?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Electrochemical impedance spectroscopy (EIS), potentiodynamic polarization (PDP), and gravimetric analysis techniques were used to evaluate the ability of Lippia javanica leaf extract to inhibit aluminium (Al) corrosion in 1 M HCl solution. The formation of the protective film was confirmed using Ultraviolet-visible spectroscopy (UV–Vis), Fourier transform-infrared spectroscopy (FTIR) and contact angle studies. According to EIS, the presence of L. javanica extract in the acidic solution decreased the corrosion current density (icorr) and the double-layer capacitance (Cdl) due to the formation of a protective adsorption layer on the Al surface. PDP curves revealed anodic polarization passivation of the Al substrate, a well-known property of a highly entropic alloy like Al. Gravimetric results showed that the maximum protective efficacy of the L. javanica extract was 91.89% when the optimal concentration of the extract was 800 ppm. The contact angle increased to 123° in the presence of ALJPE, indicating the hydrophobic nature of the Al surface due to the formation of a protective film. The FTIR spectra revealed the involvement of C=O, C=C, O–H, =C–O–C, S=O, and C–H during the adsorption process, leading to the formation of ALJPE–Al3+ protective complexes. It was discovered that the adsorption mechanism of the extract on the Al surface follows the Langmuir adsorption isotherm description process. Using density functional theory (DFT), different dsorption positions were studied, finding that verbascoside preferentially binds to the Al(111) surface via the phenylethanoid group, with the oxygen atom close to the Al surface, leading to a binding energy of 114.620 kcal/mol.
1. Introduction
Aluminium (Al) is one of the most adaptable engineering and construction materials due to its unique properties. It has a wide range of applications due to its high strength-to-weight ratio, formability, lightweight, exceptional electrical and thermal conductivity, low cost, and high oxygen attraction. For example, it is the most sought-after metal in transportation systems such as rail vehicles, light vehicles, and aircraft as a fuel-saving measure [Citation1,Citation2]. Al occurs naturally as bauxite (a mixture of iron oxides, clay, and Al oxides), complicating its manufacturing process [Citation1]. Due to its high affinity for oxygen, exposure of a new Al surface to the atmosphere or an oxidizing agent, as in most aqueous environments, results in the formation of a compact, tightly adherent, thin, self-healing Al oxide (Al2O3) film on its surface, which can lead to passivation of the alloy/metal [Citation3–5]. Although this film offers a high level of protection, it can be destroyed by strong acidic or alkaline environments or environments with aggressive ions such as chlorides. Once the Al surface is destroyed, it becomes vulnerable to attack when exposed to corrosive solutions in operations such as cleaning and descaling [Citation1,Citation6]. The aggressive ions can locally destroy the film, resulting in pitting corrosion commonly seen in seawater or road salt environments [Citation1]. Corrosion inhibitors, which can be added in small amounts to process fluids and solutions, can be used to reduce the susceptibility of metals to corrosion in various industrial batch processes and closed-loop systems [Citation7,Citation8]. Corrosion inhibitors generally contain heteroatoms such as nitrogen (N), sulphur (S), oxygen (O), etc., and some conjugated double or triple bonds [Citation9]. Examples of inhibitors that possess some of these properties are polymers reported by Hsissou et al. [Citation10–13], which effectively protected the carbon steel from attack by the corrosive 1 M HCl solution. The advantage of corrosion inhibitors lies in their ease of use and the fact that they can be applied in small dosages, such as parts per million (ppm) while providing an effective corrosion protection effect, which is cost-effective [Citation14,Citation15]. The most well-known effective corrosion inhibitors are synthetic, with toxic problems and a complicated synthesis process; as a result, research focus has shifted to environmentally friendly inhibitors such as plant extracts.
Plant extracts are mostly preferred over synthetic corrosion inhibitors because of their ease of manufacture, plentiful availability, low raw material costs, and biodegradable properties [Citation16]. Several plant extracts have been used as effective corrosion inhibitors for metals in acidic solutions [Citation17–24]. Shahini et al. [Citation25] used Mish cast leaf extract as mild steel corrosion inhibitors in hydrochloric acid, for example. The test results show that the anti-corrosive ability of Mish Gush leaf extract increases with concentration and exposure time, reaching an inhibition efficiency of up to 96% after 4 h of exposure at 1200 ppm. Berrissoul et al. [Citation26] studied the use of ethanolic Origanum compactum extract as a green corrosion inhibitor for mild steel in an HCl solution. Their results show that the extract was an excellent inhibitor, with 90% inhibitory potency at a dosage of 400 mg/L and that the Langmuir adsorption isotherm adequately characterized the adsorption process. This study evaluated the anti-corrosion behaviour of L. javanica leaf extract for Al surfaces in hydrochloric acid (HCl) at different temperatures. HCl was chosen as the corrosive medium for this study to mimic the conditions of the petroleum and petrochemical industrial processes. This is because most of them regularly employ a process known as pickling to remove rust and scale from pipe surfaces. The main mineral acid used for the descaling process includes HCl and H2SO4 [Citation27–Citation29]. Although pickling solutions effectively remove corrosion products from pipeline surfaces, the pickling process still exposes the pipeline surface to corrosion if proper precautions are not taken. Additionally, the corrosive effects resulting from the pickling process can be circumvented by adding an inhibitor, such as L. javanica leaf extract, to the pickling solution. The inhibitor molecules protect against corrosion by adsorbing to the metal surface either through physical or chemical action or a combination of both, effectively isolating the metal from the corrosive solution [Citation30].
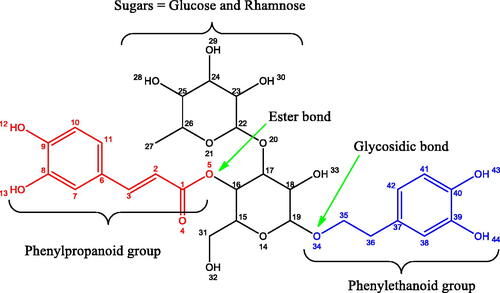
In Africa, infusions made from L. javanica leaves are used to make tea, which is then used to treat respiratory conditions such as colds, coughs, bronchitis, and fevers. It is also used to treat a variety of conditions, such as chest discomfort, stomach problems, measles, flu, malaria, skin rashes, and headaches [Citation31–34]. Vhavenda people from Limpopo province in South Africa use leaf infusions from the plant for respiratory and febrile diseases and prophylactics against dysentery, diarrhoea, and malaria [Citation33,Citation35]. Because of these uses, the plant can be considered environmentally safe. According to the literature, the plant contains a wide range of compounds that can be extracted and isolated, with one study identifying up to 173 different compounds [Citation36]. The complex nature of the chemical makeup of the L. javanica plant makes it difficult to ascribe inhibitory activity to any single specific component. Therefore, the contribution to corrosion inhibition through the mutual influence of the connections and their interaction cannot be ruled out. Verbascoside (VBS) is a compound that is abundant in L. javanica extracts, and the high antioxidant activities of the plant can be attributed in part to it () [Citation37]. Although recent research has focused on plant extracts as metal inhibitors, such studies have mainly focused on the discovery of an efficient inhibitor, leaving other important aspects of corrosion inhibitors unexplored, such as the stability of the protective film they produce. To describe corrosion protection, calculation methods that are characterized by force fields, such as molecular mechanics, are still used based on classical mechanics’ laws for predicting structures and properties. The force field approach to molecular modelling does not provide insight into chemical processes involving bond formation or breaking. Therefore, this work investigates the anti-corrosion potential of L. javanica for Al and also the stability of its protective layer, as well as the bond formation mechanism between VBS and the Al(111) surface using quantum mechanical methods and density functional theory (DFT).
2. Materials and methods
2.1. Production of plant extracts and test solutions
Fresh leaves of the L. javanica plant were shade-dried and pulverized into small pieces with a blender. 25 g of the crushed material was extracted three times with 200 mL of 100% acetone (99.5% pure) solution, each time using a fresh acetone solution. The extraction process was carried out for 3 h using the Soxhlet extraction method, and after that, the extracted liquid was filtered to get rid of the powdered plant material. The acetone L. javanica plant extract (ALJPE) yielded 2.5504 g, representing a 10.20% yield of a concentrated crude solid product after removing the acetone solution on a rotary evaporator. The solid ALJPE product was then characterized by FTIR spectroscopy using the ALPHA FTIR Bruker spectrophotometer. 1 M HCl solution was prepared by diluting analytical grade 32% HCl solution with distilled water. The 1 M HCl solution was then used to perform experimental tests for the uninhibited system and to prepare the desired ALJPE inhibitor solution of a concentration range of 200–800 ppm for the inhibited system tests. The ALJPEs were soluble in the corrosive 1 M HCl medium without adding any organic solvents.
2.2. Preparation of the Al test specimens
The test specimens were cut to a height of 3 cm, a length of 2 cm, and a thickness of 0.04 cm, with a 0.375 cm radius hole at the top for inserting the glass hooks used to hang the metals. The Al samples had the same weight percent composition as those reported by Nesane et al. [Citation38]. Except for the electrochemical measurements, where a 1 cm2 working area of the sample was cut and embedded in epoxy resin until dry, the Al specimens were used without further modification. The dried samples were sanded with different emery paper and cleaned with distilled water to expose the working area surface before each electrochemical test.
2.3. Electrochemical measurements
The electrochemical techniques used in this study are limited to electrochemical impedance spectroscopy (EIS) and potentiodynamic polarization (PDP). The techniques involved immersing epoxy-embedded 1 cm2 Al samples (working electrodes) in uninhibited and inhibited 1 M HCl solutions for approximately 1 h to reach a steady-state open-circuit potential (OCP). This was done in the presence of the reference and auxiliary electrodes, which were Ag/AgCl and graphite electrodes, respectively. A single-channel potentiostat/galvanostat BioLogic–SP150 with a built-in frequency response analyzer (FRA) was used for the analysis. The PDP and EIS data were examined using EC-Lab (version 11.42) and Zsim (version 3.3) software, respectively. The Tafel curves from PDP were measured in the potential range between ±10 (V) relative to the Eocp and a scan rate of 1 mV.s−1. The frequency (f) range chosen for EIS measurements was 100 kHz ≤ f ≤ 10 mHz with 10 mV applied potential signal amplitude around the resting potential. Both PDP and EIS were run at 303 K. Tafel curves lines (anodic and cathodic) were extrapolated to obtain the corrosion current densities (icorr) and other Tafel parameters. The icorr values for the uninhibited () and inhibited (
) systems were used to calculate the PDP corrosion inhibition efficiency, IEPDP(%), of ALJPE, using EquationEquation (1)
(1)
(1) :
(1)
(1)
From EIS measurements, resistance to charge transfer for the uninhibited () and inhibited (
) systems were used to evaluate the EIS corrosion inhibition efficiency, IEEIS(%), of ALJPE, using EquationEquation (2)
(2)
(2) :
(2)
(2)
2.4. Gravimetric and statistical analysis
Two sets of gravimetric analysis tests were performed at a constant temperature (303 K to 333 K) controlled by a thermostat water bath. In one test, the Al samples were immersed in 60 ml 1 M HCl, while in the other, the samples were immersed in 1 M HCl containing different concentrations of ALJPE (200–800 ppm). After 7 h in the water bath, samples were abducted from both the uninhibited and inhibited caustic solution, rinsed, air-dried, and accurately weighed, and the weight loss (Δm) in grams was calculated using the following EquationEquation (3)(3)
(3) :
(3)
(3)
where m1 is the initial mass of the Al sample before immersion and m2 is the final mass of the sample after immersion in the uninhibited and inhibited 1 M HCl solutions. The obtained ΔM at various temperatures was used to compute the corrosion rate (CR) using EquationEquation (4)
(4)
(4) .
