 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Behind armor blunt trauma (BABT) is a non-penetrating injury caused by the rapid deformation of body armor, by a projectile, which may in extreme circumstances cause death. Although there is not a high incidence of high energy BABT, the understanding of the mechanisms is still low, in relation to what is needed for safety threshold levels. BABT is also useful as a model for blunt thoracic trauma, with a compressive speed between traffic accidents and blast caused by explosives. High velocity projectile BABT causes severe hypoxia. The mechanisms are not fully known. We investigated the acute pulmonary consequences in the individual lungs, and the effects of alveolar recruitment.
Methods
12 swine (mean weight 62.5 kg) were randomized to groups BABT by 7.62 × 51 mm NATO-type bullets (mean velocity 803 m/s) to a military grade ceramic plate armor (n = 7) or control (n = 5). Modified double lumen tracheal tubes provided respiratory dynamics in the lungs separately/intermittently for two hours, with alveolar recruitment after one hour.
Results
Venous admixture increased 5 min after BABT (p < .05) and correlated with increased cardiac output. Static compliance decreased 5 minutes after BABT (p < .05) and further by recruitment (p < .005). Physiological dead space decreased 5 minutes after BABT (p < .01) and further by recruitment (p < .01), while not in the contralateral lung. V′A/Q′ decreased 5 minutes after BABT (p < .05), also shown in phase III volumetric capnography (p < .05). Most effects regressed after one hour.
Conclusions
High velocity projectile BABT caused hypoxia by a severe and transient decrease in V′A/Q′ to <1 and increased venous admixture in the exposed lung. Alveolar recruitment was hemodynamically and respiratory tolerable and increased V′A/Q′. Body armor development should aim at ameliorating severe pulmonary consequences from high projectile velocities which also needs to include further understanding of how primary and secondary effects are distributed between the lungs.
Introduction
Behind armor blunt trauma (BABT) is a non-penetrating injury caused by the rapid deformation of body armor by a projectile.Citation1 When the body armor inhibits the projectile from penetrating the thorax, shock waves propagate and kinetic energy from the ballistic impact is transferred to the chest wall and to the internal organs, causing injury through rapid acceleration and deceleration of the tissue.Citation2 BABT may lead to cardiopulmonary failure and in extreme circumstances death.Citation1,Citation3,Citation4 BABT injuries risk increasing with the escalation of the energy of bullets, more frequent use of body armor in law enforcements and international conflicts and novel low weight armor designs, which is why increased research efforts of injury mechanisms are needed to ameliorate severe pulmonary injury.Citation1,Citation3,Citation5 BABT is well described in gelatin models but the correlation with human physiology is low.Citation3,Citation6–8 We have described an immediate and severe hypoxia following high velocity projectile BABT in swine.Citation9 The mechanisms are not sufficiently elucidated. Hypoxia may result from hypoventilation, low inspired air O2 (PIO2), diffusion limitation, low ventilation-perfusion (V′A/Q′) regions or shunt.Citation10 Increased shunt was described in porcine chest trauma by a captive bolt gun.Citation11,Citation12 However, BABT injury mechanisms differ from direct thoracic trauma, and may include characteristics of primary blast injury.Citation1 It is not known how an impact of BABT type affects the V′A/Q′ relationship and if a mismatch contributes to the hypoxia. We therefore investigated the acute consequences in both lungs separately, and the effects of alveolar recruitment after high velocity projectile BABT caused by NATO-grade 7.62 × 51 mm bullets with a mean velocity of 803 m/s, impacting a military grade ceramic plate armor.Citation8,Citation9 Double lumen tracheal tubes were modified for porcine single lung ventilation and provided respiratory dynamics in the left and right lung separately and intermittently for 2 hours. Alveolar recruitment was performed after one hour. Quantitative estimates of the effect of V′A/Q′ mismatch on gas exchange was measured and computed by venous admixture and physiological dead space (wasted ventilation).
Material and methods
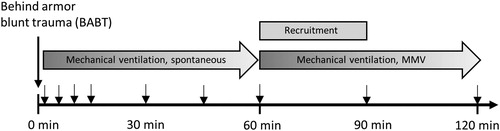
The study was approved by, and conducted in accordance with, the Swedish regional ethics approval board for animal research in Stockholm (approval no S22-13), and included five phases: preparation, behind armor blunt trauma, monitoring for 60 minutes, alveolar recruitment and monitoring for 60 minutes (). The animals were housed in an accredited animal facility for at least 5 days prior to the experiment and fed a standard diet with free access to tap water. The ambient room temperature was maintained at 21–22 °C with 12-hour light/12-hour darkness cycles. A total of 12 Swedish landrace swine (females or castrated males) with mean weight 62.5 (range 56–70) kg were anesthetized and randomized to groups “BABT”, receiving BABT (n = 7), or “control”, receiving no trauma (n = 5). At completion of the experiments, the animals were euthanized with 70 mL pentobarbital (100 mg/mL).
