Abstract
Background: Lung ischemia–reperfusion injury (LIRI) is among the complications observed after lung transplantation and is associated with morbidity and mortality. Preconditioning of the donor lung before organ retrieval may improve organ quality after transplantation. We investigated whether preconditioning with metformin (Met) ameliorates LIRI after lung transplantation. Methods: Twenty Lewis rats were randomly divided into the sham, LIRI, and Met groups. The rats in the LIRI and Met groups received saline and Met, respectively, via oral gavage. Subsequently, a donor lung was harvested and kept in cold storage for 8 h. The LIRI and Met groups then underwent left lung transplantation. After 2 h of reperfusion, serum and transplanted lung tissues were examined. Results: The partial pressure of oxygen (PaO2) was greater in the Met group than in the LIRI group. In the Met group, wet-to-dry (W/D) weight ratios, inflammatory factor levels, oxidative stress levels and apoptosis levels were notably decreased. Conclusions: Met protects against ischemia–reperfusion injury after lung transplantation in rats, and its therapeutic effect is associated with its anti-inflammatory, antioxidative, and antiapoptotic properties.
Introduction
Lung transplantation is the ultimate treatment for many kinds of end-stage lung diseases, such as chronic obstructive pulmonary disease, cystic fibrosis, idiopathic pulmonary fibrosis, bronchiectasis, and primary pulmonary hypertension.Citation1 However, ischemia and subsequent reperfusion are inevitable during lung transplantation. Ischemia can lead to a series of hypoxia events, which in turn leads to different degrees of cell damage and the release of toxic substances, and reperfusion can aggravate these changes. Therefore, ischemia–reperfusion can lead to severe lung inflammation, oxidative stress, impaired alveolar capillary permeability and apoptosis. Lung ischemia–reperfusion injury (LIRI), characterized by alveolar injury, pulmonary edema and hypoxemia, is the main cause of graft dysfunction (PGD) after surgery, and it is also the main cause of postoperative mortality and morbidity.Citation2,Citation3 Moreover, LIRI is associated with an increased risk of dysfunction in chronic lung allografts.Citation4,Citation5 Therefore, alleviating LIRI is highly important for improving the survival rate after lung transplantation.
Metformin(Met) is the first-line medication for the treatment of type 2 diabetes. Metformin activates AMP-activated protein kinase (AMPK) by directly or indirectly activating the AMP pathway, which has many downstream effects on the regulation of metabolic proteins, autophagy-related proteins, mitochondrial biogenesis-related proteins and transcription factors, including the inhibition of inflammatory reactions, oxidative stress, autophagy and apoptosis.Citation6 Met can decrease the phosphorylation of NF-κB and the expression of proinflammatory cytokines [TNF-α and IL-6] by increasing AMPK activity. Moreover, Met can directly reduce the level of apoptosis by reducing the ratio of apoptotic genes (Bax/Bcl-2) and inhibiting the activation of caspase3 and caspase12. Studies have shown that Met can inhibit mTOR by activating the AMPK signaling pathway and exert a protective effect against endotoxin-induced acute lung injury (ALI).Citation7 Similarly, in lipopolysaccharide-induced ALI, Met reduces lung tissue damage by promoting the expression of AMPKα1 in lung tissue and reducing the release of inflammatory cytokines.Citation8 In addition, Met has been suggested to mitigate ischemia reperfusion injury in multiple organs, such as the heart, brain, liver, kidney, and viscera.Citation9,Citation10 Therefore, we speculate that metformin may have a protective effect against LIRI. The purpose of this study was to investigate whether Met can alleviate ischemia-reperfusion injury in a rat lung transplantation model.