(4)
(4)
S is the Al sample’s geometrical surface area (cm2), and t is the total corrosion test period (h). The calculated CR for the inhibited CR(ALJPE) and uninhibited CR(unh) 1 M HCl solution was then used to calculate the gravimetric inhibition efficiency, IE(%), of ALJPE using EquationEquation (5)(5)
(5) .
(5)
(5)
All gravimetric analyses tests were performed in triplicate except for time variation studies and statistical evaluation of the mass loss data was also performed to determine the precision of the CR data [Citation13,Citation39]. The standard deviations for the efficiencies (SDIE) were calculated according to EquationEquation (6)(6)
(6) :
(6)
(6)
where x are values in the data set, µ is the mean of the data set and n is the average number of data points.
2.5. Vibrational spectroscopy
Fourier transform-infrared spectroscopy (FTIR) was used to characterize ALJPE functional groups responsible for the inhibition process based on the vibrational frequencies using the ALPHA FTIR Bruker spectrometer. For this purpose, the samples were exposed to electromagnetic radiation in the frequency range of 400–4000 cm−1, where molecules absorb specific radiation frequencies due to the functional groups and the symmetry within the molecule [Citation40,Citation41]. The analysis was performed with the OPUS version 7.0, and the IR spectra were recorded as % transmittance versus wavelength.
2.6. Electronic spectroscopy
Ultraviolet-visible spectroscopy (UV–Vis) was performed to help elucidate the electronic absorption properties of the ALJCE and its adsorption behaviour on the Al surface. As molecules, electrons are known to absorb energy from the visible to the UV range (200–780 nm), which excites them from the ground state to a higher energy state; UV analysis in this work was performed from 199.9 nm to 1000 nm using a Jenway 7305 spectrophotometer in a quartz cell at room temperature. For the experiments, the optimal concentration of the extract (800 ppm) was analyzed without performing a gravimetric analysis. To examine the ALJPE stability on the Al surface, the Al sample was immersed in 60 ml of 1 M HCl containing an effective concentration (800 ppm) of the L. javanica extract and subjected to gravimetric analysis at 303 K for 7 h. After 7 h, the test samples were drawn out, and the solution was analyzed with UV–Vis. The solution after immersing the Al sample in 1 M HCl in the absence of ALJPE was also analyzed in addition to the 1 M HCl control for comparison purposes.
2.7. Contact angle measurements
Contact angle measurements were performed to evaluate ALJPE’s ability to form hydrophobic films on Al coupons capable of repelling water and preventing corrosion. A smartphone was used to capture the water droplet on the surface of the Al sample. The droplet was created by dispensing a small drop of ultrapure water with a pipette near the edge of the Al surface. While capturing the water droplet image, the smartphone with a macro lens mount was placed 1–2 cm from the water droplet to ensure a high-resolution image was captured. The captured images were analyzed with an image editor (ImageJ version 1.53), which allows for the calculation of the contact angle directly from the image. The image was converted to 32-bit to ensure that the image was grayscale, and after that, the droplet analysis LBADSA plugin [Citation42] was used to measure the static contact angle. Measurements were made for three conditions: the first was for the untreated polished Al surface, the second was for the polished sample subjected to gravimetric analysis in 1 M HCl without ALJPE, and lastly, for the sample subjected to gravimetric analysis in 1 M HCl was subjected to 800 ppm ALJPE.
2.8. DFT adsorption energy calculation
Material Studio 2020 was used to perform the computational of the DMol3 module [Citation43]. To ensure the reliability of the measurement and to define the thermodynamic properties of molecular systems, positive commutative correlation functionals such as Perdew-Burke-Ernzerhof (PBE) and generalized gradient approximation (GGA) were selected during the calculations [Citation44,Citation45]. While the double extended base of the double numeric basis plus polarization (DNP) extended orbit was selected as the numerical atomic base group for consideration of atomic orbitals [Citation46]. DFT semi-core pseudopotential (DSPP) and iterative subspace direct inversion (DIIS) were used to account for the core electrons and also to accelerate the convergence of the self-consistent field (SCF) charge density [Citation47]. The Monkhorst–Pack grid (3 × 3 × 1) was used to sample the Brillouin zone. For achieving geometry optimization, 0.00001 Ha (Ha is Hartree), 0.002 Ha/Å, and 0.005 Å were set as the values for the electronic energy convergence criteria, gradient, and atom displacement, respectively. To accommodate the large VBS molecule, the Al(111) slab surfaces were modelled by three layers repeated in a 7 × 7 surface cell unit with a 30 Å separation between clean slabs to ensure no contact between the sorbate and its periodic image. The uppermost layer of the Al atoms was relaxed along with the adsorbates, and the remaining two layers were constrained.
3. Results and discussion
3.1. Open circuit potential vs. time
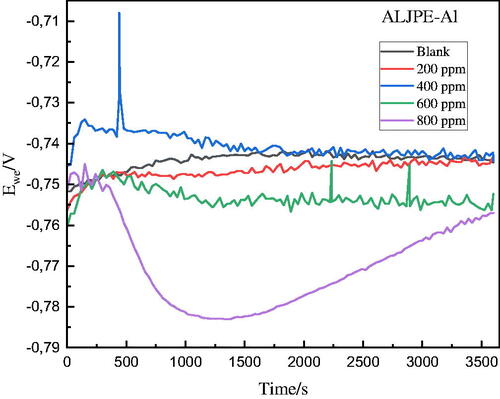
Before each electrochemical test, the potential of the Al working electrode versus the reference electrode was observed with no external current or potential to achieve a stable OCP value. shows the OCP curves run for 1 h, and they indicate that the introduction of ALJPE into the caustic 1 M HCl solution causes a shift of the OCP to more negative values as the concentration of ALJPE is increased, indicating the formation of a protective inhibitor film on the Al surface ().
3.2. Potentiodynamic polarization measurements
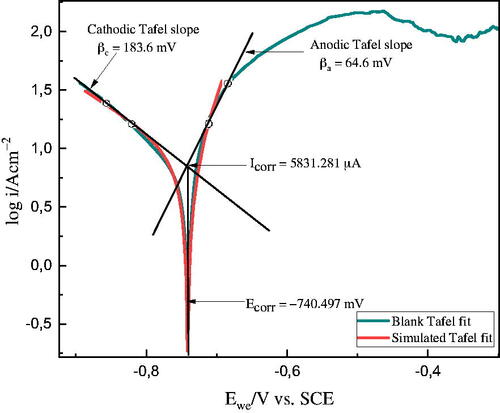
Polarization curves were recorded to study the kinetics of the anodic and cathodic corrosion reactions. The analysis was performed for Al samples in 1 M HCl at 303 K for various concentrations of the ALJPE, and obtained the curves are presented in . While the corrosion kinetic parameters derived from these plots are shown in . The CR is one of the pieces of information that can be extracted from PDP scans. The anodic and cathodic Tafel slope reactions (βa and βc) occurring at the open circuit for the inhibited and uninhibited systems were obtained from the linear region of the polarization curves. shows the Tafel extrapolation for Al (working electrode) in the 1 M HCl solution using the EC − Lab 11.42 software. The figure shows that by extrapolating back from the anodic and cathodic branches to the point where the anodic and cathodic reaction rates are equivalent, other parameters, such as the corrosion current density (icorr) and the corrosion potential (Ecorr), can be obtained.
Figure 4. PDP-Tafel plots for the uninhibited and ALJPE-inhibited (200–800 ppm) Al system in 1 M HCl at 303 K.
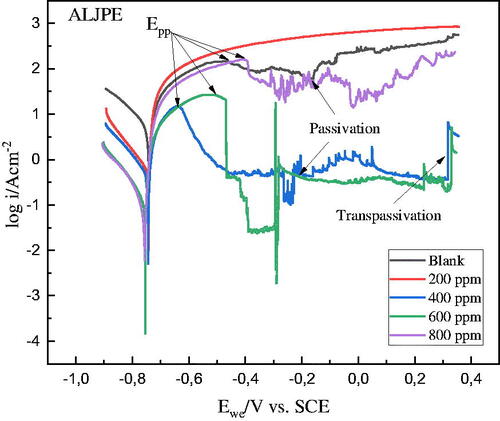
Table 1. PDP parameters for the uninhibited and ALJPE-inhibited Al system.
The Tafel fit shown in gives values for Ecorr (−740.497 mV vs. SCE), Icorr (5831.281 μA.cm−2), βa (64.6 mV), βc (183.6 mV). The CR was calculated by the software to be 2492.66 mils/year using the equivalent weight (E.W = 8.994 g/eq.), the density of the material (d = 2.7 g/cm3), and the exposed surface area of the working Al electrode (S = 1 cm2). The introduction of various concentrations of ALJPE into the corrosive 1 M HCl solution led to a decrease in the CR due to the control of the anodic and cathodic branches. This is more evident at the maximum concentration of the inhibitor used (800 ppm), where the CR decreased to 62.5649 mils/year, and the values for Ecorr (−757.873 mV vs. SCE), Icorr (145.823 μA.cm−2), βa (anodic Tafel slope = 19.4 mV), βc (cathodic Tafel slope= 122.8 mV) were obtained.
A high entropic alloy such as Al is prone to passivation and pitting due to the dissolution of its passive natural oxide layer, which protects it from corrosion. The anodic Tafel curves emphasize the passivation behaviour of the Al substrate under anodic polarization. The occurrence of passivation without and with the addition of ALJPE in the anodic branch was observed from −500 mV up to 300 mV. The diagram shows that when the system’s potential increases beyond Ecorr, the Icorr initially rises due to activation-controlled behaviour and reaches a maximum at the passivation potential (Epp). Thereafter, a rapid decrease in icorr with increasing potential indicates the formation of a protective or passive film on the Al surface (i.e. passivation took place). According to the curves, a low constant icorr is maintained regardless of the applied potential when the Al metal is in the passive state. The curves in the presence of 400 and 600 ppm ALJPE inhibitors show that the passive/protective film breaks down at higher potentials, which leads to the onset of the trans-passive region or pitting. The anodic current remains almost steady with the addition of a low concentration (200 ppm) of ALJPE even after the critical current has been attained, resulting in the absence of distinct features of the active-passive region. As shown in , adding ALJPE to the corrosive solution had little effect on the Ecorr values. The free corrosion potential of the corrosive 1 M HCl solution without ALJPE was −740.497 mV. After adding different concentrations of ALJPE, it stayed around this value up to 400 ppm. Still, when the concentration was increased to 600 and 800 ppm, the Ecorr reached more active values than those obtained without the extract, namely −756.712 and −757.873 mV. Though the Ecorr values were not significantly affected, the icorr values improved (decreased) with the addition of various concentrations of ALJPE. The icorr values decreased from 5831.281 (blank) down to 594.661 μA.cm−2 (200 ppm), and the lowest icorr value obtained was at the addition of 800 ppm (145.823 μA.cm−2). The reduction in the icorr values with ALJPE concentrations can be linked to the adsorption of the extracts on the Al surface [Citation48–50].