Figure 1. Experimental protocol and temporal overview of sampling. Arrows depict sampling times. MMV = mandatory minute ventilation mode.
Preparation
The animals were sedated with 150 mg tiletamine/zolazepam (Zoletil 100 Vet) and 6 mg medetomidine (Domitor). Anesthesia was induced with pentobarbital 6 mg/kg, atropine 0.02 mg/kg and fentanyl 2.5 µg when required. Ringer´s Acetate (500 ml/hour during the first hour and then 180 mL/hour during the rest of the experiment) was infused for hydration. A 50 mg/mL glucose solution (500 mL/hour during the first hour and then 180 mL/hour during the rest of the experiment) was infused for normoglycemia. Anesthesia was maintained with ketamine 25 mg/kg/hour and midazolam 0.042 mg/kg/hour. Tracheotomy was performed to enable mechanical ventilation, using a modified double lumen tracheal tube for monitoring of respiratory dynamics in the left and right lung separately, during temporary one lung ventilation. Commercially available double lumen tracheal tubes are not suitable for use in swine without modification. In swine, a tracheal bronchus (right cranial lobe bronchiole) originates from the right side of the trachea. In the left lung the cranial lobe bronchioles are usually absent. Short left main bronchus branches out into several bronchioles forming the bi-lobed middle and caudal lobes.Citation13 The ventilator (Hamilton C2, Hamilton Medical) was adjusted to volume-control mode for normoventilation. During the first 60 minutes the mode was spontaneous triggering of breaths, to allow for monitoring of the pulmonary physiological response. To allow for alveolar recruitment, the mode was changed to mandatory minute ventilation for the consecutive 60–120 minutes. Settings were FiO2 21%, PEEP 6 cm H2O, tidal volume 6–8 mL/kg and inspiratory/expiratory ratio 1:2. An optical pulmonary thermodilution catheter, 7.5 F/110 cm (Edwards Lifescience) was inserted in the right internal jugular vein by cut-down technique and measured mean pulmonary artery pressure (MPAP), cardiac output (CO) and mixed venous saturation (SvO2) with a Vigilance II-monitor (Edwards Lifescience). The left carotid artery was cannulated with a polyethylene catheter (Portex Ltd) through a cervical cut-down for measurements of mean arterial pressure (MAP). A BIOPAC monitoring system (MP150, BIOPAC systems) recorded physiological parameters. Arterial blood samples were analyzed for arterial hemoglobin O2 saturation (SaO2), arterial O2 pressure (PaO2), arterial CO2 pressure (PaCO2) using a blood gas analyzer (GEM 3000, Instrumentation Laboratories). After the preparation, animals were not manipulated for 30 minutes to enable for physiological steady state. Data were registered at baseline prior to BABT, and at 1, 5, 10, 15, 30, 45, 60, 90 and 120 minutes after BABT. At each time, the airflow in the left and then the right lumen of the tracheal tube was occluded by surgical forceps, enabling measurements for each lung separately.
BABT
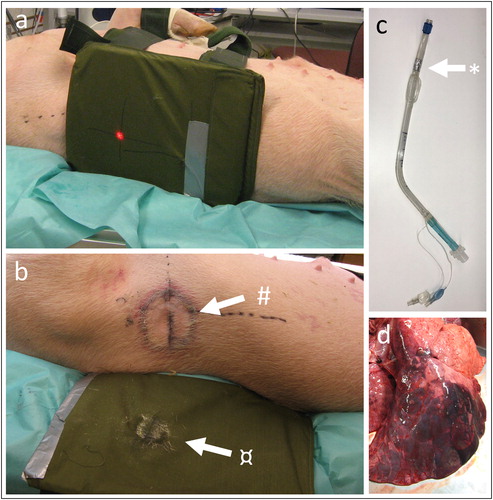
For animals randomized to BABT, a focally severe pulmonary contusion was induced, as previously describedCitation8,Citation9 by a gunshot perpendicular to a body armor panel fixed on the mid-lateral right thorax with two 3-cm wide girdles. The armor protection consisted of a 300 × 255 mm ceramic ballistic plate (weight 3 Kg) and 14 additional 0.24 mm aramid layers placed behind the plate (Åkers Krutbruk Protection Inc.) (). A Swedish Armed Forces AK4 assault rifle, equipped with a laser aiming device (Diode laser type S1889, Melles Griot), was fixed to a gun-carriage and placed 10 m from the ballistic plate. 7.62 mm × 51 mm NATO-type bullets (M/94, Ammunitions-Arsenalet) with a mean bullet velocity 803 (range 800–806) m/s, measured by an optical shutter device (Chronograph Beta model, Shooting Chrony, Inc.) were fired. The target was costa 8 and firing was synchronized with the end of inspiration. Airway suctioning was started after three minutes and was performed when required. After the shot, the animals were reconnected to the ventilator with a positive end-expiratory pressure of 6 cm H2O and mandatory minute ventilation mode.
