Abstract
Progress in the processing of wet tissues, without the need of fixation and complex preparation procedures, may facilitate the microscopic examination of tissues and cells. Microscopic examination of tissues is a central tool in clinical diagnosis as well as in diverse areas of research. The authors present the application of Wet SEM, a technology for imaging fully hydrated samples at atmospheric pressure in a scanning electron microscope (SEM). The technique is based on 2 principles. First, samples are imaged in sealed specimen capsules and are separated from the evacuated interior of the electron microscope by a thin, electron-transparent partition membrane that is strong enough to sustain a 1-atm pressure difference. Second, imaging is done in a SEM, based on detection of backscattered electrons, which penetrate a few microns into the specimen and thus give information on the cellular level.
Progress in the processing of wet tissues, without the need of fixation and complex preparation procedures, may facilitate the microscopic examination of tissues and cells for clinical diagnosis as well as diverse areas of research in the life sciences. Microscopic examination of tissues is a central tool in clinical diagnosis as well as in diverse areas of research. We present here the application of Wet SEM, a technology for imaging fully hydrated samples at atmospheric pressure in a scanning electron microscope (SEM).
Light microscopy (LM) is performed with thin sections of tissue (several microns), stained with chemicals, antibodies, and/or enzymes to reveal general tissue architecture, to identify normal or abnormal cell types, and to localize specified molecules. Transmission electron microscopy (TEM) requires specially prepared ultrathin sections (typically 50 nm), and reveals a wealth of subcellular information. Each of the aforementioned techniques has limitations: the resolution of light microscopy is limited by diffraction to 0.25 µm; the use of TEM is encumbered by extensive processing of the sample, which may alter its structure significantly. Preparation of samples for standard TEM also requires specific skills and takes at least a few days to achieve. The very thin slices present a very limited (and often arbitrary) portion of the sample, necessitating the imaging of multiple serial sections [Citation[1], Citation[2]]. SEM occupies a more restricted niche, usually limited to surface imaging. It is used to investigate surface topography, and imaging of intracellular structure by SEM requires specialized preparatory techniques such as fracturing and etching. All those techniques usually require fixation and complex methods for preparation of the tissue. In marked contrast to the above described methods, we present a brief preparation of fixed or unfixed tissues for evaluation by SEM.
Our technique is based on 2 principles. First, samples are imaged in sealed specimen capsules () and are separated from the evacuated interior of the electron microscope by a thin, electron- transparent partition membrane that is strong enough to sustain a 1-atm pressure difference (QuantomiX, Israel). Second, imaging is done in an SEM, based on detection of backscattered electrons (BSE). This setup results in 3 unique characteristics:
Samples can be imaged in an electron microscope, at resolutions up to 20 nm, in a fully wet state, without dehydration or coating.
Backscattered electrons result from interactions of the scanning electron beam with an internal layer of up to approximately 2 µm into the sample; any material present beyond this layer is irrelevant to the imaging process. Consequently, thick specimens, for example, tissue fragments of a few millimeters thickness, can be imaged without thin sectioning, embedding, or freezing.
Backscattering efficiency is sensitive to material differences within the sample, allowing contrast mechanisms that are distinct from those operative in other microscopy methods. In particular, biological features may be distinguished without staining, or with heavy metal staining, and gold-linked immunolabels are very clearly resolved.
For the evaluation of the method, we chose the mucosal surface of the large bowel, comparing normal colon to inflamed colon diagnosed as inflammatory bowel disease (IBD). Three cases of normal colon resection procedures and 3 cases of Crohn disease affecting the colon were selected from the pathology repository of the Department of Pathology, Sheba Medical Center after taking samples for diagnostic purposes. A few small fragments of a few millimeters each were taken from each specimen. Tissue samples were washed several times in water, incubated for 5 min with 1% tannic acid, and stained with uranyl acetate, 0.1%, pH 3.5, for 10 min. Samples were placed in the wet SEM specimen capsule and visualized in the SEM.
The SEM was used in a few studies for the understanding of the morphology and evolution of IBD lesions throughout the gastrointestinal tract [Citation[3–5]]. In lesions such as “aphthoid” ulcers, the SEM demonstrated variety of villous abnormalities in regions adjacent to ulcers [Citation[3]]. In addition, the macroscopically unaffected mucosa was found to be distorted by irregularities in the normal polygonal units. The individual absorptive cells were found to lose their pentagonal–hexagonal cell borders [Citation[4]]. Moreover, quantitative techniques were used to develop more objective criteria for the diagnosis of dysplasia in IBD using SEM [Citation[5]]. In addition, the SEM was useful in the study of inflammatory processes, such as cell migration in the lamina propria in samples of IBD [Citation[6]].
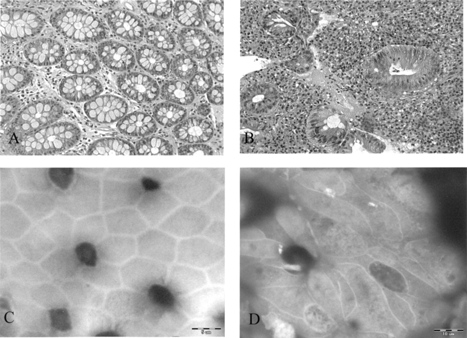
By our method, using wet tissue, en face photographs of colonic Crohn disease show marked irregularity of the cell borders with elongation of the crypt orifices and cell contours (), similar to what was observed in previous studies [Citation[4]].
Fig. 2 (A, B) Hematoxylin and eosin stain of normal colon (A) and IBD (B), ×200. (C,D) Using wet tissue for SEM: (C) “en face” SEM photograph of normal colon; (D) “en face” SEM photographs of colon affected by Crohn disease show marked irregularity of the cell borders with elongation of the crypts’ orifices, ×3200.
This novel method offers a potential source of quick examination of various tissues. A few preliminary studies have already been performed in cultures of cell lines [Citation[7]], cytologic material, and human tissue biopsies of thyroid, colon, and other organs. Tissues such as thyroid and liver are evaluated for quick examination for purposes that may replace the frozen sections during operation. It will be important to evaluate the role that this method can play in quick diagnosis of additional pathological specimens.
REFERENCES
- Rosai J, Rodriguez HA.. Application of electron microscopy to the differential diagnosis of tumors. Am J Clin Pathol. 1968; 50: 535–562
- Williams MJ, Uzman BG.. Uses and contributions of diagnostic electron microscopy in surgical pathology: a study of 20 veterans administration hospitals. Hum Pathol. 1984; 15: 738–745. [PUBMED], [INFOTRIEVE]
- Rickerts RR, Carter HW.. The “early” ulcerative lesion of Crohn's disease: correlative light- and scanning electron-microscopic studies. J Clin Gastroenterol. 1980; 2: 11–19
- Myllarniemi H, Nickels J.. Scanning electron microscopy of Crohn's disease and ulcerative colitis of the colon. Virchows Arch A Pathol Anat Histol. 1980; 385: 343–350. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Shields HM, Bates ML, Goldman H, et al, Scanning electron microscopic appearance of chronic ulcerative colitis with and without dysplasia. Gastroenterology. 1985; 89: 62–72. [PUBMED], [INFOTRIEVE]
- McAlindon ME, Gray T, Galvin A, Sewell HF, Podolsky DK, Mahida YR.. Differential lamina propria cell migration via basement membrane pores of inflammatory bowel disease mucosa. Gastroenterology. 1998; 115: 841–848. [PUBMED], [INFOTRIEVE], [CSA]
- Thiberge S, Zik O, Moses E.. (submitted) An apparatus for imaging liquids, cells and other wet samples in the scanning electron microscope..