Materials and methods
Reagents and antibodies
Metformin (purity >97%), which was purchased from Sigma–Aldrich (St. Louis, MO, USA), was dissolved in sterile saline and administered orally at a dose of 300 mg/kg. The primary antibodies used were anti-β-actin (ab227387; Abcam), anti-NF-κB (GB11142; Servicebio), anti-Bax (50599-2-Ig; Proteintech), anti-Bcl-2 (60178-1-Ig; Proteintech), and anti-cleaved caspase-3 (9661; Cell Signaling Technology) antibodies. All the antibodies were produced in rabbits. Reactive oxygen malondialdehyde (MDA) and total superoxide dismutase (SOD) detection kits were purchased from Aidisheng (JS, CHN), and a myeloperoxidase (MPO) detection kit was purchased from Jiancheng (Nanjing, JS, CHN). ELISA kits for tumor necrosis factor-a (TNF-α), interleukin-1β (IL-1β), interleukin-6 (IL-6) and monocyte chemotactic protein 1 (MCP-1) were purchased from Jiangsu Meimian industrial (JS, CHN).
Animals
Male Lewis rats (270–300 g) were purchased from Beijing Vitonglihua Experimental Animal Technology Co., Ltd. (Beijing, China); they were housed in the specific pathogen-free (SPF) facility of Wuxi People’s Hospital Affiliated with Nanjing Medical University (Wuxi, China) at room temperature (22 ± 1 °C) on a 12/12 h light/dark cycle and provided access to food and water ad libitum.
The rats were randomly divided into the sham group (n = 4), LIRI group (n = 8), and Met group (n = 8). In the Met group, the donor rats received Met intragastrically (300 mg/kg body weight) at a fixed time every morning for 7 days. In the other groups, the donor rats were given the same volume of saline at a fixed time for 7 days. The pretreatment dose of metformin was 300 mg/kg body weight, as treatment at this dose results in sufficient serum levels of the drug.Citation11
Donor graft preparation and lung transplantation
Rats in the sham group underwent anesthesia and thoracotomy, while rats in the LIRI and Met groups underwent orthotopic left lung transplantation.
The donor rats were anesthetized with 5% isoflurane, intubated, and then mechanically ventilated (flexiVent, SCIREQ, Montreal, CA). The respiratory parameters were a tidal volume of 10 ml/kg, a respiratory rate of 70 breaths/min, and a PEEP of 2 cm H2O. General anesthesia during the surgical procedures was maintained with isoflurane (2%). After median laparotomy, 50 IU of heparin was injected into the inferior vena cava. Then, median thoracotomy was performed, and the pulmonary artery was cannulated for anterograde perfusion with 20 mL of low-potassium dextran (LPD) solution (Perfadex, Vitrolife, China) at 4 °C under constant pressure (20 cm H2O). Before perfusion, the inferior vena cava was sectioned to decrease venous return, and the left atrial appendage was amputated to drain the LPD. After perfusion, the trachea was clamped to maintain the lungs in an inflated state. The lungs were then stored at 4 °C for 8 h until transplantation.
Just before each recipient procedure, the donor lungs were prepared by placing three cuffs into the left pulmonary artery, left atrium, and left main bronchus. Recipient rats were anesthetized and ventilated with the same procedure used for the donor rats. A left thoracotomy was performed. The cuffs in the donor lung were placed into the corresponding structures in the recipient through ventral incisions, and the organs were anastomosed with 7-0 polypropylene ties. Subsequently, the native lung was removed (via left pneumonectomy). The lungs were then reinflated and ventilated with 100% oxygen. The tidal volume of the recipient rats was maintained at 6 ml/kg during lung transplantation period and returned to 10 ml/kg immediately after reperfusion. The recipient rats were ventilated and subjected to reperfusion for 2 h.
Lung sample collection
After 2 h of reperfusion, all the rats were anesthetized via an intraperitoneal injection of 3% pentobarbital sodium (30 mg/kg body weight) and cannulated. Left arterial blood gas analysis was performed, and peripheral blood was collected.
