Abstract
This study presents the first palynological characterisation of the five endemic plant genera of the Madeira archipelago: Chamaemeles Lindl, Melanoselinum Hoffm., Monizia Lowe, Musschia Dumort and Sinapidendron Lowe. Pollen grain morphology of ten endemic species was studied using light and scanning electron microscopy techniques. The size and shape of pollen grains, the polar axis, the equatorial diameter, and the exine ornamentation were measured and described. We found that the pollen grains of the five endemic genera are all medium-size monads. The close relative apiaceous Melanoselinum and Monizia differ in polar (P) and equatorial (E) diameter size and exine ornamentation while Sinapidendron species show differences in P, E, and P/E ratios. The pollen grains of the two Musschia species are very similar to each other, but differ in morphology and ornamentation from the Macaronesian endemic bellflowers Azorina vidalii and Canarina canariensis. This study unveiled differences between the endemic taxa and with their close related species, thus providing support to previous taxonomic findings.
1. Introduction
The archipelago of Madeira, like other Macaronesian archipelagos, is included in one of the worldwide biodiversity hotspots mostly due to the uniqueness of its plant biodiversity (Médail and Quézel Citation1999; Myers et al. Citation2000). In fact, over a thousand plant taxa have been reported to this archipelago and nearly two hundred of them are exclusive to Madeira or Macaronesia (Press and Short Citation1994; Jardim and Francisco Citation2000; Jardim and Sequeira Citation2008). Amongst the Macaronesia endemic taxa, we found paleoendemics with a subtropical Tertiary Tethysian origin (e.g. trees of genera Apollonias Nees, Clethra L., Heberdenia Banks ex A. DC., Ocotea Aubl., Persea Mill., Picconia A. DC.) and neoendemics that have resulted from radiation processes (e.g. shrubs included in genera Argyranthemum Webb ex Sch. Bip., Helichrysum Mill., Sinapidendron Lowe, Sonchus L.) (Jardim and Sequeira Citation2008). Five endemic plant genera have been reported to the Madeira archipelago, namely Chamaemeles Lindl, Melanoselinum Hoffm., Monizia Lowe, Musschia Dumort and Sinapidendron Lowe (Press and Short Citation1994; Jardim and Sequeira Citation2008). The former three are monotypic while three species of Musschia and five of Sinapidendron are known from the archipelago. Here, we still treat Melanoselinum and Monizia as distinct genera, but recent studies provide evidence that (altogether with the Canarian endemic Cryptotaenia elegans Webb ex Bolle) they constitute a distinct evolutionary lineage (the Macaronesian clade) within the Daucus s.l. complex (Banasiak et al. Citation2016).
The study plants are diverse taxonomically (belonging to four plant families), present different ecology (with specific phenologies and habitat preferences) and differ morphologically, ranging from perennial herbs to large woody shrubs (Press and Short Citation1994; Jardim and Francisco Citation2000). The three monotypic genera include:
Chamaemeles coriacea Lindl. (Rosaceae), an evergreen shrub with spoon-shaped coriaceous leaves and elongate to pyramidal inflorescences that can be found at low altitude (between 0–400 m) inhabiting coastal rocky cliffs and inland ravines on Madeira, Porto Santo and Desertas islands (Press and Short Citation1994; Jardim and Francisco Citation2000).
Melanoselinum decipiens (Schrad. & J.C. Wendl.) Hoffm. (Apiaceae), a tall monocarpic plant with a ridged smooth stem and a large umbel which occurs on shady rocks and banks in ravines in northern Madeira (Press and Short Citation1994; Jardim and Francisco Citation2000).
Monizia edulis Lowe (Apiaceae), a perennial plant with triangular leaves and a paniculate inflorescence with white petals. This plant is restricted to cliff areas in Madeira, Porto Santo (Ilhéu de Cima) and Deserta Grande (Press and Short Citation1994; Jardim and Francisco Citation2000; Fernandes and Carvalho Citation2014).