The Al substrate undergoes an anodic and cathodic localized corrosion reaction process by EquationEquations (7)(7)
(7) and Equation(8)
(8)
(8) as follows [Citation51]:
(7)
(7)
(8)
(8)
Outside the pits, other cathodic reactions are possible and include hydrogen evolution and oxygen reduction, which are shown by EquationEquations (9)(9)
(9) and Equation(10)
(10)
(10) , respectively:
(9)
(9)
(10)
(10)
According to the literature [Citation52], a corrosion inhibitor can prevent metal dissolution by controlling either the anodic or the cathodic reaction, and the value of Ecorr shift determines this. If the Ecorr is greater than 85 mV compared to the blank, the inhibitor is classified as cathodic or anodic. In the current study, the maximum Ecorr difference (−17.376 mV at 800 ppm) is much smaller. This classifies the L. javanica plant extract as a mixed-type Al corrosion inhibitor [Citation53], which affects the corrosion rates of both the anodic and cathodic branches, lowering the overall rate.
3.3. Impedance spectroscopy
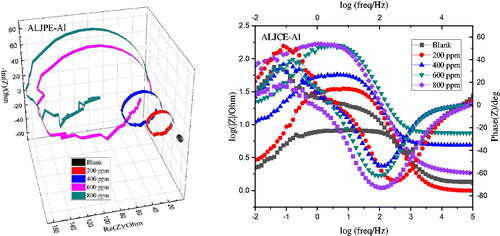
EIS measurements were used as a complementary electrochemical technique to understand the charge transfer mechanism of the ALJPE inhibitors and to quantitatively assess the effectiveness of extract performance as corrosion inhibitors for Al substrates. The EIS spectra () obtained for the blank Al sample (without ALJPE) were significantly similar to those obtained in the presence of different concentrations of ALJPE, indicating the formation of passivation films on the Al surface does not influence the corrosion mechanism. The semi-circular curves in the Nyquist plots for Al show that the charge transfer process controls the process of Al dissolution. Al Nyquist plots are known to consist primarily of a small inductive loop in the intermediate frequency (IF) compared to a large capacitive loop in the higher frequency (HF) ranges [Citation54]. However, the shape of the curves in the present study showed an inductive loop in the IF ranges that is almost as large as the capacitive loop in the HF ranges [Citation55]. Other researchers studying Al corrosion in HCl observed similarly shaped EIS plots [Citation56–59]. The depressed semi-circular nature of the Nyquist curves with a centre below the real axis can be attributed to the scattering/dispersing effect, which is one of the properties of solid-state electrodes like Al [Citation60,Citation61]. The diameter of the semi-circular Nyquist plots increased with the addition of different concentrations of ALJPE, indicating that ALJPE increased Al’s resistance to anodic oxidation. The corrosion resistance that ALJPE imparts to the Al surface can be attributed to the protective film barrier formed as a result of the adsorption of the extract.
Figure 5. EIS-Nyquist (a) and Bode (b) plots for the uninhibited and ALJPE-inhibited (200–800 ppm) Al system in 1 M HCl at 303 K.
shows the equivalent circuit used to simulate the measured impedance data for Al with and without ALJPE for Bode and Nyquist plots to find the electrochemical parameters. The simulation process was achieved using the ZSimp 3.3 software with low values (×10−3) of the squared differences between theoretical and experimental points (χ2) obtained for both the uninhibited and the inhibited systems, indicating that the selected equivalent circuit is ideal for fitting the EIS data [Citation62,Citation63].
Figure 6. Equivalent circuit for simulating the EIS data with a focus on the uninhibited corrosive Al system (1 M HCl).
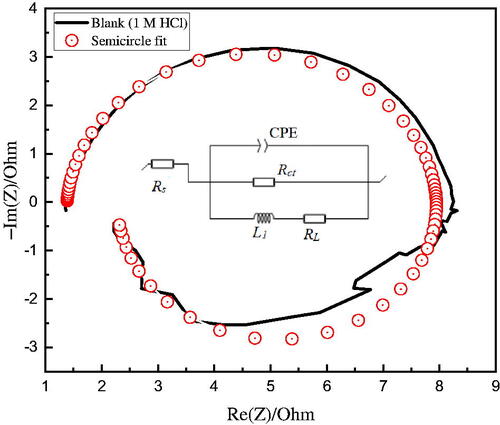
The circuit is similar to the modified Randel circuit {with a constant phase element (CPE), solution resistance (Rs), and charge transfer resistance (Rct)} except for the addition of inductance resistance (RL) and inductance element (L) to compensate for the inductive loop in lower frequency ranges. The CPE was used instead for a pure capacitor (Cdl, i.e. double-layer capacitance) as it accounts for the dispersion effects [Citation64]. While the polarization resistance (Rp) was calculated by EquationEquation (11)(11)
(11) :
(11)
(11)
The icorr and Rp are said to be related inversely [Citation65], implying that the impedance percentage efficiency of inhibition, IERp(%), can be calculated using EquationEquation (12)(12)
(12) as shown,
(12)
(12)
where Rp(unh) and Rp(ALJPE) are polarization resistance for the uninhibited and ALJPE-inhibited systems, respectively. Because impedance plots for composite metals such as Al are affected by frequency dispersion effects that occur during analysis, EquationEquation (13)
(13)
(13) can be used to find the true value of the capacitance.
(13)
(13)
where fmax is the frequency at which the imaginary part of the impedance is highest.
shows that the addition of ALJPE causes an increase in Rct and Rp values, which further increases as the concentration of ALJCE increases, indicating a slowdown in the CR process due to ALJPE adsorption on the Al surface. For instance, the Rp value increases from 0.9255 (blank) to 82.7249 Ω.cm2 (800 ppm) resulting in protection efficiency of 98.88%. The ALJPE components are considered to be significantly larger than those of the water molecules, which is supported by the structure of the VBS compound. Therefore, ALJPE components replace the water molecules on the Al electrode during the adsorption process [Citation66]. The adsorption and subsequent protection against Al corrosion by ALJPE rely on increased corrosion resistance by reducing electron flow and lowering Cdl with increasing ALJPE concentration. The decrease in Cdl values with increasing ALJPE concentration can be attributed to a decrease in dielectric constant or an increase in protective layer thickness value since more extract molecules are available with increasing ALJPE concentration [Citation67].
Table 2. EIS parameters for the uninhibited and ALJPE-inhibited Al system.
The downward trend in Cdl values with increasing ALJPE concentrations is thought to result from a reduction in the local dielectric constant (ε) or an increase in the electrical double layer. This implies the components of the extract are adsorbed at both the anodic and cathodic sites on the MS surface [Citation68]. The relation of the thickness of the protective film (d) formed on the MS surface to the Cdl cab be expressed according to EquationEquation (14)(14)
(14) [Citation69]:
(14)
(14)
where εo is the permittivity of the free space.
The Bode impedance and phase angle plots shown in were used to analyze the impedance data further. From these plots, the slope and phase angle values were obtained and evaluated to determine if the Al-inhibited system behaved like an ideal capacitor (phase angle = −90°; slope = −1) as the concentration of ALJPE was increased [Citation70]. The slope and phase angle for the uninhibited system were found to be −0.5775 and −41.5066°, respectively. The introduction of ALJPE into the system increased these values to a maximum of −0.8797 (slope) and −73.1285° (phase angle) at 800 ppm, indicating a deviation from the ideal capacitive behaviour. However, the increase in the slope and phase angle values with ALJPE concentration also suggests movement toward ideal behaviour as more ALJPE molecules are introduced into the system. Two peaks are indicated from the phase angle plots for both the uninhibited and inhibited solutions, demonstrating the manifestation of two relaxation processes. Such relaxation processes result from the adsorption of the ALJPE components at the Al/solution interface. One relaxation process is attributed to the Rct values, and the other corresponds to the adsorption of ALJPE at the Al/solution interface, forming a protective film [Citation66]. Since the second relaxation processes in the presence of ALJPE are ascribed to its adsorption process, the relaxation process observed in its absence could be attributed to the oxide layer formed during the passivation process.
3.4. Gravimetric results
3.4.1. Impact of ALJPE concentration on the CR and IE(%) of Al
Gravimetric measurements performed at different temperatures (303 K to 333 K) were used to provide a preliminary framework for the anti-corrosive nature of ALJPE for Al in 1 M HCl solution. This was done to see how the concentration of the extracts (200–800 ppm) affected the CR and IE(%). A review of the data in shows an increase in the IE(%) along with a decrease in CR as the concentration of ALJPE was increased to 800 ppm, where the maximum inhibition of 91.89% and the lowest CR of 7.0499 × 10−4 g.cm−2.h−1 was achieved at 303 K. The results in show that ALJPE is effective even at the lowest concentration of extract used, as a protection efficiency of 85.13% was observed at 303K.
Figure 7. The dependence of (a) IE(%) (solid lines) and (b) CR (dashed lines) on the concentration of ALJPE after 7 h of immersion of Al samples in 1 M HCl at different temperatures.
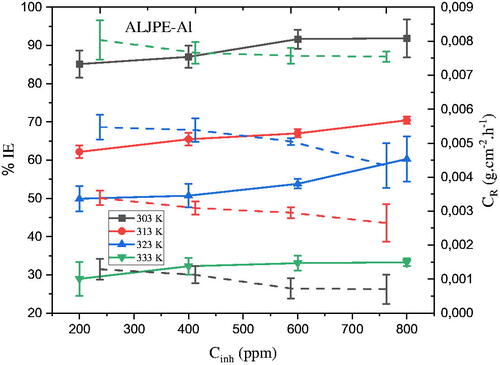
Table 3. Gravimetric parameters for the uninhibited and ALJPE-inhibited Al system in 1 M HCl corrosive solution at 303–333 K.
The decrease in CR with increasing ALJPE concentration indicates that the plant extract successfully reduced the Al surface area available for attack by HCl molecules. This is more evident at 800 ppm, which could be due to the fact that more ALJPE molecules are available for adsorption on the Al surface at this concentration. The high IE(%) is determined by how strongly corrosion inhibitor molecules, such as those found in L. javanica plant extracts, interact with the Al surface [Citation71–73]. For example, the main component of the plant extract, VBS, contains oxygen as a heteroatom that contains lone pairs of electrons, facilitating their adsorption on the surface of the Al metal.
3.4.2. Influence of immersion time on the protection stability of ALJPE for Al corrosion
A time study gives an indication of the stability of the adsorption film; therefore, the effect of long-term exposure (6–24 h) of Al to 1 M HCl solution in the presence of 800 ppm ALCE was examined using gravimetric analysis. The results are shown in and show that the IE(%) of Al was found to increase from 91.03% (6 h) to 97.22% (7 h).
Figure 8. Variations in %IE from gravimetric analysis with immersion time for 800 ppm ALJPE at 303 K.