Wet-to-dry weight ratio
The wet-to-dry (W/D) weight ratio of left lung tissues was calculated 2 h after reperfusion. Tissues were excised from the upper third of the lung. The wet weight (in mg) was measured first, and the dry weight (in mg) was measured after the tissue had been dried for 3 days at 60 °C. The WDR was calculated as wet weight/dry weight.
Histological analysis
A portion of each lung sample was embedded in paraffin, and the lung tissue was prepared for HE staining to evaluate histological injury, which was scored by 2 independent investigators. The lung injury score included 5 variables: lung hemorrhage, peribronchial infiltration of inflammatory cells, pulmonary interstitial edema, pneumocyte hyperplasia, and intra-alveolar infiltration of inflammatory cells. Each variable was scored on a semiquantitative scale of 0–4, where 0 = normal, 1 = minimal change, 2 = mild change, 3 = moderate change and 4 = severe change. The overall histological score was calculated by summing the scores for variables 1 through 5.
Oxidative stress response
The activities of myeloperoxidase (MPO) and superoxide dismutase (SOD) and the concentration of malondialdehyde (MDA) in the homogenates were measured with specific kits (Nanjing Jiancheng, Nanjing, China) per the manufacturer’s instructions.
Inflammation assays
Systemic and local inflammation were assessed by measuring cytokine levels in homogenates and serum. The levels of tumor necrosis factor-a (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6) in the homogenates, as well as the level of monocyte chemotactic protein 1 (MCP-1) in the serum, were determined via commercial enzyme-linked immunosorbent assay (ELISA) kits following the manufacturer’s protocols. The expression of NF-κB in the lung tissue was determined via Western blotting.
Western blotting
The samples were homogenized in ice-cold RIPA buffer (CWBIO, Beijing) supplemented with a protease inhibitor mixture, and the supernatant was collected after centrifugation (12,000 rpm, 10 min, and 4 °C). A BCA Protein Assay Kit was used to determine the concentrations of the proteins in the supernatants, after which the proteins were denatured at 100 °C for 5 min. Equal amounts of protein (40 µg) were loaded onto SDS-PAGE gels, separated, and transferred to PVDF membranes, which were incubated at 4 °C overnight with primary antibody. Then, the membranes were incubated with an horseradish peroxidase (HRP)-conjugated goat anti-rabbit secondary antibody. The protein bands were visualized and analyzed with a ChemiDocTM XRS Plus luminescence image analyzer (Bio-Rad, USA) using an enhanced chemiluminescence (ECL) system (Millipore, USA).
Apoptosis assays
Terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling (TUNEL) staining was used to detect apoptosis in LIRI-induced lung tissues. The appropriate amount of TdT (reagent 1) or dUTP (reagent 2) from the TUNEL kit was added to the slices at a 1:9 ratio. After TUNEL staining, the lung tissue was counterstained with diaminobenzidine (DAB), a color development reagent, to detect nuclei. Under a light microscope, TUNEL-positive apoptotic cells and the nuclei of these apoptotic cells (stained dark brown and yellow brown) in the lung tissues were observed.
Statistical analysis
Statistical analysis was performed with GraphPad Prism® 9.0 software (GraphPad Software, Inc., California, USA). All the data are presented as the mean ± standard deviation (SD). Statistical significance was calculated using ANOVA followed by with Bonferroni multiple comparison post hoc test. p < 0.05 indicated statistical significance.
Results
Metformin increases oxygenation and alleviates pulmonary edema in the grafted lungs of rats
As shown in A, the LIRI group and the Met group had significantly reduced PaO2-to-FiO2 ratios after reperfusion. Compared with those in the LIRI group, the PaO2 to FiO2 ratios in the Met group were significantly greater. Similarly, the wet/dry weight ratio in lung tissues was worsened in the LIRI and Met groups.