Figure 2. (a) Photo of ceramic armor plate placement. Red dot marks point of projectile impact. (b) Photo of imprint of the skin after BABT (#) and imprint in the ceramic plate (¤). (c) Photo of double lumen tracheal tube with porcine modification (*). (d) Photo of the right lung displaying contusion after BABT (dark area).
Alveolar recruitment
Alveolar recruitment was performed at 60 minutes after BABT by increasing PEEP gradually to 15 cm H2O and decreasing the respiratory rate from 20 to 10 breaths per minute. Pressure control was increased gradually from 5 to 12 cm H2O. After 30 minutes, pressure support was decreased until tidal volumes were 6–8 ml/kg and the respiratory rate was adjusted to achieve normocapnia (PaCO2 4–7 kPa).
Physiologic calculations
Venous admixture (Qs/Qt) was calculated by the shunt equation (Berggren equation):Citation14
where Qs/Qt = shunt fraction (shunt flow divided by total cardiac output), CcO2 = pulmonary end capillary O2 content, same as alveolar O2 content = SaO2 × Hb × 1.3. SaO2 is assumed at 100% in the lungs. CaO2 = arterial O2 content (SaO2 × Hb × 1.3), CvO2 = mixed venous O2 content (SvO2 × Hb × 1.3). V′A/Q′ = alveolar minute ventilation/cardiac output. Units: CcO2/CaO2/CvO2 = mL/L, Hb = g/L, SaO2 = %.
Ventilator calculations
Ventilation parameters were obtained and computed by a Hamilton C2 ventilator. Physiological dead space, alveolar ventilation and CO2 slope, indicative of V′A/Q′ mismatch were computed by volumetric capnography, providing a noninvasive and continuous display of the fractional concentration or partial pressure of expired CO2 versus exhaled volume. A volumetric capnogram is divided into three phases. Phase I is the first gas exhaled that comes from the conducting airways and contains no CO2. Phase II represents gas exhaled from conducting airways mixed with gas from fast-emptying alveoli. Phase III represents gas exhaled from the alveoli, and the slope represents the changing time constant of the emptying alveoli; i.e., alveoli with a low V′A/Q′ ratio empty last and contain the highest amount of CO2.Citation15 The physiological dead space calculation is derived from phase III.Citation16 Physiological dead space from volumetric capnography corresponds to the Bohr equation.Citation17 Alveolar ventilation = minute ventilation – physiological dead space.
Statistical analyses
Statistical analyses were done using GraphPad Prism version 8.4.1 (GraphPad Software). Baseline values are the first values at time 0 hours. The primary outcome was venous admixture (Qs/Qt). All temporal data sets were analyzed with mixed effects model, fixed effects (type III). Time 0 − 60 minutes were compared (observation phase), and 60 − 120 min were compared (alveolar recruitment phase). Qs/Qt to CO were fitted with linear regression. Plotted bars display the standard error of the mean. p < 0.05 was considered significant. A post-hoc power analysis for continuous endpoint, two independent sample study resulted in 88.5% power for venous admixture (Qs/Qt) (group 1: 12.73% SE 3.8, group 2: 37.33%, SE 17.1, alpha 0.05).
Results
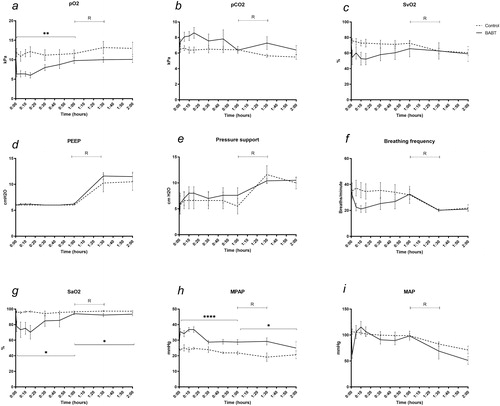
describes general lung- and circulation parameters. pO2 decreased in BABT compared to controls during the initial 60 minutes (p < .01). No difference was detected after recruitment (). pCO2 increased in BABT compared to controls but did not reach statistical significance (). SvO2 decreased in BABT compared to controls during the initial 60 minutes but did not reach statistical significance (). PEEP was kept at 6 cm H2O during the first 60 minutes and increased to a maximum 11.6 cm H20 at recruitment (). Pressure support was kept at a mean 6.6 cm H2O during the first 60 minutes and increased to a maximum 11.6 cm H2O during recruitment (). Breathing frequency was triggered spontaneously during the first 60 minutes and mandatory during the recruitment phase and was between 20–40 breaths per minute during the spontaneous phase. A decrease was detected in BABT although not reaching statistical significance (). SaO2 decreased in BABT compared to controls during the first 60 minutes (p < .05), and during the recruitment phase (p < .05) (). Mean pulmonary arterial pressure (MPAP) increased in BABT compared to controls during the first 60 minutes (p < .001), and during the recruitment phase (p < .05) (). MAP decreased in BABT during the first 5 minutes but did not reach statistical significance when comparing the first 60 minutes, or at recruitment ().