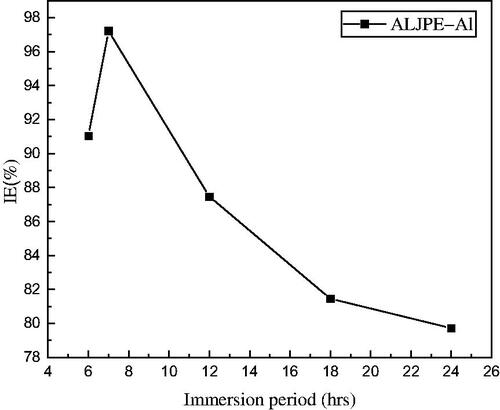
From 7 h to 24 h, the protective effect for Al corrosion showed a decreasing trend. However, the efficiency between 18 and 24 h is relatively similar, indicating that a stable adsorption film was formed, keeping the IE(%) around 80%.
3.4.3. Temperature effect, activation, and thermodynamic parameters
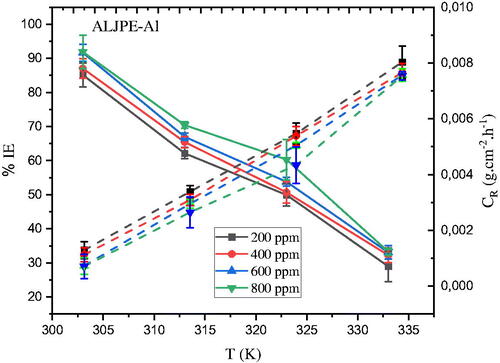
Monitoring the temperature response to corrosion kinetics can provide valuable information about the electrochemical properties of Al under various conditions [Citation74]. Since most Al applications are conducted under conditions involving high temperatures, it is essential to study the effect of temperature on ALJPE’s inhibition ability. and show that temperature influences the course of CR and IE(%) in the temperature range from 303–333 K. The results also show that the Al surface has the highest CR values with increasing system temperature, and it can be assumed that this is due to the desorption process of the ALJPE from the Al surface at higher temperatures. This is implied by the general rule of chemical kinetics at a higher temperature, which states that higher temperatures result in accelerated etching and desorption of inhibitor molecules from the metal surface [Citation75–78]. For instance, the CR at 200 ppm increased from 1.2924 × 10−3, 3.3875 × 10−3, 5.4723 × 10−3, up to 8.0348 × 10−3 g.cm−2.h−1 at 303, 313, 323, and 333 K, respectively. The decrease in IE(%) with the increasing temperature of the test system indicates that the equilibrium between the adsorption and desorption of ALJPE on the Al surface has been moved towards desorption. At higher temperatures, this leads to lower IE(%) values. The decrease in IE(%) with temperature is thought to be due to electrostatic interactions (physical adsorption) that disappear at elevated temperatures [Citation18]. This process takes place even at 800 ppm, where the IE(%) decreases from 91.89% (303 K) to 33.29% (333 K). The IE(%) decreased at a faster rate as the system temperature increased, with the lowest IE(%) of 28.95% obtained at the highest temperature of 333 K and the lowest extract concentration of 200 ppm.
Figure 9. The dependence of (a) IE(%) (solid lines) and (b) CR (dashed lines) on temperature after 7 h of immersion of Al samples in 1 M HCl at different ALJPE concentrations.
Thermodynamic and activation parameters were calculated to provide qualitative insight into the inhibitory behaviour of ALJPE in a 1 M HCl solution. Transition state functions and the Arrhenius equation were used to calculate values of apparent activation enthalpy (ΔHa), activation energy (Ea), and entropy (ΔSa) values for Al undergoing corrosion in 1 M HCl, and the formulas used are described by EquationEquations (15)(15)
(15) and Equation(16)
(16)
(16) [Citation79]:
(15)
(15)
(16)
(16)
where A denotes the Arrhenius parameter, h denotes the Planck constant (6.626176
1
J s), N denotes the number of Avogadro (6.02252
1
mo
), R denotes the universal molar gas constant (8.3145 J.mol−1.K−1), T is the temperature (K), and 2.303 is a transformation factor from natural log to log10. The Arrhenius (log CR vs 1/T) and transition state (log CR/T vs 1/T) plots for the uninhibited and ALJPE-inhibited systems produced straight-line graphs, as shown in , respectively. The log CR vs 1/T enabled the calculation of activation energy values from the slopes using Ea = – (slope) 2.303 R. While the slope (–ΔHa/2.303R) and intercept [log (R/Nh) + ΔSa/2.303R] of log CR/T vs 1/T were used to determine the values of ΔHa and ΔSa.
Figure 10. Arrhenius (a) and Transition (b) state diagrams for the uninhibited (blank) and ALJPE-inhibited (200–800 ppm) Al system in corrosive 1 M HCl solution.
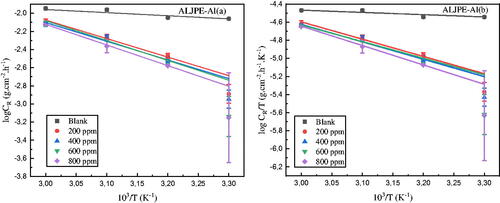
The ΔHa, ΔSa, and Ea values obtained are listed in . It has been found in the literature [Citation80,Citation81] that temperature, IE(%), and Ea of the inhibited system are all related in the following ways:
Table 4. Gravimetric parameters for the uninhibited and ALJPE-inhibited Al system in 1 M HCl corrosive solution at 303–333 K.
If the Ea remains constant in the presence and absence of an inhibitor, then the IE(%) does not vary with system temperature.
If the Ea of the inhibited solution is less than that of the uninhibited system, then the IE(%) increases with temperature.
If the Ea of the inhibited solution is greater than that of the uninhibited system, the IE(%) decreases as the temperature rises.
shows that the reported Ea values are higher in the presence of ALJPE than in its absence. The increase in Ea values in the presence of the extract/inhibitor could be due to the participation of ALJPE molecules during the adsorption process on the Al surface, leading to the formation of a physical barrier, which in turn blocks the corrosive charge and mass transfer [Citation82], leading to a decrease in its rate of dissolution. An analogous increase in apparent Ea in the presence of ALJPE compared to its absence, along with a decrease in IE(%) with increasing temperature, can be interpreted as indicating the formation of electrostatic adsorption (physisorption) nature. The electrostatic adsorption of ALJPE on the Al surface leads to the formation of a barrier that prevents charge and mass transfer processes. The mean value of (Ea−ΔH) can be used to describe unimolecular reactions from a thermodynamic and kinetic standpoint (Ea−ΔHa = RT) [Citation83–85]. The Ea values for ALJPE are greater than those of ΔHa by a value similar to that of RT, where T is the lowest temperature of the experiment (T = 303 K). This indicates that the corrosion of Al in 1 M HCl solution for the uninhibited and ALJPE-inhibited systems occurs via a by a unimolecular reaction. Higher negative ΔSa values were obtained for the inhibited 1 M HCl solution compared to that of the uninhibited solution, indicating a reduction in disorder or randomness as the inhibited corrosive reaction moves from the reagents to the activated complex [Citation86]. The increase in the positive values of ΔHa in the presence of ALJPE indicates the endothermic nature of the dissolution process of the Al surface, indicating a slow corrosion process as a result of an energy barrier created [Citation87]. The amount of energy required for the extract components to move from the bulk solution to the Al surface, also known as the heat of adsorption (Qads), was calculated using EquationEquation (17)(17)
(17) [Citation88,Citation89]:
(17)
(17)
where θ1 and θ2 are the degrees of surface coverage at temperatures T1 and T2. Positive Qads values often denote chemisorption adsorption, which is connected to rising inhibition efficiency with system temperature. Negative Qads values, conversely, signify physisorption adsorption, which is associated with a decline in inhibitory efficiency with rising system temperature [Citation90]. The negative Qads values in indicate that physisorption was the primary mechanism followed during the adsorption of ALJPE on the surface of Al.
3.5. Comparison of IE(%) in the present study with those in literature
The IE(%) obtained in this work was compared with those in the literature, and the results are shown in . The results show that ALJPE inhibits Al corrosion more effectively than other plant extracts. Although some of the comparative studies have been performed with different concentration units and corrosive electrolytes, the results still indicate that ALJPE offers high protection against Al corrosion and has an IE(%) of up to 97.22% at the highest concentration used (800 ppm) reached. Whereases others obtained maximum IE(%) of 85.9% (300 ppm) [Citation91], 94% (400 ppm) [Citation92], 95.7% (0.5 g/L) [Citation93] and 76.6% (1 g/L) [Citation94].
Table 5. Comparison of IE(%) for Al obtained by gravimetry in this work with literature.
3.6. Thermodynamic adsorption parameters
The most frequent way that anti-corrosion inhibitor compounds reduce the CR of metals is by adhering to the metal surface, which can be evaluated using adsorption isotherms. As a result, the adsorption behaviour of ALJPE on the Al surface was investigated by fitting the experimental data to a variety of known adsorption isotherms. The inhibition process has been described as occurring through two mechanisms [Citation95]. One of the mechanisms is believed to involve geometric blocking, which reduces the metal surface exposed to attack by corrosive species as a result of the adsorption of anti-corrosion inhibitor molecules. The energy effect is another mechanism involving changes in anodic or cathodic reaction energies. No method has been described in the literature that can be used to describe which of the two mechanisms is entirely responsible for the inhibitor’s ability to be inhibited. Theoretically, however, the corrosion potential cannot be changed if the energy effect is weaker than the geometric blockage [Citation95,Citation96]. The Al corrosion inhibitory effect of ALJPE occurs through a process in which the ALJPE molecules displace the water molecules with dissolved corrosive species from the metal interface, and such a process can be explained according to EquationEquation (18)(18)
(18) below [Citation97,Citation98]:
(18)
(18)
Among the isotherms fitted, the Langmuir adsorption model was found to have the best linear regression coefficient (R2) and was used to describe the adsorption mechanism of ALJPE onto the Al surface. The isotherm plots were evaluated by fitting experimental data CALJPE/θ versus CALJPE, and the results are shown in (gravimetric) and 11b (EIS and PDP). According to the isotherm, the surface coverage {i.e. θ = IE(%)/100} is related to the concentration of ALJPE, CALJPE (g.L−1), and the adsorption equilibrium constant, Kads through the expression described by EquationEquation (19)(19)
(19) :
(19)
(19)
Figure 11. Gravimetric (a), EIS, and PDP (b) Langmuir adsorption isotherms for Al system with different concentrations of ALJPE in corrosive 1 M HCl solution.
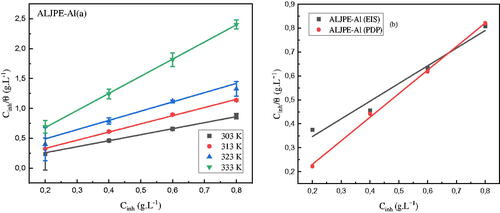
The Gibbs free energy of adsorption (ΔG°ads) was calculated from the Kads values using EquationEquation (20)(20)
(20) :
(20)
(20)
In the equation, 1000 represents the water concentration in the solution (g.L−1). Kads and ΔG°ads values from gravimetric, EIS, and PDP data at various temperatures are shown in .
Table 6. Gravimetric, EIS, and PDP Langmuir adsorption isotherm parameters for ALJPE-inhibited Al system in 1 M HCl corrosive solution at different temperatures.