Figure 1. Metformin increases oxygenation and alleviates pulmonary edema in the grafted lungs of rats. (A–B) Rats in the sham group underwent anesthesia and thoracotomy, while rats in the LIRI and Met groups underwent orthotopic left lung transplantation and were treated with oral saline or metformin. (A) The PaO2/FiO2 ratios of each group were calculated. (B) The wet/dry weight ratios of lung samples from the rats were determined (B). *p < 0.05, vs. the sham group; #p < 0.05, vs. the LIRI group. ![]()
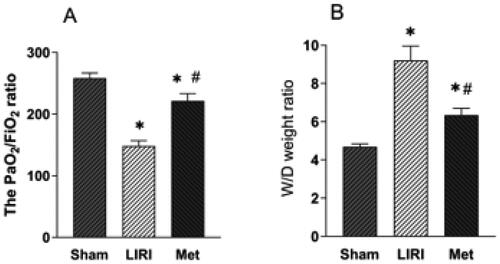
However, the increase in the wet/dry weight ratio was mitigated in the Met group compared with the LIRI group ().
Met mitigated histological injury to grafted lungs in rats
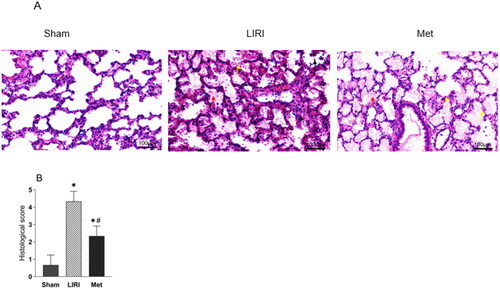
Typical histological injury was observed in the grafted lungs and sham lungs. The histological changes in the lungs included abnormalities in the infiltration of inflammatory cells, severe alveolar and mesenchymal edema, broken alveoli, and even hemorrhage. Pathological injury was significantly alleviated in the Met group compared with the LIRI group, as evidenced by significantly decreased cellular infiltration, ameliorated lung edema, and decreased alveolar injury (). Although the Met group still demonstrated significantly greater histological injury scores than the sham group, Met markedly decreased these scores in the rats that underwent lung transplantation ().
Figure 2. Metformin attenuated lung pathological injury in rats after lung transplantation (A) Representative images of H&E-stained lung tissues from rats. Many inflammatory cells were observed in the transplanted lung tissues, and severe thickening and breakage of the alveolar wall were observed. Severe edema and hemorrhage were observed in alveolar tissues in the LIRI and Met groups (magnification, 100 µm.) (B) The histological injury scores are shown. *p < 0.05, vs. the sham group; #p < 0.05, vs. the LIRI group. ![]()
Met suppressed the oxidative stress response in grafted rat lungs
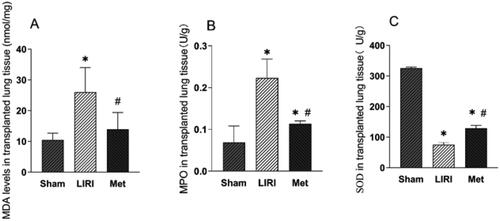
We investigated the impacts of Met on the oxidative and inflammatory responses in grafted lungs. Transplantation caused severe oxidative stress in the lung tissues, as compared with those from the sham group, lung tissues from the LIRI group and the Met group had significantly increased levels of MDA () and MPO (). Compared with those in the LIRI group, the levels of MPO and MDA in the Met group were markedly lower. However, treatment with Met resulted in higher levels of SOD than treatment with the other agents ().
Figure 3. Metformin inhibited the oxidative stress response in grafted lung tissues in rats (A–C) the concentrations of MDA (A), MPO (B) and SOD (C) in lung tissues from the indicated groups were measured. *p < 0.05, vs. the sham group; #p < 0.05, vs. the LIRI group. ![]()
Met attenuated local and systemic inflammation
Systemic and local inflammation were assessed by determining cytokine levels in tissue homogenates and serum samples, respectively. Met treatment downregulated the expression of MCP-1 in the lung tissue of rats after lung transplantation and reperfusion, although the Met-treated rats still had significantly greater levels of MCP-1 than the rats in the sham group (). However, while the same trend was observed for the plasma concentration of MCP-1, the difference was not significant (). In addition, after reperfusion, the levels of TNF-α, IL-1β, and IL-6 in lung homogenates and plasma were significantly greater than those in the sham group. Compared with those in the LIRI group, the TNF-α, IL-1β, and IL-6 levels in the Met group were significantly decreased (). Furthermore, the expression level of NF-κB in rat lung tissues was significantly upregulated by ischemia and reperfusion. Compared with that in the LIRI group, the expression level of NF-κB in the Met group was significantly downregulated ().