Figure 3. (a–c) BABT causes hypoxia, hypercapnia and a decrease in SvO2. (d–e) PEEP and pressure support were kept constant during the 60 minutes observation phase and increased during the 60 min alveolar recruitment phase. (f) Breathing frequency decreased after BABT during the first 60 minutes. (g) SaO2 decreased after BABT and during alveolar recruitment. (h) MPAP increased because of hypoxic pulmonary vasoconstriction after BABT and during alveolar recruitment. (i) MAP decreased 5 minutes after BABT. R means alveolar recruitment. *p < .05, **p < .01, ****p < .001.
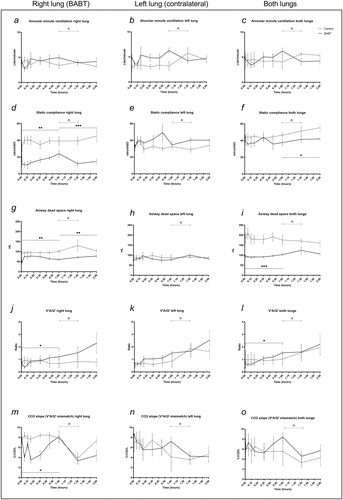
describes the results from one lumen ventilation. Alveolar minute ventilation did not differ between groups after BABT or at recruitment (). Static compliance increased the right lung in BABT compared to controls during the initial 60 minutes (p < .01) and during the recruitment phase (p < .005) (). No difference was seen in the left lung (). A decrease was detected in BABT compared to controls after recruitment in both lungs combined (p < .05) (). Physiological dead space decreased the right lung in BABT compared to controls during the initial 60 minutes (p < .01) and during the recruitment phase (p < .01) (). No difference was seen in the left lung (). A decrease was detected in BABT compared to controls during the first 60 minutes in both lungs combined (p < .005) (). V′A/Q′ decreased the right lung in BABT compared to controls during the initial 60 minutes (p < .05). No difference was detected in the recruitment phase (). No difference was seen in the left lung (). A decrease was detected in BABT compared to controls during the first 60 minutes in both lungs combined (p < .05) (). The CO2 slope (V′A/Q′ mismatch) decreased the right lung in BABT compared to controls during the initial 60 minutes (p < .05) (). No difference was seen in the left lung () or both lungs combined ().
Figure 4. Individual dynamic airway measurements in the lungs. (a–c) Alveolar minute ventilation remained stable between groups. (d–f) Static compliance decreased after BABT in the right lung which was compensated by an increase in the left lung. (g–i) Physiological dead space decreased in the right lung after BABT while the left lung was unchanged, and both lungs combined displayed a net decrease in dead space after BABT. (j–l) V′A/Q′ decreased in the right lung and both lungs combined following BABT while not in the left lung. (m–o) CO2 slope, indicative of V′A/Q′ mismatch, decreased in the right lung following BABT while the left lung and both lungs combined remained unchanged. R means alveolar recruitment. *p < .05, **p < .01, ***p < .005.
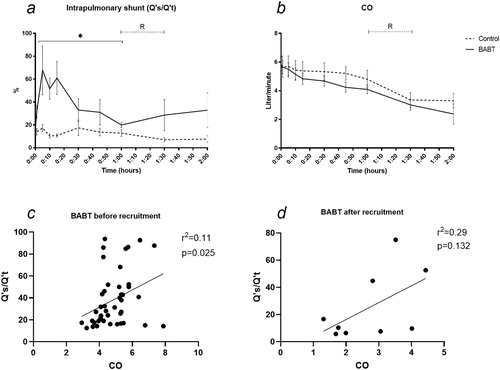
Venous admixture increased in BABT compared to controls (mean 67.7% SD 56.2 in exposed at 5 min, mean 17.3% SD 7.0 in control at 5 min) (p < .05). No difference was detected after recruitment (). MAP did not differ between groups after BABT or at recruitment (). Qs/Qt correlated with CO with a slope 7.88 and R2 0.11 before recruitment (p < .05). Qs/Qt did not correlate significantly with CO with a slope 12.42 and R2 0.29 after recruitment ().
Figure 5. (a) Venous admixture (Qs/Qt) increased from a mean17.3% to 67.7% at 5 minutes after BABT. No difference was detected after alveolar recruitment.(b) CO decreased during the 120 min observation and was not affected by alveolar recruitment, allowing for comparison with venous admixture. (c–d) Qs/Qt correlated with CO with a slope 7.88 and R2 0.11 before recruitment (p < .05). Qs/Qt related to CO with a slope 12.42 and R2 0.29 after recruitment, not showing statistical significance. R means alveolar recruitment. *p < .05.