With ALJPE as an Al corrosion inhibitor, the ΔG°ads values are above −20 kJ.mol−1 [Citation99] but below −40 kJ.mol−1, suggesting that the adsorption mechanism of ALJPE is the combination of physical and chemical adsorption processes, with physical adsorption on the surface of Al being dominant. The slope values of the Langmuir plots deviate from unity, indicating that the isotherm cannot be applied implicitly, leading to consideration of the physical property of the adsorption isotherm, which is relevant to the best fit of an isotherm, along with the slope and R2 values [Citation100–102].
The dimensionless constant separation factor (KL) is defined by the relationship shown in EquationEquation (21)(21)
(21) :
(21)
(21)
When KL > 1 or KL = 1, the adsorption process is unfavorable or considered irreversible and does not agree with the Langmuir adsorption isotherm [Citation101]. If KL ˂ 1, the experimental data are favourable and fit the Langmuir adsorption isotherm. The results in show that the KL values are less than one, implying that the Langmuir adsorption isotherm can interpret the adsorption process of ALJPE on the Al surface.
Table 7. The KL values for various concentrations of ALJPE at 303 to 333 K from gravimetric Langmuir isotherm parameters.
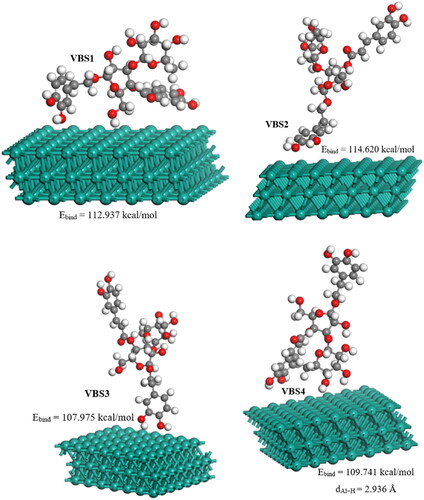
3.7. Binding structures and energies of the VBS/Al(111) system
The binding structures and energies of the VBS molecule on the Al(111) surface were evaluated to find the highest energy adsorption sites. For this study, the face-centered cubic (FCC) surface plane Al(111) was chosen as it is one of the most stable planes for Al [Citation103]. Four interaction sites were evaluated to discern the equilibrium adsorption configuration of VBS on the Al(111) surface. The PBE-optimized geometry structures of the VBS/Al(111) system are shown in . From the side view, the VBS molecules are located vertically (VBS1, VBS2, and VBS3) and parallel (VBS4) on the Al(111) surface. The interaction energy (Eint) of VBS molecules with the Al surface was obtained using EquationEquation (22)(22)
(22) :
(22)
(22)
where Etotal is the total energy of the VBS molecules upon interaction with the A(111) surface, Esurface is the energy of the Al(111) surface without adsorption of molecules, and Emolecule is the energy of the isolated VBS molecule. The binding energies (Ebind = −Eint) [Citation104] were found by taking the positive sign value of Eint, and the results are shown in .
Table 8. Calculated optimized energies for stable VBS adsorption sites on Al(111) surface.
The results show that the binding to the metal surface occurs preferentially through the phenylethanoid group, with the oxygen atom located near the Al surface (Al − O43) through the oxygen atom (VBS2), resulting in the highest binding energy (114.620 kcal/mol). The other three adsorbed geometries at different adsorption sites also showed high binding energies with an oxygen atom close to the Al surface. Binding energies can be interpreted to indicate whether the mode of adsorption is physisorption or chemisorption. Chemisorption has been associated with large binding energies and is thought to occur coupled with physisorption when the molecule of interest is close to the metal surface, and chemical bonds are formed [Citation105]. The binding of the oxygen atom to the surface of metals by chemisorption occurs due to the interaction between oxygen in its 2p state and the sp band of the metal, such as Al [Citation105]. It has been reported that binding energy of about 13 kcal/mol indicates a chemisorption binding nature on the surface of metals [Citation105]. All four binding geometries indicated binding energies greater than 13 kcal/mol for the VBS/Al(111) systems, indicating a chemisorption binding process. The experimental results (PDP, EIS, and gravimetric analysis) suggested that the inhibition of Al corrosion in 1 M HCl occurred via a mixed-type inhibition process involving both physical and chemical processes. This supports the assumption that the chemisorption binding process is normally coupled with the physisorption binding process. Compared to the others, the high binding energy for the VBS2/Al(111) system can also be attributed to the aromatic rings, whose structure contains π-electrons from the conjugated double bonds that interact with the sp band of the metal.
The bond distance for the optimized VBS2/Al(111) structure between the O43 atom and the Al atom is 2.590 Å. As a result of the interaction process, the initial bond distances of C41 = C40 and C40–O43 of the non-interacting phenylethanoid group were lengthened from 1.391 and 1.388 Å to 1.395 and 1.399 Å, and that of C39–C40 was compressed from 1.406 to 1.405 Å, respectively. Although these bond lengths indicate a slight or minor distortion of the VBS molecule when it binds through the phenylethanoid group, the small change is indicative of the stability of the final, binding structure.
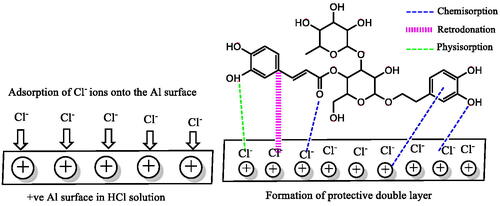
3.8. Corrosion inhibition mechanism
Most corrosion inhibitors are believed to inhibit corrosion primarily through adsorption and coordination processes [Citation106]. The π-electron clouds in the aromatic ring of organic compounds and the lone pair of electrons in the donor atoms can form a strong interaction bond between the inhibitor and the metal surfaces such as Al [Citation107]. Although lone pairs of electrons in O, S, and N heteroatoms play an essential role in the formation of the protective layer [Citation108], the absence of d orbitals in the outer shell of Al suggests that few compounds containing such atoms directly covalent bonds can be formed on the Al surface via the chemisorption mechanism [Citation109]. On the other hand, several studies have shown that Al3+ chelate complexes can be easily created through coordination with heterocyclic, hydroxy, and carboxyl groups [Citation106,Citation110,Citation111]. Al corrosion’s inhibition mechanism in the presence of ALJPE in the corrosive solution was modelled with VBS and presumably occurred, as suggested in . In acidic corrosive solutions, the Al surface possesses a positive charge that creates an attractive force for the negatively charged Cl− ions adsorbed on the Al surface [Citation38,Citation112], which act as adsorption centres for the protonated extract components (VBS+). This adsorption type is linked to the proposed physical adsorption mode [Citation25]. Chelation of freshly oxidized Al3+ ions (EquationEquation 7(7)
(7) ) with ALJPE components can lead to the formation of Al3+−ALJPE complexes as proposed in EquationEquation (23)
(23)
(23) , similar to that reported by Nnaji et al. [Citation112]:
(23)
(23)
The formation of Al3+-ALJPE complexes led to the passivation of the Al surface, as supported by the PDP results in this work. Nnaji et al. [Citation112] also suggested possible reactions that might be involved during the corrosion process. These include acid ionization (EquationEquation 24(24)
(24) ), water hydrolysis (EquationEquation 25
(25)
(25) ), metal oxidation or corrosion (EquationEquation 26
(26)
(26) ), formation of corrosion products (EquationEquations 27
(27)
(27) and Equation28
(28)
(28) ), and protective film formation (EquationEquations 29
(29)
(29) and Equation30
(30)
(30) ).
(24)
(24)
(25)
(25)
(26)
(26)
(27)
(27)
(28)
(28)
(29)
(29)
(30)
(30)
where x is the number of inhibitor molecules and n can be 1, 2, 3, or 4 for the different components of ALJPE.
Another process that might aid in Al corrosion inhibition is the intermolecular interactions between the ALJPE components, such as hydrogen bonding between the VBS molecules. This process is supported by the Langmuir slope’s departure from unity. During the Al corrosion inhibition process in 1 M HCl, VBS molecules can interact via the hydroxyl functional groups by forming hydrogen bonds between the partial negative charge (δ−) of the oxygen atom and the partial positive charge (δ+) of the hydrogen atom. Intermolecular interactions are usually observed for larger molecules such as the VBS, and when this occurs, the observed bond energies are also affected by the length of the hydrogen bond in cases where the intermolecular contacts are similar [Citation113].
3.9. Contact angle measurement
Adsorption of inhibitors to metal surfaces or corrosion products via the metal-surface-oriented hydrophilic portion of the molecule and the liquid-phase oriented hydrophobic portion results in a more hydrophobic metal surface, thereby reducing liquid water wettability [Citation114]. The contact angle is one of the simplest ways to determine the hydrophobicity of a substance [Citation115]. Different contact angles can be observed on the solid surface, including a small contact angle propagating along the solid surface of the material and a large contact angle observed when the liquid rolls/beads off the surface. When the surface contact angle is less than 90°, wetting is favourable, and the liquid tends to spread over a large surface area of the material/solid. A contact angle greater than 90° indicates unfavourable surface wetting, resulting in a smaller surface-liquid contact area and a compact liquid droplet. Complete wetting of the solid surface occurs when the contact angle is 0° because the placement of the water droplet on the surface results in a shallow puddle of water. For superhydrophobic surfaces, the contact angle formed by a tactile drop of water is usually greater than 150°, indicating that the contact between the drop and the solid surface is very small [Citation116]. Contact angle measurements for polished untreated metal substrate and metal substrate immersed in 1 M HCl medium with (800 ppm) and without ALJPE for 7 h at 303 K are shown in . The figure reveals that the contact angle for the untreated metal was 66° and decreased to 48° when the metal was exposed to the corrosive medium. The reduction in the contact angle indicates an increase in the surface roughness of Al due to the attack of the exposed surfaces by Cl– ions. Immersing the Al metal in the corrosive solution in the presence of the optimum concentration of ALJCE (800 ppm) increased the contact angle, indicating an increase in the hydrophobicity of the Al surface. The contact angle increased to 123° in the presence of ALJPE, indicating that the Al has become hydrophobic due to the formation of an adsorption film which inhibits its corrosion [Citation117,Citation118].
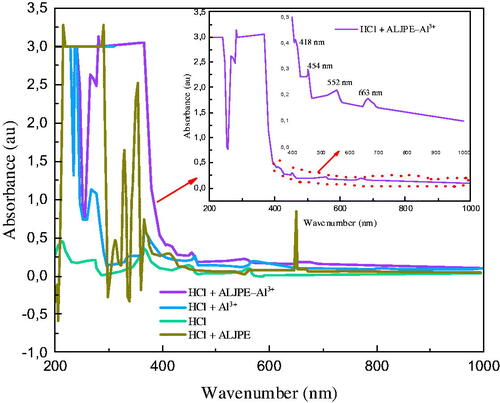
3.10. UV-visible spectroscopy: interaction of ALJPE with Al3+ cations
The possible interaction at the interface between the ALJPE molecules and the Al surface was investigated using a UV–vis test. The tests were performed for the ALJPE-inhibitor acidic solution before (HCl + ALJPE), and after (HCl + ALJPE–Al3+) the Al samples were immersed for 7 h (). For control and comparison purposes, the tests were also performed for 1 M HCl solution before and after immersion of the Al sample in the absence of ALJPE. The UV traces show that the long-wavelength band observed prior to immersion of the Al metal in a corrosive solution containing ALJPE molecules was shifted from the 215–290 to 265–366 nm region due to a bathochromic shift produced by the introduction of the Al metal. These absorbance peaks could be interpreted as indicating the presence of π–π* and n–π* transitions, which could result from the C=C and C=O groups present in the plant extract [Citation119,Citation120]. UV traces for Al immersed in 1 M HCl (HCl + Al3+) revealed absorption peaks at 240 and 268 nm associated with the intra-orbital d-d transitions in the Al cation, which correspond to those reported by Marhamati et al. [Citation121] for the iron cation UV curves. The shift in the long-wavelength band (265–366 nm) also indicates the occurrence of intramolecular electron transfer from a ligand to inhibitor compounds, also known as ligand-to-metal charge transfer (LMCT) [Citation122–124]. Other absorption peaks at 418 and 663 nm that point to the adsorption of ALJPE onto the Al surface may be due to the n–p* transitions of carbonyl and other functional groups found in the extract [Citation125,Citation126]. Based on these findings, it can be concluded that one method of inhibiting Al corrosion is the formation of a coordinated complex as a result of the interaction of the p-orbital of Al with non-bonding electrons of the oxygen atoms found in the hydroxyl group of one of the ALJPE compounds, such as the VBS.