Figure 4. Metformin attenuated the inflammatory response associated with LIRI in rats (A-B) the concentration of MCP-1 in the serum of the rats was measured. (C-H) The concentrations of TNF-α, IL-1β, and IL-6 in the lung tissue and plasma were measured. *p < 0.05, vs. the sham group; #p < 0.05, vs. the LIRI group. ![]()
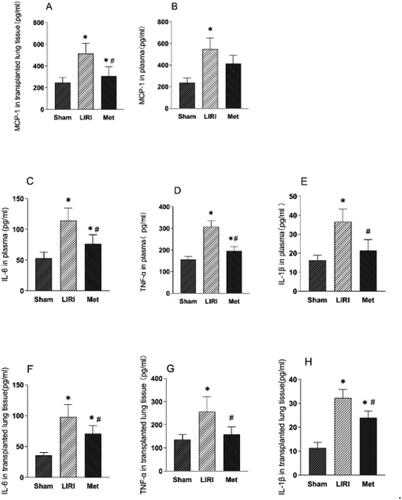
Figure 5. Metformin attenuated lung tissue apoptosis in rats after lung transplantation (A) merged immunofluorescence images showing apoptosis in each group (magnification, 200×). (B) The percentage of apoptotic cells in each group was determined. (C) Representative Western blot images are shown. *p < 0.05, vs. the sham group; #p < 0.05, vs. the LIRI group. ![]()
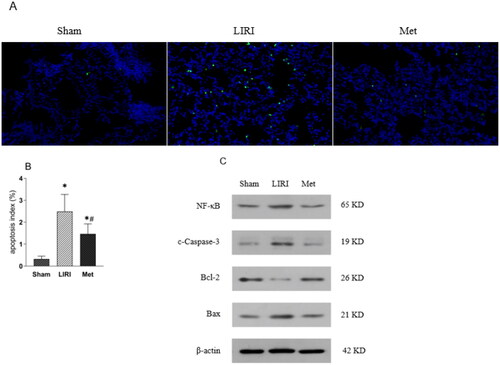
Met attenuated lung tissue apoptosis in rats after lung transplantation
There were many apoptotic cells in the transplanted lung tissue after reperfusion. The number of apoptotic cells (was significantly alleviated in the Met group compared with the LIRI group (). Compared with that in the LIRI group, the apoptosis index in the Met group decreased significantly (). After reperfusion, the protein expression of Bax and cleaved caspase-3 was significantly upregulated and the protein expression of of Bcl-2 was downregulated in the LIRI and Met groups. Compared with that in the LIRI group, the protein expression of the Bax and cleaved caspase-3 in the Met group was significantly downregulated, but the protein expression of Bcl-2 was upregulated ().
Discussion
The outcomes of lung transplantation are the worst among those of all types of solid organ transplantation. The success of lung transplantation is mainly limited by primary graft dysfunction due to lung ischemia–reperfusion injury.Citation12 Although the pathological mechanism of LIRI is complex and still not fully understood, inflammation, the oxidative stress response and apoptosis have been shown to play key roles in LIRI.Citation13,Citation14 In the present study, oral administration of metformin to donor rats for 7 days before lung transplantation attenuated lung ischemia–reperfusion injury. We found that metformin significantly alleviated lung injury after lung transplantation in rats by increasing oxygenation and alleviating pulmonary edema, inhibiting local and systemic inflammation, alleviating oxidative stress, and reducing apoptosis. This is the first study to confirm the protective effect of Met against LIRI.