The genus Musschia Dumort (Campanulaceae) includes two species very different from each other. Musschia aurea (L.) Dumort is a small glabrous plant with bright yellow flowers while M. wollastonii Lowe is a pubescent monocarpic plant with reddish brown flowers. Both species are rare and generally occur on rocky cliffs, but M. aurea inhabits coastal and inland valleys while M. wollastonii is restricted to humid shaded valleys in laurel forests (from 400 to 900 m) (Press and Short Citation1994; Jardim and Francisco Citation2000).
The genus Sinapidendron Lowe (Brassicaceae) contains five species. They are perennial herbs or small shrubs, with toothed or simple leaves and 7–10 mm long yellow petals with a claw, but differ in size, leaf morphology and habitat (Press and Short Citation1994; Jardim and Francisco Citation2000). Sinapidendron gymnocalyx Rustan is a perennial shrub that occurs mostly on cliffs along the north coast of Madeira, but can also be found on a few inland cliffs. The other four species (S. angustifolium Lowe, S. frutescens Lowe, S. rupestre Lowe and S. sempervivifolium Menezes) have narrow geographic distributions and are severely threatened of extinction, being classified as endangered or critically endangered by the IUCN Red List (Carvalho Citation2011a, Citation2011b; Kell Citation2011a, Citation2011b).
Despite the ongoing research on Madeiran plants, including the description of new taxa, very few palynological studies targeted endemic species of this archipelago. To our knowledge, there are just two studies on Madeiran Asteraceae (Pinheiro de Carvalho et al. Citation2001, Citation2003), a few other studies included several endemic taxa (e.g. León-Arencibia & Serna-Ramos Citation1992), but no comprehensive palynological study is available on the Madeiran flora. On the other hand, a considerable number of palynological studies were carried out in the Canaries (e.g. La Serna-Ramos et al. Citation1994; La Serna-Ramos Citation1996; La Serna-Ramos & Padron-Mederos Citation2008; Padrón-Mederos and La Serna-Ramos Citation2011) and, more recently, several investigations on pollen morphology were conducted in Azores (Morgado et al. Citation2014, Citation2015), where a pollen inventory of the endemic species was also performed (Morgado et al. Citation2017). In this study we aim to characterize and compare the pollen morphological features of ten species from the endemic plant genera of Madeira archipelago and contribute to a better understanding of their taxonomy.
2. Materials and methods
The archipelago of Madeira lies in the northeastern Atlantic Ocean at the southwest of the Iberian Peninsula, between latitudes 33° 10′ − 33° 20′ N and longitudes 16° 10′ − 17° 20′ W and is composed by three island groups (Madeira, Porto Santo and Desertas). The islands present several habitat types, including the emblematic Laurisilva, and have a valuable biodiversity, including 172 endemic plants (Jardim and Francisco Citation2000; Borges et al. Citation2008; Boieiro et al. Citation2015). Fieldwork took place in Madeira proper and in Deserta Grande (Desertas islands), where the target plant species are known to occur. Sampling took place in 2018, 2019 and 2022, during the flowering season of the different endemic species which lasted between April and August (Jardim and Francisco Citation2000). Most samples were collected in the field from natural populations, but the Jardim Botânico da Madeira – Eng. Rui Vieira provided additional plant material of M. edulis.
This study targeted the species included in the five endemic plant genera of Madeira archipelago. Three genera – Chamaemeles, Melanoselinum and Monizia – are monotypic while the genus Sinapidendron has radiated in the archipelago and five valid species are known, with one being restricted to Desertas (Jardim and Sequeira Citation2008). The genus Musschia is represented by two species in Madeira island, M. aurea occurring predominatly in coastal areas and M. wollastoni restricted to the laurel forest and, in Desertas, we can find M. aurea and the rare M. isambertoi (Menezes de Sequeira et al. Citation2007). We were not able to collect samples from Musschia isambertoi M. Seq., R. Jardim, Magda Silva & L. Carvalho, a critically endangered species known from a few localities of difficult accesss in Deserta Grande, where it is severely threatened by invasive species (Menezes de Sequeira et al. Citation2007, Citation2021).