3.11. Characterization of pure ALJPE and the adsorption film on the Al surface by FTIR
The characterization of possible functional groups of compounds contained in ALJPE was done by FTIR, and the results are shown in . This work mainly focuses on the determination of the functional groups responsible for the adsorption of ALJPE compounds on the Al surface and, subsequently, its protection from the aggressive 1 M HCl solution. To achieve this, analysis was conducted for the solution, which was used to run gravimetric analysis in the absence (Blank–Al3+ solution) and presence of ALJPE inhibitor (ALJPE–Al3+ solution), as shown in . The results show that the two spectra are identical, indicating that no functional group from ALJPE was identified. These two spectra, however, are similar to that of liquid water, which is known to exhibit a broad but intense adsorption peak in the mid-infrared range of the FTIR spectrum. Around the 3000–4000 cm−1 wavelength, a peak representing an O–H was observed. At 2000 cm−1, a less intense peak representing the coupling of the scissor bending and near-infrared liberation band was observed [Citation127,Citation128]. The scissors bending of the water molecule (H–O–H) can also result in a small intense peak at the 1600 cm−1 region, observed in these spectra [Citation129]. This makes it somewhat difficult to assign any contribution to the functional groups found in the two solutions, and the next step is to analyze the surface of Al after immersion in an acidic solution with (ALJPE–Al3+) and without (Blank–Al3+) ALJPE. This was achieved by allowing the metals sufficient time to dry completely and then after scratching the surface and analyzing the powder obtained with FTIR. The Blank–Al3+ spectrum is also identical to that of liquid water except for the two peaks identified at 1151 and 502 cm−1, which in our previous paper [Citation38] on the inhibition of Al, these peaks were stated to represent the γ–AlO4 and γ–AlO6, respectively, indicating the presences of both octahedral and tetrahedral coordination of alumina in γ–Al2O3 [Citation130]. The ALJPE–Al3+ spectrum is distinctively different from the other spectra showing the participation of ALJPE in the dissolution process of Al.
Figure 16. FTIR spectra for uninhibited (Blank–Al3+) and ALJPE-inhibited (ALJPE–Al3+) Al systems and their solutions (a) and the full-scale ALJPE-inhibited (ALJPE–Al3+) Al system (b).
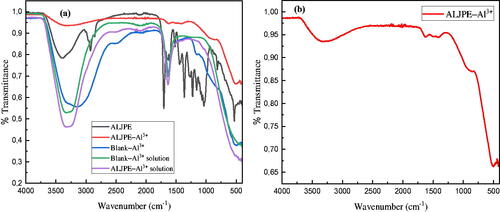
Table 9. FTIR spectral peaks and their identification for ALJPE, Blank–Al3+ corrosion products, and ALJPE–Al3+ adsorption film on Al in 1 M HCl.
The spectrum showed a reduction in the intensity of the Al2O3.×H2O peak found at about 3000–4000 cm−1 to a narrower peak. This indicates that the presence of ALJPE in the corrosive solution decreased Al’s CR, which the O–H group could have caused in the crude extract. A less intense peak at 1620 cm−1 is evidence that the ALJPE adsorption layer protected the Al surface since this peak can be linked to the scissor band of water molecules. Additionally, the uniqueness of this peak from that of water could be the result of the interaction of ALJPE with the Al surface via the C=O and C=C groups. The characteristic absorption bands around the 1496 and 1406 cm−1 can be linked to the adsorption of ALJPE via the S=O, C–H C=C, and =C–O–C functional groups in the extract. The absorption band observed around 1151 cm−1 in the Blank–Al3+ spectrum has shifted to around 900 cm−1 in the presence of ALJPE, while the peak at 502 cm−1 has shifted to higher transmittance value. This indicates that the extract successfully altered the formation of both the octahedral and tetrahedral coordination of alumina.
4. Conclusions
The current study demonstrated that L. javanica extract is an excellent inhibitor of Al corrosion in 1 M HCl because it can promote the formation of a passive or protective layer on the Al surface and is inexpensive to produce, biodegradable and non-toxic. Some of the key findings from this work are highlighted below:
ALJPE provided maximum inhibition protection for the Al surface at 303 K of 97.22%, 98.88% and 97.50% as indicated from the gravimetric, EIS and PDP analysis, respectively.
Experimental results from gravimetric and electrochemical (EIS and PDP) analysis indicated that the inhibition of Al corrosion was achieved through a complex process involving physical and chemical adsorption processes. This is in agreement with theoretical results since the chemical binding process indicated by the binding energies is said to also involve a physical interaction process.
The negative ΔG°ads values indicated that ALJPE adsorption on Al surface obeyed the Langmuir adsorption isotherm and occurred through a spontaneous process.
EIS measurements showed that an increase in extract concentration is accompanied by an increase in Rct and consequently IE(%) values, leading to a decrease in Cdl values.
Contact angle tests indicated the adsorption of L. javanica leaf extract to the active sites of Al in 1M HCl solution, as their presence resulted in a reduction in surface hydrophilicity at contact angles greater than 90°.
The functional groups responsible for the formation of the protective layer and the occurrence of an intramolecular electron transfer from the inhibitor compounds to the Al surface were identified by FTIR analysis and UV-Vis test.
DFT calculations of the binding process of the VBS molecule on the Al surface indicated that the molecule contains oxygen atoms located at the carbonyl and hydroxyl groups capable of donating their lone pair electrons to the Al surface. The calculations also indicated the participation of the aromatic π-bonds during the interaction of the molecule with the Al surface resulting in high binding energies.
Acknowledgments
T.N thanks the Sasol scholarship and the Sasol-National Research Foundation of South Africa for financial support. The authors also thank the Centre of High-Performance Computing (CHPC), Cape Town, South Africa for the Materials Studio 2020 license and platform to perform computational calculations.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Gándara MF. Aluminium: the metal of choice. Mater Tehnol. 2013;47:261–265.
- Xhanari K, Finšgar M, Hrnčič MK, et al. Green corrosion inhibitors for aluminium and its alloys: a review. RSC Adv. 2017;7(44):27299–27330.
- Martin JH, Yahata BD, Hundley JM, et al. 3D printing of high-strength aluminium alloys. Nature. 2017;549(7672):365–369.
- Anbarasi CM, Divya G. A green approach to corrosion inhibition of aluminium in acid medium using azwain seed extract. Mater Today. 2017;4:5190–5200.
- He X, Jiang Y, Li C, et al. Inhibition properties and adsorption behavior of imidazole and 2-phenyl-2-imidazoline on AA5052 in 1.0 M HCl solution. Corros Sci. 2014;83:124–136.
- Mishra A, Godhani D, Sanghani A. Inhibitive properties of nitrogen containing heterocyclic compounds over aluminium alloys in organic acid environment. J Chem Pharm Res. 2011;3:388–396.
- Alvarez PE, Fiori-Bimbi MV, Neske A, et al. Rollinia occidentalis extract as green corrosion inhibitor for carbon steel in HCl solution. J Ind Eng Chem. 2018;58:92–99.
- Miralrio A, Espinoza Vázquez A. Plant extracts as green corrosion inhibitors for different metal surfaces and corrosive media: a review. Processes. 2020;8(8):942.
- Guo L, Tan J, Kaya S, et al. Multidimensional insights into the corrosion inhibition of 3, 3-dithiodipropionic acid on Q235 steel in H2SO4 medium: a combined experimental and in silico investigation. J Colloid Interface Sci. 2020;570:116–124.
- Hsissou R, Abbout S, Seghiri R, et al. Evaluation of corrosion inhibition performance of phosphorus polymer for carbon steel in [1 M] HCl: computational studies (DFT, MC and MD simulations). J Mater Res Technol. 2020;9(3):2691–2703.
- Hsissou R, Benhiba F, Dagdag O, et al. Development and potential performance of prepolymer in corrosion inhibition for carbon steel in 1.0 M HCl: outlooks from experimental and computational investigations. J Colloid Interface Sci. 2020;574:43–60.
- Hsissou R, Abbout S, Benhiba F, et al. Insight into the corrosion inhibition of novel macromolecular epoxy resin as highly efficient inhibitor for carbon steel in acidic mediums: synthesis, characterization, electrochemical techniques, AFM/UV–visible and computational investigations. J Mol Liq. 2021;337:116492.
- Hsissou R, Benhiba F, Abbout S, et al. Trifunctional epoxy polymer as corrosion inhibition material for carbon steel in 1.0 M HCl: MD simulations, DFT and complexation computations. Inorg Chem Commun. 2020;115:107858.
- Lgaz H, Masroor S, Chafiq M, et al. Evaluation of 2-mercaptobenzimidazole derivatives as corrosion inhibitors for mild steel in hydrochloric acid. Metals. 2020;10(3):357.
- Dehghani A, Bahlakeh G, Ramezanzadeh B, et al. Integrated modeling and electrochemical study of myrobalan extract for mild steel corrosion retardation in acidizing media. J Mol Liq. 2020;298:112046.
- Mo S, Luo H-Q, Li N-B. Plant extracts as “green” corrosion inhibitors for steel in sulphuric acid. Chem Pap. 2016;70:1131–1143.
- Omran MA, Fawzy M, Mahmoud AED, et al. Optimization of mild steel corrosion inhibition by water hyacinth and common reed extracts in acid media using factorial experimental design. Green Chem Lett Rev. 2022;15(1):216–232.
- Ghazi I, Zefzoufi M, Siniti M, et al. Corrosion inhibition of carob pod pulp (Ceratonia siliqua L.) on carbon steel surface C38 in hydrochloric acid. J Bio- Tribo-Corros. 2022;8:1–23.
- Chaubey N, Singh VK, Quraishi M. Papaya peel extract as potential corrosion inhibitor for aluminium alloy in 1 M HCl: electrochemical and quantum chemical study. Ain Shams Eng J. 2018;9(4):1131–1140.
- Al Otaibi N, Hammud HH. Corrosion inhibition using harmal leaf extract as an Eco-Friendly corrosion inhibitor. Molecules. 2021;26(22):7024.
- Manh TD, Huynh TL, Thi BV, et al. Corrosion inhibition of mild steel in hydrochloric acid environments containing sonneratia caseolaris leaf extract. ACS Omega. 2022;7(10):8874–8886.