Ischemia–reperfusion injury can lead to breakdown of the endothelial and epithelial barriers through a variety of mechanisms, leading to life-threatening edema and organ dysfunction.Citation15 Specifically, Ming et al.Citation16 demonstrated that metformin promotes microvascular repair in acute lung injury by stimulating AMPK-α1, thus alleviating pulmonary edema in acute lung injury. In this study, we measured capillary permeability and histological damage and found that metformin significantly reduced lung tissue damage, increased the PaO2/FiO2 ratio, and decreased the wet/dry weight ratio of lung tissue, suggesting that metformin also has a protective effect against vascular damage induced by lung transplantation.
Oxidative stress is an important mechanism leading to lung ischemia–reperfusion injury.Citation12,Citation16,Citation17 During oxidative stress, oxygen-free radicals react with cellular lipids to form malondialdehyde (MDA), which accumulates in large quantities in cells. SOD is a metal enzyme that plays an antioxidant role in cells. It can catalyze the generation of oxygen and hydrogen peroxide by oxygen-free radicals.Citation18 Moreover, neutrophils play an important role in LIRI-related oxidative stress by acting on the MPO system. In activated neutrophils, MPO generates hypochlorite through the production of hydrogen peroxide and the release of oxygen-free radicals. MPO and its derived oxidants are important factors in the pathogenesis of organ ischemia–reperfusion injury.Citation19,Citation20 Many studies have confirmed that metformin has antioxidant effects in various organs following ischemia–reperfusion injury.Citation21–23 In this study, we also observed that metformin could inhibit oxidative stress by reducing MDA levels, increasing SOD activity, and reducing MPO content, thus exerting protective effects against LIRI.
NF-κB is a key transcription factor in the inflammatory response. During ischemia–reperfusion, NF-κB is activated, which can induce the release of MCP-1 and other chemokines into the serum, cause the recruitment of inflammatory cells into the lung tissue to secrete many inflammatory factors, and eventually lead to organ damage. Li et al.CitationCitation24 showed that metformin can protect against lung tissue injury by reducing the inflammatory response in lung tissue in an acute lung injury model. In this study, we found that metformin could reduce the release of MCP-1, especially in lung tissue, and further reduce the secretion of TNF-α, IL-1β and IL-6 in lung tissue and serum. Moreover, metformin significantly reduced the inflammatory response by downregulating the expression of NF-κB. This finding is consistent with study.Citation24
Apoptosis is a form of programmed cell death.Citation25 Studies have shown that the degree of apoptosis is affected by the duration of cold ischemia. Cold ischemia 6–12 h before reperfusion leads to more apoptosis than necrosis in lung tissue, while cold ischemia 24 h before reperfusion leads to more necrotic cell death.Citation26 Bcl-2 and caspase family members are pivotal mediators of apoptosis. Bax is a key proapoptotic protein that can activate the endogenous apoptosis pathwayCitation27 and further induce the cleavage of caspases. Bcl-2 is an important antiapoptotic protein that can block endogenous apoptosis by inhibiting the release of Bax.Citation28 Caspase-3 is the most critical apoptosis executor, and cleaved caspase-3 is the activated form of caspase-3. We found that metformin significantly downregulated the expression of Bax and cleaved caspase-3 but upregulated the expression of Bcl-2. These findings indicate that the protective effect of metformin against lung ischemia–reperfusion injury is related to its antiapoptotic effect.
The present study has several limitations. First, we did not induce brain death in the donor animals. As such, we did not exactly replicate clinical pulmonary transplantation. Second, due to technical insufficiency, respiratory mechanics and related data could not be investigated. Third, this was a descriptive study lacking in vitro experimental verification and in-depth study of the molecular mechanisms involved. However, these findings may help our team initiate additional new experimental studies.