Pollen grains from each species were studied using both light microscopy (LM) and scanning electron microscopy (SEM). For the LM study, pollen grains were prepared following the acetolysis method (Erdtman Citation1952, Citation1966), and then mounted on permanent slides to be used for observations and morphological measurements under light microscopy (Zeiss Primo Star with micrometric ocular) at the University of the Azores. We randomly selected 25 undamaged pollen grains from each study species to measure polar and equatorial diameters in equatorial view and calculate P/E ratios. Additionally, we measured the apocolpium/apoporium (LA) and the equatorial diameter in polar view (DEPV), the size of the exine layers, and aperture width and length from a random sample of pollen grains (Morgado et al. Citation2017). Unfortunately, we were not able to obtain data on LA and DEPV for M. decipiens. Acetolysed pollen grains were photomicrographed at 100X magnification under LM (Leica DM 2500 with digital camera DFC 495). The data on pollen grain morphology were summarised using descriptive statistics, namely the arithmetic mean, standard deviation of the mean, range and coefficient of variation, and interspecific differences in standard pollen morphological metrics were assessed using Kruskal-Wallis tests.
Pollen samples also underwent SEM analysis at the Microscopy Laboratory in the Faculty of Sciences of the University of Lisbon. Without previous chemical treatment, pollen grains were metalised with gold using an ion-putter coater (JEOL JFC 1200) and then analysed by standard techniques using a scanning electron microscope (JEOL – JSM5200LV), which provides detailed information on pollen morphology, particularly pollen ornamentation. Five undamaged pollen grains of each plant species were selected at random to perform SEM photomicrographs, allowing us to describe pollen grain shape and ornamentation. The detailed description of pollen grains includes information on pollen unit, size, class, polarity, shape, polar view, equatorial view, aperture number and exine ornamentation. The terminology used to describe pollen grain morphology follows Punt et al. (Citation2007) and pollen grain size classes follow the classification proposed by Erdtman (Citation1952). The samples used in this study were deposited in the herbarium of the University of the Azores (AZU) (Angra do Heroísmo, Azores, Portugal) ().
Table 1. List of the endemic study species and the herbarium reference number of the samples examined.
3. Results
Pollen grain morphology of ten endemic species of the Madeira archipelago is described below. Pollen grain description follows the sequence adopted in Morgado et al. (Citation2017), namely pollen grain size, polarity, shape, aperture (number), and ornamentation. Standard morphological characteristics of pollen grains are presented for all study species (). For the polytypic genera Sinapidendron and Musschia, the differences in pollen morphological variables supported by statistical analysis are highlighted in the respective genus sections while for the monotypic Melanoselinum and Monizia (both in Apiaceae) the results from statistical analysis on interspecific comparisons are shown in the Monizia section.
Table 2. Morphological features of pollen grains from endemic plant taxa of Madeira archipelago.
Table 3. Measurements of ectoaperture (pore or colpus) and exine (sexine and nexine) from endemic plant taxa of Madeira archipelago.
Table 4. Measurements of pollen grains from endemic plant taxa of Madeira archipelago in polar view (DEVP = equatorial diameter of polar view; LA = apocolpium/apoporium side; PAI = polar area index).