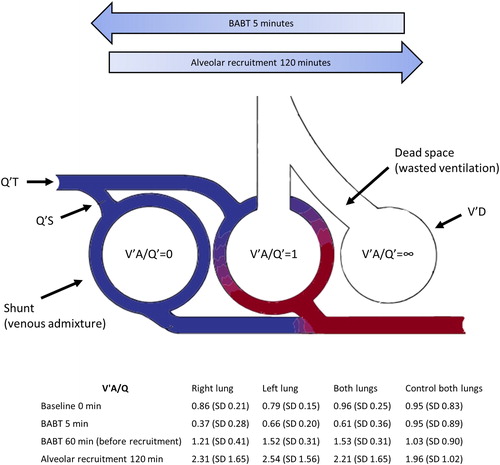
illustrates the three-compartment model proposed by Riley et al.Citation18 and adapted from Petersson and Glenny,Citation10 to quantify VA/Q mismatch as venous admixture and wasted ventilation.
Figure 6. High velocity BABT caused a severe and transient decrease in V′A/Q′ to <1 and shunt formation in the exposed lung. Illustration of three-compartment model proposed by Riley et al.Citation18 and adapted from Petersson and Glenny,Citation10 to quantify VA/Q mismatch as venous admixture and wasted ventilation. Q′T = total pulmonary capillary blood flow, Q′S = total blood flow through shunt, V′D = physiological dead space ventilation.
Discussion
In this study we show that high velocity impact on the thorax, caused by a high energy BABT, caused hypoxia by a severe decrease in V′A/Q′ to <1 and increased venous admixture in the exposed lung while the contralateral lung remained largely unaffected.
Mechanisms related to immediate and severe hypoxia after BABT were determined by dynamic respiratory changes in the exposed (right) and the contralateral (left) lung. FiO2 was kept at 21% and alveolar minute ventilation was equal in exposed animals and controls. Alveolar oxygen pressure (PAO2) was therefore assumed to equal PaO2. The alveolar gas equation calculates PAO2 from PIO2, PaCO2, and FiO2 with a constant R for the respiratory exchange ratio, defined as the ratio between the amount of CO2 and O2 exchange. R was not assumed at a specific level (normally 0.8) due to the heterogenous BABT-injury.Citation19 SaO2 was assumed at 100% in the lungs and CcO2 was assumed to equal alveolar O2 content.
The effect of V′A/Q′ mismatch on gas exchange can be illustrated as lung consisting of three compartments without different V′A/Q′ ratios: one compartment with ideal V′A/Q′ matching (V′A/Q′=1.0), one totally without ventilation (V′A/Q′ = 0, shunt) and one without blood flow (V′A/Q′ = ∞, physiological dead space).Citation18 Using this illustration, BABT caused a left shift in the first 5 minutes to a V′A/Q′ < 1, which was spontaneously reversed to V′A/Q′ >1 after 60 minutes. Alveolar recruitment further right-shifted V′A/Q′ after 120 minutes. The three-compartment model is convenient but assume that the effects of V′A/Q′ mismatch on PaO2 and PaCO2 are entirely due to shunt and physiological dead space ventilation, and that all gas exchange occurs in units with ideal V′A/Q′ matching. This is a limitation, as it does not reflect the true situation as it ignores gas exchange in units with other V′A/Q′ ratios, which may be assessed with multiple inert gas elimination technique.Citation20 Also, the calculated shunt corresponds to the amount of shunt of mixed venous blood that would result in the observed arterial oxygenation in the absence of low V′A/Q′ regions. Therefore, venous admixture may be increased even in the absence of true shunt.
Venous admixture (Qs/Qt) increased after BABT in the first 5 minutes. The amount of venous admixture is quantified as the fraction of cardiac output distributed to nonventilated units. The venous admixture correlated with cardiac output after BABT, similarly to shunt fraction correlating with increasing cardiac output in healthy lungs.Citation21 Interestingly, this relation between venous admixture and cardiac output was detected only before alveolar recruitment.
Compliance decreased in the exposed lung. A compensation occurred in the contralateral lung, which is why both lungs together did not display differences between BABT and controls before recruitment. Physiological dead space decreased in the exposed lung specifically, while the contralateral lung remained largely unaffected. Gas exchanging units, with little or no blood flow (high V′A/Q′ regions), result in physiological dead space and increased waisted ventilation, i.e. less efficient CO2 removal. Because of the respiratory drive to maintain a normal arterial pCO2, the most frequent result of waisted ventilation is increased minute ventilation and work of breathing, not hypercapnia.Citation10 We detected hypercapnia and decreased breathing frequency, which were likely related to increased venous admixture.