- Ramirez-Arteaga M, Valladares M, Gonzalez Rodriguez J. Use of prosopis laevigata as a corrosion inhibitor for Al in H2SO4. Int J Electrochem Sci. 2013;8:6864–6877.
- Gk S, Jacob JM, P R, et al. Synergistic effect of salts on the corrosion inhibitive action of plant extract: a review. J Adhes Sci Technol. 2021;35(2):133–163.
- Tk B. Corrosion inhibition of mild steel in hydrochloric acid by leaves extract of Tephrosia purpurea. J Adhes Sci Technol. 2020;34:2424–2447.
- Shahini M, Ramezanzadeh M, Bahlakeh G, et al. Superior inhibition action of the Mish Gush (MG) leaves extract toward mild steel corrosion in HCl solution: theoretical and electrochemical studies. J Mol Liq. 2021;332:115876.
- Berrissoul A, Ouarhach A, Benhiba F, et al. Assessment of corrosion inhibition performance of origanum compactum extract for mild steel in 1 M HCl: weight loss, electrochemical, SEM/EDX, XPS, DFT and molecular dynamic simulation. Ind Crops Prod. 2022;187:115310.
- Meriem Z, Hana F, Souad D, et al. Experimental and theoretical evaluation of the adsorption process of some polyphenols and their corrosion inhibitory properties on mild steel in acidic media. J Environ Chem Eng. 2021;9(6):106482.
- Fan G, Liu H, Fan B, et al. Trazodone as an efficient corrosion inhibitor for carbon steel in acidic and neutral chloride-containing media: facile synthesis, experimental and theoretical evaluations. J Mol Liq. 2020;311:113302.
- Guo W, Talha M, Lin Y, et al. Effect of phosphonate functional group on corrosion inhibition of imidazoline derivatives in acidic environment. J Colloid Interface Sci. 2021;597:242–259.
- Tan B, Lan W, Zhang S, et al. Passiflora edulia Sims leaves extract as renewable and degradable inhibitor for copper in sulfuric acid solution. Colloids Surf A Physicochem Eng Asp. 2022;645:128892.
- Van Wyk B-E, Oudtshoorn B, Gericke N. Medicinal Plants of South Africa: Briza; 1997:304.
- Watt J, Breyer-Brandwijk M. Africa. PPoS. The Medicinal and Poisonous Plants of Southern Africa; 1932.
- Hutchings A. Press NJV, flora. Zulu medicinal plants. An Inventory. 1998:27, 464.
- Hutchings A, van Staden J. Plants used for stress-related ailments in traditional Zulu, Xhosa and Sotho medicine. Part 1: plants Used for Headaches. 1994;43:89–124.
- Pascual M, Slowing K, Carretero E, et al. Lippia: traditional uses, chemistry and pharmacology: a review. J Ethnopharmacol. 2001;76(3):201–214.
- Maroyi A. Lippia javanica (Burm. F.) Spreng.: traditional and commercial uses and phytochemical and pharmacological significance in the african and indian subcontinent. Evid Based Complement Alternat Med. 2017;2017:1–34.
- Olivier D, Shikanga E, Combrinck S, et al. Phenylethanoid glycosides from Lippia javanica. S Afr J Bot. 2010;76(1):58–63.
- Nesane T, Mnyakeni-Moleele SS, Murulana LC. Exploration of synthesized quaternary ammonium ionic liquids as unharmful anti-corrosives for aluminium utilizing hydrochloric acid medium. Heliyon. 2020;6(6):e04113.
- Rbaa M, Lakhrissi B. Novel oxazole and imidazole based on 8-hydroxyquinoline as a corrosion inhibition of mild steel in HCl solution: insights from experimental and computational studies. Surf Interfaces. 2019;15:43–59.
- Silverstein R, Webster F, Kiemle D. Proton NMR spectrometry. Spectrometric identification of organic compounds, 7th ed. New York, NY: John Wiley & Sons Inc. 2005. p. 142.
- Smith ND. Fourier transform infrared spectroscopy. Analyst. 1996;121:83N.
- Stalder AF, Melchior T, Müller M, et al. Low-bond axisymmetric drop shape analysis for surface tension and contact angle measurements of sessile drops. Colloids Surf A Physicochem Eng Asp. 2010;364(1–3):72–81.
- Delley B. From molecules to solids with the DMol 3 approach. J Chem Phys. 2000;113(18):7756–7764.
- Hehre WJ, Ditchfield R, Pople JA. Self—consistent molecular orbital methods. XII. Further extensions of gaussian—type basis sets for use in molecular orbital studies of organic molecules. J Chem Phys. 1972;56(5):2257–2261.
- Francl MM, Pietro WJ, Hehre WJ, et al. Self‐consistent molecular orbital methods. XXIII. A polarization‐type basis set for second‐row elements. J Chem Phys. 1982;77(7):3654–3665.
- Mashhadzadeh AH, Ahangari MG, Dadrasi A, et al. Theoretical studies on the mechanical and electronic properties of 2D and 3D structures of beryllium-oxide graphene and graphene nanobud. Appl Surf Sci. 2019;476:36–48.
- Wang J, Zhou Q, Lu Z, et al. Gas sensing performances and mechanism at atomic level of Au-MoS2 microspheres. Appl Surf Sci. 2019;490:124–136.
- Ji G, Anjum S, Sundaram S, et al. Musa paradisica peel extract as green corrosion inhibitor for mild steel in HCl solution. Corros Sci. 2015;90:107–117.
- Ngobiri N, Oguzie E, Li Y, et al. Eco-friendly corrosion inhibition of pipeline steel using Brassica oleracea. Int J Corros. 2015;2015:1–9.
- El Hamdani N, Fdil R, Tourabi M, et al. Alkaloids extract of Retama monosperma (L.) Boiss. seeds used as novel eco-friendly inhibitor for carbon steel corrosion in 1 M HCl solution: electrochemical and surface studies. Appl Surf Sci. 2015;357:1294–1305.
- Gudić S, Smoljko I, Kliškić M. The effect of small addition of tin and indium on the corrosion behavior of aluminium in chloride solution. J Alloys Compd. 2010;505(1):54–63.
- Li W-h, He Q, Zhang S-T, et al. Some new triazole derivatives as inhibitors for mild steel corrosion in acidic medium. J Appl Electrochem. 2008;38(3):289–295.
- Ibrahim TH, Gomes EE, Obot IB, et al. Mild steel green inhibition by Ficus carica leaves extract under practical field conditions. J Adhes Sci Technol. 2017;31(24):2697–2718.
- Lorenz W, Mansfeld F. Determination of corrosion rates by electrochemical DC and AC methods. Corros Sci. 1981;21(9–10):647–672.
- Li X, Deng S, Fu H. Inhibition by tetradecylpyridinium bromide of the corrosion of aluminium in hydrochloric acid solution. Corros Sci. 2011;53(4):1529–1536.
- Murthy HA. Electroanalytical study on the corrosion behaviour of TiO2 particulate reinforced Al 6061 composites. Mat Sci Res India. 2015;12(2):112–126.
- Charitha B, Rao P. Carbohydrate biopolymer for corrosion control of 6061 Al-alloy and 6061Aluminum-15%(v) SiC (P) composite—green approach. Carbohydr Polym. 2017;168:337–345.
- Charitha B, Rao P. Pullulan as a potent green inhibitor for corrosion mitigation of aluminum composite: electrochemical and surface studies. Int J Biol Macromol. 2018;112:461–472.
- Shetty D, Kumari PP, Rao SA, et al. Anticorrosion behaviour of a hydrazide derivative on 6061 Al-15%(v) SiC (P) composite in acid medium: experimental and theoretical calculations. J Bio-Tribo-Corros. 2020;6:1–15.
- Pourbaix M. Atlas of electrochemical equilibria in aqueous solution. NACE. 1974:307, 648.
- Butler JAV. Studies in heterogeneous equilibria. Part II.—the kinetic interpretation of the nernst theory of electromotive force. Trans Faraday Society. 1924;19(March):729–733.
- Elgyar OA, Ouf AM, El-Hossiany A, et al. The inhibition action of viscum album extract on the corrosion of carbon steel in hydrochloric acid solution. Biointerface Res Appl Chem. 2021;11:14344–14358.
- Lahbib H, Ben Hassen S, Gerengi H, et al. Corrosion inhibition performance of dwarf palm and cynara cardunculus leaves extract for St37 steel in 15% H2SO4: a comparative study. J Adhes Sci Technol. 2021;35(7):691–722.
- Liu X, Okafor PC, Pan X, et al. Corrosion inhibition and adsorption properties of cerium-amino acid complexes on mild steel in acidic media: experimental and DFT studies. J Adhes Sci Technol. 2020;34(19):2047–2074.
- Bairy M, Pais M, Kumari PP, et al. Hydrazinecarbothioamide derivative as an effective inhibitor for corrosion control: electrochemical, surface and theoretical studies. J Bio- Tribo-Corros. 2022;8:1–15.
- Tan B, Zhang S, Cao X, et al. Insight into the anti-corrosion performance of two food flavors as eco-friendly and ultra-high performance inhibitors for copper in sulfuric acid medium. J Colloid Interface Sci. 2022;609:838–851.
- Xia G, Wan J, Zhang J, et al. Cellulose-based films prepared directly from waste newspapers via an ionic liquid. Carbohydr Polym. 2016;151:223–229.
- McCafferty E, Hackerman N. Double layer capacitance of iron and corrosion inhibition with polymethylene diamines. J Electrochem Soc. 1972;119(2):146.
- Hassan HH. Perchlorate and oxygen reduction during Zn corrosion in a neutral medium. Electrochim Acta. 2006;51(26):5966–5972.
- Verma C, Quraishi MA, Kluza K, et al. Corrosion inhibition of mild steel in 1M HCl by D-glucose derivatives of dihydropyrido [2, 3-d: 6, 5-d′] dipyrimidine-2, 4, 6, 8 (1H, 3H, 5H, 7H)-tetraone. Sci Rep. 2017;7:44432.
- Salghi R, Jodeh S, Ebenso EE, et al. Inhibition of C-steel corrosion by green tea extract in hydrochloric solution. Int J Electrochem Sci. 2017;12:3283–3295.
- Bouoidina A, El-Hajjaji F, Drissi M, et al. Towards a deeper understanding of the anticorrosive properties of hydrazine derivatives in acid medium: experimental, DFT and MD simulation assessment. Metall and Mat Trans A. 2018;49(10):5180–5191.
- Bashir S, Sharma V, Lgaz H, et al. The inhibition action of analgin on the corrosion of mild steel in acidic medium: a combined theoretical and experimental approach. J Mol Liq. 2018;263:454–462.
- Chafiq M, Chaouiki A, Al-Hadeethi MR, et al. Naproxen-based hydrazones as effective corrosion inhibitors for mild steel in 1.0 M HCl. Coatings. 2020;10(7):700.
- Aslam R, Mobin M, Shoeb M, et al. Novel ZrO2-glycine nanocomposite as eco-friendly high temperature corrosion inhibitor for mild steel in hydrochloric acid solution. Sci Rep. 2022;12(1):19.
- Bommersbach P, Alemany-Dumont C, Millet J-P, et al. Formation and behaviour study of an environment-friendly corrosion inhibitor by electrochemical methods. Electrochim Acta. 2005;51(6):1076–1084.