3.1. Melanoselinum decipiens (Schrad. & J.C. Wendl.) Hoffm
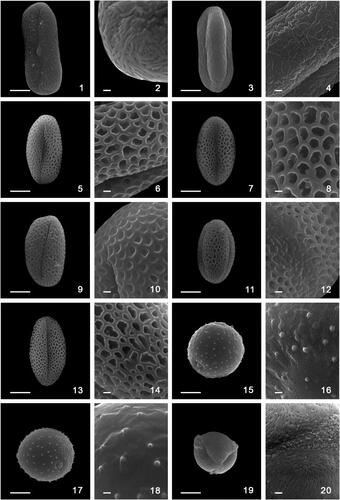
The pollen grains are medium-sized (44.7 µm), monads, isopolar symmetric with a prolate shape (, figures 1, 2). The pollen grain shows an elliptic equatorial outline (boat-shaped) and an amb triangular polar view. The polar axis ranges from 41.0 to 48.0 µm while the equatorial axis from 20.0 to 26.0 µm (). The aperture is tricolporate type with endoaperture lalongate (transversely elongated endoaperture). The sexine ornamentation is rugulate-microreticulate, with elongated ornamentation elements irregularly arranged (, figure 2).
Plate 1. Optical microscopy photographs of pollen grains of Madeira endemic species. 1,2. Melanoselinum decipiens: 1. equatorial view, 2. endoaperture lalongate. 3,4. Monizia edulis: 3. equatorial view, 4. polar view; 5,6. Sinapidendron angustifolium. 5. equatorial view, 6. polar view; 7,8. Sinapidendron frutescens. 7. equatorial view, 8. polar view. 9,10. Sinapidendron gymnocalyx. 9. equatorial view, 10. polar view. 11,12. Sinapidendron rupestre. 11. equatorial view, 12. polar view. 13,14. Sinapidendron sempervivifolium. 13. equatorial view, 14. polar view. 15,16. Musschia aurea. 15. equatorial view, 16. polar view. 17,18. Musschia wollastonii. 17. equatorial view, 18. polar view. 19,20. Chamaemeles coriacea. 19. equatorial view, 20. polar view. Scale bars: 10 µm.
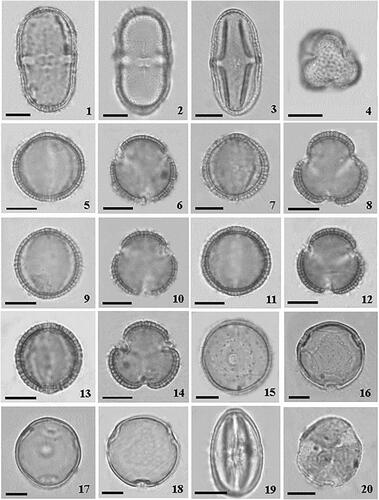
Plate 2. Scanning electron microscopy photographs of pollen grains of Madeira endemic species. 1,2. Melanoselinum decipiens: 1. equatorial view; 2. exine ornamentation. 3,4. Monizia edulis: 3. equatorial view, 4. exine ornamentation; 5,6. Sinapidendron angustifolium. 5. equatorial view, 6. exine ornamentation. 7,8. Sinapidendron frutescens. 7. equatorial view, 8. exine ornamentation. 9,10. Sinapidendron gymnocalyx. 9. equatorial view, 10. exine ornamentation. 11,12. Sinapidendron rupestre. 11. equatorial view, 12. exine ornamentation. 13,14. Sinapidendron sempervivifolium. 13. equatorial view, 14. exine ornamentation. 15,16. Musschia aurea. 15. equatorial view, 16. exine ornamentation. 17,18. Musschia wollastonii. 17. equatorial view, 18. exine ornamentation. 19,20. Chamaemeles coriacea. 19. equatorial view, 20. exine ornamentation. Scale bars: 10 µm (1, 3, 5, 7, 9, 11, 13, 15, 17, 19), 1 µm (2, 4, 6, 8, 10, 12, 14, 16, 18, 20).