Alveolar recruitment was performed after 60 minutes to determine if the procedure was safe and could ameliorate a ventilation-perfusion mismatch. Alveolar recruitment did not alter lung compliance, ventilation (pCO2), oxygenation (pO2/SaO2) or venous admixture (Qs/Qt). Physiological dead space was not affected. Respiratory compliance, but not gas exchange, correlates with changes in lung aeration after alveolar recruitment in porcine saline lavage lung injury.Citation22 Monitoring of physiological dead space is useful for detecting lung collapse and for establishing open-lung PEEP after alveolar recruitment.Citation23 The spontaneous regression of V′A/Q′ mismatch after 60 minutes and before alveolar recruitment likely decreased the effects. Alveolar recruitment may result in severe hypotension.Citation24 We show that alveolar recruitment was hemodynamically tolerable without vasopressor support after BABT. It is possible that alveolar recruitment is more beneficial in the delayed phase. ARDS is characterized by severe hypoxemia due to shunt that might exceed 50%.Citation25 PEEP reduces shuntCitation10 but recruitment in ARDS is disputed.Citation26 Future studies should therefore investigate if alveolar recruitment is beneficial in the delayed phase of lung trauma.
MPAP increased by 36%, 5 minutes after BABT (34.2 vs 25.1 mm Hg). Hypoxia causes pre-capillary pulmonary vasoconstriction in units with low PAO2 (<8 kPa) such as those with low V′A/Q′ or shunt. The effect is to divert blood flow away from hypoxic units, improving V′A/Q′ matching and arterial oxygenation.Citation27 It is therefore likely that MPAP increased secondary to a locally severe hypoxia in the lung. Hypoxic vasoconstriction is of little importance for V′A/Q′ matching in normal lungs, but can be of critical importance in disease causing regional alveolar hypoxia.Citation27,Citation28
Some limitations need to be discussed. First, for methodological reasons FiO2 was kept at a constant 21%, and alveolar recruitment was prioritized as intervention instead of increasing FiO2. The rationale was that hypoxia related to increased venous admixture may improve with alveolar recruitment but responds poorly to oxygen.Citation10 O2 could also be used as an indication of lung function, because hypoventilation, while keeping FiO2 at 21% will result in hypoxemia before PaCO2 increases to high levels.Citation10 An intervention by increasing FiO2 to 100% may instead differentiate hypoxia due to low V′A/Q′ regions from the effect of a true shunt, because the venous admixture from low V′A/Q′ regions is abolished in this situation.Citation10 However, FiO2 of 100% may also increase shunt due to absorption atelectasis so the degree of shunt while breathing a lower FiO2 might be overestimated.Citation10 FiO2 of 100% may also raise the PAO2 in low V′A/Q′ units, inhibiting regional hypoxic pulmonary vasoconstriction and increasing blood flow to these units, causing an increase in PaCO2 despite constant minute ventilation. Blood is diverted away from better ventilated regions, converting them to high V′A/Q′ units, which increases wasted ventilationCitation29 and could therefore interfere with the study. To clarify these aspects, future studies should investigate FiO2 increases to 100% in relation to BABT.
Second, a primary diffusion limitation as cause for hypoxia was not calculated. A diffusion limitation can occur in interstitial lung diseases by impaired equilibration between PAO2 and expired CO2 tension (PeCO2), creating increased PA–aO2 and may cause hypoxemia even in the absence of V′A/Q′ mismatch.Citation10 A primary diffusion limitation was however not suspected after BABT. Diffusion limitation, as measured by carbon mon-oxide rebreathing, did not contribute significantly to hypoxemia in patients with flail chest, and did not contribute to hypoxia after bolt-gun induced pulmonary contusion.Citation12 Also, the animals had healthy lungs prior to BABT, and had normal cardiac output and alveolar minute ventilation. At very high cardiac outputs, the transit time might be too short for complete equilibration between PAO2 and PeCO2.Citation30
Third, the observation time was limited to two hours. The study was designed to determine acute lung function impairments that may cause immediate respiratory failure, which is an important aspect of treatment strategies to prevent death in prehospital or tactical environments and respiratory mechanics are affected early after pulmonary contusion.Citation31 Future studies should include longer observation times to investigate delayed effects of BABT trauma, which may be characterized by lung hemorrhage, alveolar collapse, pulmonary edema and an inflammatory response.Citation31–33
Fourth, the similar size and anatomy of pig and human organs make this model particularly beneficial for translational research. For the respiratory medicine field, the similarities between pig and human lungs give the porcine model potential for advancing translational medicine. However, interspecies differences in lung function, such as decreased pulmonary compliance in swine, may limit extrapolation to humans with BABT.Citation34,Citation35
We report severe changes in V′A/Q′ matching after high-energy BABT, with unique features compared to experimental pulmonary contusion with lower impact energy. The V′A/Q′ mismatch was faster and more severe than earlier described and may cause acute respiratory distress. The mismatch peaked at 5 minutes with 60% venous admixture, compared to a 35% shunt peaking at 30 minutes after a similar, but lower energy, experimental pulmonary contusion.Citation11 It is likely that the energy transfer from a projectile to the thorax is speed and kinetic energy dependent, and that BABT-specific models are required for adequate assessments of injuries related to protective equipment. Body armors validated for handgun- and grenade fragment protection are still used, where high velocity weapons are a realistic threat, and few reports include data of energy levels equal to- or higher than 7.62 mm rifle bullets.Citation3 Light body armors may not provide enough protection from the kinetic energy transferred from a high energy projectile and the development and increased use of light-weight body armors may increase injuries.Citation4 Body armor designs and injury assessments should therefore include relevant BABT-models to encompass the severe injury spectrum from high velocity projectile BABT, which also needs to include further understanding of how primary and secondary effects are distributed between the lungs.
