KA, Vnučec D, Kutnar A, et al. Bonding of THM modified Moso bamboo (Phyllostachys pubescens Mazel) using modified soybean protein isolate (SPI) based adhesives. Eur. J. Wood Wood Prod. 2015;73:781–792.10.1007/s00107-015-0938-5
- Kumar R, Choudhary V, Mishra S, et al. Enzymatically modified soy protein. J. Therm. Anal. Calorim. 2004;75:727–738.10.1023/B:JTAN.0000027169.19897.30
- Wu WU, Hettiarachchy NS, Qi M. Hydrophobicity, solubility, and emulsifying properties of soy protein peptides prepared by papain modification and ultrafiltration. J. Am. Oil Chem. Soc. 1998;75:845–850.10.1007/s11746-998-0235-0
- Li K, inventor; State of Oregon, assignee. Formaldehyde-free lignocellulosic adhesives, their manufacture and composites made from the adhesives. United States patent US 7,252,735 B2. 2007 Aug 7.
- Li K, Peshkova S, Geng X. Investigation of soy protein-kymene® adhesive systems for wood composites. J. Am. Oil Chem. Soc. 2004;81:487–491.10.1007/s11746-004-0928-1
- Allen AJ, Spraul BK, Wescott JM. Improved CARB II-compliant soy adhesives for laminates. In: Frihart CR, Hunt CG, Moon RJ, editors. International conference on wood adhesives 2009. Proceedings; 2009 Sep 28–30; Lake Tahoe, Nevada (US). Madison (WI): Forest Product society; 2010. p. 176–184.
- Wescott JM, Birkeland MJ, Yarvoski J, et al. Recent advances in soy containing PB and MDF. In: Frihart CR, Hunt CG, Moon RJ, editors. International conference on wood adhesives 2009. Proceedings; 2009 Sep 28–30; Lake Tahoe, Nevada (US). Madison (WI): Forest Product society; 2010. p. 136–141.
- Frihart CR, Satori H. Soy flour dispersibility and performance as wood adhesive. J. Adhes. Sci. Technol. 2013;27:2043–2052.10.1080/01694243.2012.696948
- Yang G, Yang B, Yuan C, et al. Effects of preparation parameters on properties of soy protein-based fiberboard. J. Polym. Environ. 2011;19:146–151.10.1007/s10924-010-0246-4
- Solenis: soyad™ adhesive technology [Internet]. Wilmington: Solenis; [cited: 2015 Dec 12]. Available from: http://solenis.com/en/industries/specialties-wood-adhesives/innovations/soyad-adhesive-technology/
- Cargill: Soy flour [Internet]. Minneapolis (MN): Cargill, Incorporated; [cited: 2015 May 15]. Available from: http://www.cargill.com/products/industrial/adhesives/soyflour/