3.2. Monizia edulis Lowe
The pollen grains of M. edulis are medium-sized (40.7 µm), monads, radially symmetrical and isopolar (, figures 3). The pollen grain shows an elliptic equatorial outline and an amb (sub) triangular polar view (, figure 4). The shape is prolate with polar axis ranging from 35.0 to 45.4 μm and equatorial axis from 18.0 to 21.0 μm (). Pollen grains are tricolporate with lalongate endoaperture and surface rugulate-microreticulate (, figure 4). We found significant differences in polar and equatorial diameter size (both p < 0.001) between M. decipiens and M. edulis (). The two Apiaceae species have similar colpus length, and nexine and sexine layers thickness (p > 0.05) (), but sexine ornamentation is different, being long rugulae in M. decipiens and shorter rugulae in M. edulis (, figures 2, 4).
3.3. Sinapidendron Lowe
The pollen grains of the five Sinapidendron species are monads, isopolar, and medium size (, figures 5–14). The shape of all pollen grains is spheroidal, tricolpate and the sexine reticulate with free-standing columellae (, figure 8; ). Sinapidendron pollen grains show a spheroidal equatorial outline and an amb subcircular polar view (, figures 5, 7, 9, 11, 13; ). We found significant differences in pollen grain size and shape measurements (P, E, P/E, DEVP) between Sinapidendron species, but not in colpus size and in the thickness of exine layers. The average polar diameter varies from 23.4 μm in S. gymnocalyx to 25.6 μm in S. frutescens, while the average equatorial diameter was largest in S. frutescens (25.0 μm) and lowest in both S. gymnocalyx and S. sempervivifolium (22.8 μm) (; ). The polar diameter of S. frutescens is significantly larger compared to the other congeners (all p < 0.05). Similarly, the equatorial diameter (E) is significantly different between S. frutescens and the other species (all p < 0.05), and S. gymnocalyx and S. sempervivifolium have the lowest values and do not differ significantly from each other (p > 0.05). The average colpus length ranged from 15.8 μm in S. sempervivifolium to 17.1 μm in S. rupestre. On average, the colpus length was larger in S. rupestre and lower in S. angustifolium and S. sempervivifolium, but no significant differences were detected between species. Average thickness values of sexine and nexine are similar between the Sinapidendron species and no significant differences were found in LA, despite the slight larger values recorded for S. frutescens ( and ).
3.4. Musschia Dumort
The pollen grains of the two studied Musschia species are monads and show a similar microechinate, rugulate-perforate ornamentation. The pollen grains are isopolar, spheroidal in shape, triporate and an amb circular polar view (, figures 15, 17). In both species, pollen grains present porus with operculum and annulus. In Musschia aurea, the length of the polar axis ranges between 32.0 to 36.0 μm and the equatorial diameter from 30.0 to 35.0 μm (). Pollen grains in M. aurea are furnished with microechinae (, figure 16). The average length of microechinae is 0.80 μm and the width is 0.58 μm. The porus size is 5.5 μm and sexine is thicker than nexine ( and ). The pollen grains of M. wollastonii (, figures 17, 18) show similar ranges of polar and equatorial axis sizes as M. aurea (). The average length of microechinae is 0.95 μm and the width is 0.64 μm. Porus size is 5.9 μm and sexine is thicker than nexine ( and ). No significant differences were found in most pollen grain size measurements (P, DEVP, LA), and in pore and microechinae size (length and width) between the two Musschia species (all p > 0.05) (). Only equatorial diameter is larger in M. wollastonii than in M. aurea (p = 0.02).
3.5. Chamaemeles coriacea Lindl
Pollen grains are radially symmetrical monads, isopolar with a medium size (28.2 µm). The length of the polar axis ranges from 24.0 to 32.0 μm and the equatorial diameter from 16.0 to 26.0 μm (). The pollen shape of C. coriacea is prolate (, figure 19) showing an elliptic equatorial outline and an amb subcircular polar view. The pollen grain is tricolporate with long aperture. On average, the colpus is 21.4 μm in length and 4.1 μm in width. Sexine is much thicker than nexine () and sexine ornamentation is striate-perforate with the presence of granules in the area around the ectocolpus, differing markedly from the other areas of the sexine (, figure 20).