Conclusions
High velocity projectile BABT had unique features compared to experimental pulmonary contusion, causing hypoxia by a severe and transient decrease in V′A/Q′ to <1 and increased venous admixture in the exposed lung which may induce acute respiratory distress. Alveolar recruitment was hemodynamically and respiratory tolerable and increased V′A/Q′. Body armor innovation may aim at ameliorating severe pulmonary consequences from high projectile velocities which also needs to include further understanding of how primary and secondary effects are distributed between the lungs.
Acknowledgements
We would like to thank Lars-Gunnar Olsson for excellent technical assistance.
Additional information
Funding
References
- Cannon L. Behind armour blunt trauma—an emerging problem. J R Army Med Corps. 2001;147(1):87–96. doi:10.1136/jramc-147-01-09.
- Liden E, Berlin R, Janzon B, Schantz B, Seeman T. Some observations relating to behind-body armour blunt trauma effects caused by ballistic impact. J Trauma. 1988;28(1 Suppl):S145–S8.
- Carr DJ, Horsfall I, Malbon C. Is behind armour blunt trauma a real threat to users of body armour? A systematic review. J R Army Med Corps. 2016;162(1):8–11. doi:10.1136/jramc-2013-000161.
- Gryth D, Rocksen D, Persson JK, et al. Severe lung contusion and death after high-velocity behind-armor blunt trauma: relation to protection level. Mil Med. 2007;172(10):1110–1116. doi:10.7205/milmed.172.10.1110.
- Wilhelm M, Bir C. Injuries to law enforcement officers: the backface signature injury. Forensic Sci Int. 2008;174(1):6–11. doi:10.1016/j.forsciint.2007.02.028.
- Luo S, Xu C, Wang S, Wen Y. Transient pressure wave in the behind armor blunt trauma: experimental and computational investigation. Comput Methods Biomech Biomed Eng. 2017;20(3):308–318. doi:10.1080/10255842.2016.1228908.
- Wen Y, Xu C, Wang S, Batra RC. Analysis of behind the armor ballistic trauma. J Mech Behav Biomed Mater. 2015;45:11–21. doi:10.1016/j.jmbbm.2015.01.010.
- Rocksen D, Gryth D, Druid H, Gustavsson J, Arborelius UP. Pathophysiological effects and changes in potassium, ionised calcium, glucose and haemoglobin early after severe blunt chest trauma. Injury. 2012;43(5):632–637. doi:10.1016/j.injury.2010.10.002.
- Gunther M, Sonden A, Gustavsson J, Arborelius UP, Rocksen D. Feasibility of pleural and perilesional subcutaneous microdialysis to assess porcine experimental pulmonary contusion. Exp Lung Res. 2020;46(5):117-127. doi: 10.1080/01902148.2020.1742252
- Petersson J, Glenny RW. Gas exchange and ventilation-perfusion relationships in the lung. Eur Respir J. 2014;44(4):1023–1041. England: (c)ERS 2014. doi:10.1183/09031936.00037014.
- Batchinsky AI, Jordan BS, Necsoiu C, Dubick MA, Cancio LC. Dynamic changes in shunt and ventilation-perfusion mismatch following experimental pulmonary contusion. Shock. 2010;33(4):419–425.
- Batchinsky AI, Weiss WB, Jordan BS, Dick EJ, Jr., Cancelada DA, Cancio LC. Ventilation-perfusion relationships following experimental pulmonary contusion. J Appl Physiol. 2007;103(3):895–902. doi:10.1152/japplphysiol.00563.2006.
- Nakakuki S. Bronchial tree, lobular division and blood vessels of the pig lung. J Vet Med Sci. 1994;56(4):685–689. doi:10.1292/jvms.56.685.
- Berggren S. The oxygen deficit of arterial blood caused by non-ventilating parts of the lung. Acta Physiol Scand. 1942;11(4).
- Kreit J. W. Volume capnography in the intensive care unit: physiological principles, measurements, and calculations. Ann Am Thorac Soc. 2019;16(3):291–300.