Conclusion
Metformin can alleviate ischemia–reperfusion injury after lung transplantation in rats, possibly through inhibition of oxidative stress, inflammation, and apoptosis and an increase in vascular permeability.
Disclosure statement
The authors declare that they have no competing interests.
Additional information
Funding
References
- Yusen RD, Edwards LB, Dipchand AI, International Society for Heart and Lung Transplantation, et al. The registry of the international society for heart and lung transplantation: thirty-third adult lung and heart-lung transplant report-2016; Focus Theme: primary diagnostic indications for transplant. J Heart Lung Transplant. 2016;35(10):1170–1184. doi:10.1016/j.healun.2016.09.001.
- Altun GT, Arslantaş MK, Cinel İ. Primary graft dysfunction after lung transplantation. Turk J Anaesthesiol Reanim. 2015;43(6):418–423. doi:10.5152/TJAR.2015.16443.
- Christie JD, Kotloff RM, Ahya VN, et al. The effect of primary graft dysfunction on survival after lung transplantation. Am J Respir Crit Care Med. 2005;171(11):1312–1316. doi:10.1164/rccm.200409-1243OC.
- Fiser SM, Tribble CG, Long SM, et al. Ischemia-reperfusion injury after lung transplantation increases risk of late bronchiolitis obliterans syndrome. Ann Thorac Surg. 2002;73(4):1041–1048. doi:10.1016/s0003-4975(01)03606-2.
- Huang HJ, Yusen RD, Meyers BF, et al. Late primary graft dysfunction after lung transplantation and bronchiolitis obliterans syndrome. Am J Transplant. 2008;8(11):2454–2462. doi:10.1111/j.1600-6143.2008.02389.x.
- Piwkowska A, Rogacka D, Jankowski M, et al. Metformin induces suppression of NAD(P)H oxidase activity in podocytes. Biochem Biophys Res Commun. 2010;393(2):268–273. doi:10.1016/j.bbrc.2010.01.119.
- Wu K, Tian R, Huang J, et al. Metformin alleviated endotoxemia-induced acute lung injury via restoring AMP Kdependent suppression of mTOR. Chem Biol Interact. 2018;291:1–6. doi:10.1016/j.cbi.2018.05.018.
- Zhang X, Shang F, Hui L, et al. The alleviative effects of metformin for lipopolysaccharide-induced acute lung injury rat model and its underlying mechanism. Saudi Pharm J. 2017;25(4):666–670. doi:10.1016/j.jsps.2017.05.001.
- Huang KY, Que JQ, Hu ZS, et al. Metformin suppresses inflammation and apoptosis of myocardiocytes by inhibiting autophagy in a model of ischemia-reperfusion injury. Int J Biol Sci. 2020;16(14):2559–2579. doi:10.7150/ijbs.40823.
- Leech T, Chattipakorn N, Chattipakorn SC. The beneficial roles of metformin on the brain with cerebral ischaemia/reperfusion injury. Pharmacol Res. 2019;146:104261. doi:10.1016/j.phrs.2019.104261.
- Westerkamp AC, Fujiyoshi M, Ottens PJ, et al. Metformin preconditioning improves hepatobiliary function and reduces injury in a rat model of normothermic machine perfusion and orthotopic transplantation. Transplantation. 2020;104(9):e271–e280. doi:10.1097/TP.0000000000003216.
- Chen-Yoshikawa TF. Ischemia-reperfusion injury in lung transplantation. Cells. 2021;10(6):1333. doi:10.3390/cells10061333.
- Laubach VE, Sharma AK. Mechanisms of lung ischemia-reperfusion injury. Curr Opin Organ Transplant. 2016;21(3):246–252.
- Eltzschig HK, Eckle T. Ischemia and reperfusion—from mechanism to translation. Nat Med. 2011;17(11):1391–1401. doi:10.1097/MOT.0000000000000304.