- Nnanna L, Owate I. Electrochemical study of corrosion inhibition of mild steel in acidic solution using gnetum africana leaves extracts. BJAST. 2015;5(6):556–567.
- Al-Amiery AA, Kadhum AAH, Alobaidy AHM, et al. Novel corrosion inhibitor for mild steel in HCl. Materials. 2014;7(2):662–672.
- Tezcan F, Yerlikaya G, Mahmood A, et al. A novel thiophene schiff base as an efficient corrosion inhibitor for mild steel in 1.0 M HCl: electrochemical and quantum chemical studies. J Mol Liq. 2018;269:398–406.
- Bentiss F, Lebrini M, Lagrenée M. Thermodynamic characterization of metal dissolution and inhibitor adsorption processes in mild steel/2, 5-bis (n-thienyl)-1, 3, 4-thiadiazoles/hydrochloric acid system. Corros Sci. 2005;47(12):2915–2931.
- Hegazy M, Zaky M. Inhibition effect of novel nonionic surfactants on the corrosion of carbon steel in acidic medium. Corros Sci. 2010;52(4):1333–1341.
- Chen Y, Chen Z, Zhuo Y. Newly synthesized morpholinyl mannich bases as corrosion inhibitors for N80 steel in acid environment. Materials. 2022;15(12):4218.
- Al-Amiery A, Salman TA, Alazawi KF, et al. Quantum chemical elucidation on corrosion inhibition efficiency of schiff base: DFT investigations supported by weight loss and SEM techniques. Int J Low Carbon Technol. 2020;15(2):202–209.
- Al-Amiery A, Shaker LM, Kadhum AAH, et al. Synthesis, characterization and gravimetric studies of novel triazole-based compound. Int J Low Carbon Technol. 2020;15(2):164–170.
- Yamin J, Ali ESE, Al-Amiery A. Statistical analysis and optimization of the corrosion inhibition efficiency of a locally made corrosion inhibitor under different operating variables using RSM. Int J Corros Scale Inhib. 2020;9:502–518.
- Singh AK, Ebenso EE, Quraishi M. Adsorption behaviour of cefapirin on mild steel in hydrochloric acid solution. Int J Electrochem Sci. 2012;7:2320–2333.
- Mobin M, Zehra S, Parveen M. L-Cysteine as corrosion inhibitor for mild steel in 1 M HCl and synergistic effect of anionic, cationic and non-ionic surfactants. J Mol Liq. 2016;216:598–607.
- Popova A, Sokolova E, Raicheva S, et al. AC and DC study of the temperature effect on mild steel corrosion in acid media in the presence of benzimidazole derivatives. Corros Sci. 2003;45(1):33–58.
- Avci G. Corrosion inhibition of indole-3-acetic acid on mild steel in 0.5 M HCl. Colloids Surf A Physicochem Eng Asp. 2008;317(1–3):730–736.
- Oguzie E, Njoku V, Enenebeaku C, et al. Effect of hexamethylpararosaniline chloride (crystal violet) on mild steel corrosion in acidic media. Corros Sci. 2008;50(12):3480–3486.
- El-Katori EE, Al-Mhyawi S. Assessment of the Bassia muricata extract as a green corrosion inhibitor for aluminum in acidic solution. Green Chem Lett Rev. 2019;12(1):31–48.
- Singh A, Ahamad I, Quraishi MA. Piper longum extract as green corrosion inhibitor for aluminium in NaOH solution. Arab J Chem. 2016;9:S1584–S1589.
- Deyab M. Corrosion inhibition of aluminum in biodiesel by ethanol extracts of Rosemary leaves. J Taiwan Inst Chem Eng. 2016;58:536–541.
- Khadraoui A, Khelifa A, Hachama K, et al. Thymus algeriensis extract as a new eco-friendly corrosion inhibitor for 2024 aluminium alloy in 1 M HCl medium. J Mol Liq. 2016;214:293–297.
- Junaedi S, Al-Amiery AA, Kadihum A, et al. Inhibition effects of a synthesized novel 4-aminoantipyrine derivative on the corrosion of mild steel in hydrochloric acid solution together with quantum chemical studies. Int J Mol Sci. 2013;14(6):11915–11928.
- Martinez S, Metikoš-Huković M. A nonlinear kinetic model introduced for the corrosion inhibitive properties of some organic inhibitors. J Appl Electrochem. 2003;33(12):1137–1142.
- Hosseini M, Mertens SF, Arshadi MR. Synergism and antagonism in mild steel corrosion inhibition by sodium dodecylbenzenesulphonate and hexamethylenetetramine. Corros Sci. 2003;45(7):1473–1489.
- Rudresh H, Mayanna S. Adsorption of n‐decylamine on zinc from acidic chloride solution. J Electrochem Soc. 1977;124(3):340–342.
- Galo GT, Morandim-Giannetti A, Cotting F, et al. Evaluation of purple onion (Allium cepa L.) extract as a natural corrosion inhibitor for carbon steel in acidic media. Met Mater Int. 2021;27(9):3238–3249.
- Singh A, Ansari KR, Quraishi MA, et al. Effect of electron donating functional groups on corrosion inhibition of J55 steel in a sweet corrosive environment: experimental, density functional theory, and molecular dynamic simulation. Materials. 2018;12(1):17.
- Eduok UM, Khaled M. Corrosion inhibition for low-carbon steel in 1 M H2SO4 solution by phenytoin: evaluation of the inhibition potency of another “anticorrosive drug. Res Chem Intermed. 2015;41(9):6309–6324.
- Deng S, Li X. Inhibition by Jasminum nudiflorum Lindl. leaves extract of the corrosion of aluminium in HCl solution. Corros Sci. 2012;64:253–262.
- Liu Y, Huang Y, Xiao Z, et al. Study of adsorption of hydrogen on Al, Cu, Mg, Ti surfaces in Al alloy melt via first principles calculation. Metals. 2017;7(1):21.
- Kokalj A. On the HSAB based estimate of charge transfer between adsorbates and metal surfaces. Chem Phys. 2012;393(1):1–12.
- Rocca M, Rahman T, Vattuone L. Springer handbook of surface science. Berlin: Springer; 2020.
- Cen H, Zhang X, Zhao L, et al. Carbon dots as effective corrosion inhibitor for 5052 aluminium alloy in 0.1 M HCl solution. Corros Sci. 2019;161:108197.
- Li S, Chen S, Lei S, et al. Investigation on some Schiff bases as HCl corrosioninhibitors for copper. Corros Sci. 1999;41(7):1273–1287.
- Punitha R, Kirupha SD, Vivek S, et al. Synthesis and corrosion inhibition studies of modified polyacrylic acid bearing triazole moieties on aluminium in alkaline medium. J Polym Res. 2019;26(12):3.
- Li X, Deng S, Xie X. Experimental and theoretical study on corrosion inhibition of oxime compounds for aluminium in HCl solution. Corros Sci. 2014;81:162–175.
- Abdallah M, Sobhi M, Altass H. Corrosion inhibition of aluminum in hydrochloric acid by pyrazinamide derivatives. J Mol Liq. 2016;223:1143–1150.
- Liu J, Wang D, Gao L, et al. Synergism between cerium nitrate and sodium dodecylbenzenesulfonate on corrosion of AA5052 aluminium alloy in 3 wt.% NaCl solution. Appl Surf Sci. 2016;389:369–377.
- Nnaji NJ, Ujam OT, Ibisi NE, et al. Morpholine and piperazine based carboxamide derivatives as corrosion inhibitors of mild steel in HCl medium. J Mol Liq. 2017;230:652–661.
- Zhao NN, Zhao YL, Hu Y, et al. Intermolecular interactions and thermodynamic properties of 3, 6-diamino-1, 2, 4, 5-tetrazine-1, 4-dioxide dimers: a density functional theoretical study. S Afr J Chem. 2013;66:167–172.
- Szyszka D. Study of contact angle of liquid on solid surface and solid on liquid surface. J Min Sci. 2012;135:131–146.
- Kubiak K, Wilson M, Mathia T, et al. Wettability versus roughness of engineering surfaces. Wear. 2011;271(3–4):523–528.
- Li X-M, Reinhoudt D, Crego-Calama M. What do we need for a superhydrophobic surface? A review on the recent progress in the preparation of superhydrophobic surfaces. Chem Soc Rev. 2007;36(8):1350–1368.
- Li W, Zhang Z, Zhai Y, et al. Electrochemical and computational studies of proline and captopril as corrosion inhibitors on carbon steel in a phase change material solution. Int J Electrochem Sci. 2020;15:722–739.
- Reddy CM, Sanketi BD, Kumar SN. Corrosion inhibition of mild steel by Capsicum annuum fruit paste. Perspect Sci. 2016;8:603–605.
- Majd MT, Ramezanzadeh M, Ramezanzadeh B, et al. Production of an environmentally stable anti-corrosion film based on esfand seed extract molecules-metal cations: integrated experimental and computer modeling approaches. J Hazard Mater. 2020;382:121029.
- Nematian B, Ramazani SA, Mahdavian M, et al. Adsorption of eco-friendly carthamus tinctorius on steel surface in saline solution: a combination of electrochemical and theoretical studies. Colloids Surf A Physicochem Eng Asp. 2020;601:125042.
- Marhamati F, Mahdavian M, Bazgir S. Corrosion mitigation of mild steel in hydrochloric acid solution using grape seed extract. Sci Rep. 2021;11(1):16.
- Alorabi AQ, Abdelbaset M, Zabin SA. Colorimetric detection of multiple metal ions using schiff base 1-(2-thiophenylimino)-4-(N-dimethyl) benzene. Chemosensors. 2019;8(1):1.
- Bahlakeh G, Dehghani A, Ramezanzadeh B, et al. Highly effective mild steel corrosion inhibition in 1 M HCl solution by novel green aqueous mustard seed extract: experimental, electronic-scale DFT and atomic-scale MC/MD explorations. J Mol Liq. 2019;293:111559.
- Bonardi A-H, Dumur F, Noirbent G, et al. Organometallic vs organic photoredox catalysts for photocuring reactions in the visible region. Beilstein J Org Chem. 2018;14:3025–3046.
- Elavarasan S, Gopalakrishnan M. Synthesis, structural analysis, theoretical studies of some lawsone derivatives. Spectrochim Acta A Mol Biomol Spectrosc. 2014;133:1–6.
- Singh DK, Luqman S, Mathur AK. Lawsonia inermis L.–a commercially important primaeval dying and medicinal plant with diverse pharmacological activity: a review. Ind Crops Prod. 2015;65:269–286.
- Verma PK, Kundu A, Puretz MS, et al. The bend + libration combination band is an intrinsic, collective, and strongly solute-dependent reporter on the hydrogen bonding network of liquid water. J Phys Chem B. 2018;122(9):2587–2599.
- Eisenberg D, Kauzmann W. The structure and properties of water. New York: OUP Oxford; 2005.
- Palencia M. Functional transformation of Fourier-transform mid-infrared spectrum for improving spectral specificity by simple algorithm based on wavelet-like functions. J Adv Res. 2018;14:53–62.
- Potdar HS, Jun K-W, Bae JW, et al. Synthesis of nano-sized porous γ-alumina powder via a precipitation/digestion route. Appl Catal A. 2007;321(2):109–116.