- Astrom E, Niklason L, Drefeldt B, Bajc M, Jonson B. Partitioning of dead space—a method and reference values in the awake human. Eur Respir J. 2000;16(4):659–664. doi:10.1034/j.1399-3003.2000.16d16.x.
- Tusman G, Sipmann FS, Borges JB, Hedenstierna G, Bohm SH. Validation of Bohr dead space measured by volumetric capnography. Intensive Care Med. 2011;37(5):870–874. doi:10.1007/s00134-011-2164-x.
- Riley RL, Cournand A. Ideal alveolar air and the analysis of ventilation-perfusion relationships in the lungs. J Appl Physiol. 1949;1(12):825–847. doi:10.1152/jappl.1949.1.12.825.
- Sharma S, Hashmi MF, Burns B. Alveolar Gas Equation. StatPearls. Treasure Island (FL): StatPearls Publishing; 2020.
- Wagner PD. The multiple inert gas elimination technique (MIGET). Intensive Care Med. 2008;34(6):994–1001. doi:10.1007/s00134-008-1108-6.
- Takala J. Hypoxemia due to increased venous admixture: influence of cardiac output on oxygenation. Intensive Care Med. 2007;33(5):908–911. doi:10.1007/s00134-007-0546-x.
- Henzler D, Pelosi P, Dembinski R, et al. Respiratory compliance but not gas exchange correlates with changes in lung aeration after a recruitment maneuver: an experimental study in pigs with saline lavage lung injury. Crit Care. 2005;9(5):R471–82. doi:10.1186/cc3772.
- Tusman G, Suarez-Sipmann F, Bohm SH, et al. Monitoring dead space during recruitment and PEEP titration in an experimental model. Intensive Care Med. 2006;32(11):1863–1871. doi:10.1007/s00134-006-0371-7.
- Lovas A, Szakmany T. Haemodynamic effects of lung recruitment manoeuvres. Biomed Res Int. 2015;2015:478970. doi:10.1155/2015/478970.
- Dakin J, Jones AT, Hansell DM, Hoffman EA, Evans TW. Changes in lung composition and regional perfusion and tissue distribution in patients with ARDS. Respirology. 2011;16(8):1265–1272. doi:10.1111/j.1440-1843.2011.02048.x.
- van der Zee P, Gommers D. Recruitment maneuvers and higher PEEP, the so-called open lung concept, in patients with ARDS. Crit Care. 2019;23(1):73. doi:10.1186/s13054-019-2365-1.
- Sylvester JT, Shimoda LA, Aaronson PI, Ward JP. Hypoxic pulmonary vasoconstriction. Physiol Rev. 2012;92(1):367–520. doi:10.1152/physrev.00041.2010.
- Arai TJ, Henderson AC, Dubowitz DJ, et al. Hypoxic pulmonary vasoconstriction does not contribute to pulmonary blood flow heterogeneity in normoxia in normal supine humans. J Appl Physiol. 2009;106(4):1057–1064. doi:10.1152/japplphysiol.90759.2008.
- Sassoon CS, Hassell KT, Mahutte CK. Hyperoxic-induced hypercapnia in stable chronic obstructive pulmonary disease. Am Rev Respir Dis. 1987;135(4):907–911. doi:10.1164/arrd.1987.135.4.907.
- Hopkins SR. Exercise induced arterial hypoxemia: the role of ventilation-perfusion inequality and pulmonary diffusion limitation. Adv Exp Med Biol. 2006;588:17–30.
- Raghavendran K, Notter RH, Davidson BA, Helinski JD, Kunkel SL, Knight PR. Lung contusion: inflammatory mechanisms and interaction with other injuries. Shock. 2009;32(2):122–130. doi:10.1097/SHK.0b013e31819c385c.
- Simon B, Ebert J, Bokhari F, et al.; Eastern Association for the Surgery of Trauma. Management of pulmonary contusion and flail chest: an Eastern Association for the Surgery of Trauma practice management guideline. J Trauma Acute Care Surg. 2012;73(5 Suppl 4):S351–S61. doi:10.1097/TA.0b013e31827019fd.
- Ware LB. Pathophysiology of acute lung injury and the acute respiratory distress syndrome. Semin Respir Crit Care Med. 2006;27(4):337–349. doi:10.1055/s-2006-948288.
- Woolcock AJ, Macklem PT. Mechanical factors influencing collateral ventilation in human, dog, and pig lungs. J Appl Physiol. 1971;30(1):99–115. doi:10.1152/jappl.1971.30.1.99.
- Judge EP, Hughes JML, Egan JJ, Maguire M, Molloy EL, O’Dea S. Anatomy and bronchoscopy of the porcine lung. Am J Respir Cell Mol Biol. 2014;51(3):334–343. doi:10.1165/rcmb.2013-0453TR.