4. Discussion
The comparative analysis of pollen morphology is key for understanding evolutionary affinities between study plants. Despite the ongoing study of the Madeiran flora, there is still a considerable knowledge gap on using palynology to support plant systematics. Here, we contribute to fill this gap by describing and analysing the differences in pollen morphology of ten species included in the Madeira endemic genera.
Pollen grains of Musschia are spheroidal in shape, a feature shared with other genera in Campanulaceae (Khansari et al. Citation2012). The pollen grains of the two Musschia species do not show unique morphological features that could be used for their segregation from Campanula L. Characters such as pore number, size and shape of pollen grains are usually not useful for species discrimination within Campanula (sensu lato), but the size and density of spines on exine surface, together with pore diameter, usually have taxonomic value, particularly at species level (Dunbar Citation1975a, Citation1975b; Perveen and Qaiser Citation1999; Khansari et al. Citation2012). The pollen grains of the two Musschia species are similar, showing no significant differences in overall size (P) and shape (P/E), nor in echinae and pore size. However, they can be easily separated from those of the two other Macaronesian endemic bellflowers, Azorina vidalii (H.C. Watson) Feer and Canarina canariensis (L.) Vatke (Menezes et al. Citation2018). The pollen grains of the Azorean A. vidalii are tetraporate with anulus present and spiculate ornamentation while the Canarian C. canariensis are tricolporate with microreticulate/microgemmate ornamentation (Halbritter Citation2016; Morgado et al. Citation2017).
The pollen grains of Chamaemeles coriacea show the typical features found in other genera of Maleae (Rosaceae), in general, being tricolpate and tricolporate radially symmetrical monads (Hebda and Chinnappa Citation1990, Citation1994). In this group, the colpi occupies 75–80% of pollen grain length and exine ornamentation is striate macroperforate (Hebda and Chinnappa Citation1994). The phylogenetic affinities of Chamaemeles were recently studied, highlighting its distinctiveness and close relationship (supported by the combined analysis of the nuclear regions nrITS and GBSSI-2B) with other genera of Maleae, particularly with Malus and allied genera (Campbell et al. Citation2007; Potter et al. Citation2007; Sun et al. Citation2018). Considering the sculpturing pattern, the pollen grains of C. coriacea fit the group Ia (Hebda and Chinnappa Citation1994) by presenting striate macroperforate pollen with ridges long and parallel to the colpus, a feature shared with the phylogenetically related Malus.
The pollen grains of the two Apiaceae genera endemic to Madeira (Melanoselinum and Monizia) fit the pattern found in the members of this stenopalynous plant family. In general, pollen grains of Apiaceae are tricolporate, with shape varying from prolate to perprolate, and the ornamentation is generally striate or reticulate, more rarely rugulate, perforate or pertectate (Dogan Guner et al. Citation2011; Baczynski et al. Citation2021). The two study species showed significant differences in pollen grain morphology (P and E) and ornamentation that allows their discrimination. Also, the pollen grains of these two Madeira endemic genera show differences to their closed allies within Daucinae. Recent studies place Monizia and Melanoselinum (jointly with the Canarian endemic Cryptotaenia elegans Webb ex Bolle) as members of a Macaronesian clade within Daucus s.l. species complex (Spalik and Downie Citation2007; Banasiak et al. Citation2016). The pollen grains of the two Madeira endemic genera differ from those of the Azorean endemic Daucus carota subsp. azorica Franco (a representative of clade I) which are perprolate, have striate ornamentation and their size range between 37.5–45.0 µm (Morgado et al. Citation2017).