- Chatterjee S, Nieman GF, Christie JD, Fisher AB. Shear stress-related mechanosignaling with lung ischemia: lessons from basic research can inform lung transplantation. Am J Physiol Lung Cell Mol Physiol. 2014;307(9):L668–80. doi:10.1152/ajplung.00198.2014.
- Wang M, Liu Y, Liang Y, et al. Systematic understanding of pathophysiological mechanisms of oxidative stress-related conditions-diabetes mellitus, cardiovascular diseases, and ischemia-reperfusion injury. Front Cardiovasc Med. 2021;8:649785. doi:10.3389/fcvm.2021.649785.
- Ferrari RS, Andrade CF. Oxidative stress and lung ischemia-reperfusion injury. Oxid Med Cell Longev. 2015;2015:590987–590914. doi:10.1155/2015/590987.
- Liang S, Wang Y, Liu Y. Dexmedetomidine alleviates lung ischemia-reperfusion injury in rats by activating P I3K/Akt pathway. Eur Rev Med Pharmacol Sci. 2019;23(1):370–377. doi:10.26355/eurrev_201901_16785.
- Vasilyev N, Williams T, Brennan ML, et al. Myeloperoxidase-generated oxidants modulate left ventricular remodeling but not infarct size after myocardial infarction. Circulation. 2005;112(18):2812–2820. doi:10.1161/CIRCULATIONAHA.105.542340.
- Matthijsen RA, Huugen D, Hoebers NT, et al. Myeloperoxidase is critically involved in the induction of organ damage after renal ischemia reperfusion. Am J Pathol. 2007;171(6):1743–1752. doi:10.2353/ajpath.2007.070184.
- Ghasemnejad-Berenji M, Ghazi-Khansari M, Yazdani I, et al. Effect of metformin on germ cell-specific apoptosis, oxidative stress and epididymal sperm quality after testicular torsion/detorsion in rats. Andrologia. 2018;50(2):e12846. doi:10.1111/and.12846.
- Zeng J, Zhu L, Liu J, et al. Metformin Protects against Oxidative Stress Injury Induced by Ischemia/Reperfusion via Regulation of the lncRNA-H19/miR-148a-3p/Rock2 Axis. Oxid Med Cell Longev. 2019;2019:8768327–8768318. doi:10.1155/2019/8768327.
- Cahova M, Palenickova E, Dankova H, et al. Metformin prevents ischemia reperfusion-induced oxidative stress in the fatty liver by attenuation of reactive oxygen species formation. Am J Physiol Gastrointest Liver Physiol. 2015;309(2):G100–11. doi:10.1152/ajpgi.00329.2014.
- Wu L, Cen Y, Feng M, et al. Metformin Activates the Protective Effects of the AMP K Pathway in Acute Lung Injury Caused by Paraquat Poisoning. Oxid Med Cell Longev. 2019 ;Oct 302019:1709718–1709710. doi:10.1155/2019/1709718. P MID: 31781324.
- Bertheloot D, Latz E, Franklin BS. Necroptosis, pyroptosis and apoptosis: an intricate game of cell death. Cell Mol Immunol. 2021;18(5):1106–1121. doi:10.1038/s41423-020-00630-3.
- den Hengst WA, Gielis JF, Lin JY, et al. Lung ischemia-reperfusion injury: a molecular and clinical view on a complex pathophysiological process. Am J Physiol Heart Circ Physiol. 2010;299(5):H1283–99. doi:10.1152/ajpheart.00251.2010.
- Cartron PF, Juin P, Oliver L, et al. Impact of proapoptotic proteins Bax and Bak in tumor progression and response to treatment. Expert Rev Anticancer Ther. 2003;3(4):563–570. doi:10.1586/14737140.3.4.563.
- Niture SK, Jaiswal AK. Nrf2-induced antiapoptotic Bcl-xL protein enhances cell survival and drug resistance. Free Radic Biol Med. 2013;57:119–131. doi:10.1016/j.freeradbiomed.2012.12.014.