Sinapidendron, the Madeira endemic genus with the largest number of species, presents pollen grains prolate, tricolpate and with reticulate ornamentation, which are characteristic of many other members of the stenopalynous cabbage family (Brassicaceae) (El Ghazali Citation1993; Khalik et al. Citation2002). Pollen grain morphology is relatively homogeneous in Sinapidendron and show affinities to the close allied genera (e.g. Diplotaxis and Erucastrum) within Brassiceae (Warwick and Black Citation1993; Warwick and Sauder Citation2005). We were able to identify significant differences on the average values of pollen grain size standard measurements (P and E), although range size values strongly overlap between the Sinapidendron species (). In general, S. frutescens has the largest pollen grains of this Madeira endemic genus, and S. gymnocalyx the smallest ones, but non-significant differences in pollen grain size and shape were found in some species pairs comparisons. The strong similarity between Sinapidendron pollen grains may be due to the relatively recent age of Madeira island, approximately 5MY (Fernández-Palacios Citation2010). This period allowed the plant lineage to differentiate from its ancestral taxa, but the diversification process that followed, originating the five Madeiran Sinapidendron species, was more recent and probably insufficient to be reflected in terms of pollen grain morphology.
Overall, our findings fill the knowledge gap on the pollen morphology of Madeira endemic genera, contribute to the ongoing taxonomic work on Madeira archipelago vascular plants, and provide support to previous taxonomic findings from other scientific areas (e.g. Takahata and Hinata Citation1986; Banasiak et al. Citation2016; Spínola and Castilho Citation2016; Menezes et al. Citation2018; Frankiewicz et al. Citation2020).
5. Conclusions
In this work, we characterised for the first time the pollen morphology of ten species from the five endemic genera of the Madeira archipelago and identified differences in pollen size and shape between congeners to allow species discrimination. Also, we discussed the differences in pollen morphology between the study species and their close allies. Our results show the importance of palynological studies to discriminate closely related endemic plant species that have diversified in this oceanic archipelago in the last few million years, reinforcing previous taxonomic findings from morphological, ecological, histochemical and molecular studies. This contribution to the pollen inventory of Madeira follows previous studies characterising the endemic plants from the Macaronesian islands, provides information of taxonomic value and may support studies on the vegetation dynamics in space and time in this oceanic archipelago.
Acknowledgements
The authors thank Isamberto Silva from the Instituto das Florestas e Conservação da Natureza (Funchal, Madeira, Portugal) and Célia Bairos for their help locating several plant populations. Carlos Lobo and Luisa Gouveia, from the Jardim Botânico da Madeira – Eng. Rui Vieira, kindly sent us samples of M. edulis, and Fernando Pereira helped with the deposition of samples in the herbarium of the University of the Azores. The authors are also thankful to the two reviewers for their constructive comments that helped to improve the manuscript.
Disclosure statement
The authors declare that they have no conflict of interest.
Additional information
Funding
References
- Baczynski J, Milobedzka A, Banasiak L. 2021. Morphology of pollen in Apiales (Asterids, Eudicots). Phytotaxa. 478:1–32.
- Banasiak Ł, Wojewódzka A, Baczyński J, Reduron J, Piwczyński M, Kurzyna-Młynik R, Gutaker R, Czarnocka-Cieciura A, Kosmala-Grzechnik S, Spalik K. 2016. Phylogeny of Apiaceae subtribe Daucinae and the taxonomic delineation of its genera. Taxon. 65:563–585.
- Boieiro M, Aguiar AF, Rego C, Borges PAV, Serrano ARM. 2015. The biodiversity of terrestrial arthropods in Madeira and Selvagens archipelagos. Revista Ibero Diversidad Entomologica Accessible. 6:1–21.
- Borges PAV, Abreu C, Aguiar AMF, Carvalho P, Jardim R, Melo I, Oliveira P, Sérgio C, Serrano ARM, Vieira P. 2008. A list of the terrestrial fungi, flora and fauna of Madeira and Selvagens archipelagos. Funchal and Angra do Heroísmo: Direcção Regional do Ambiente da Madeira and Universidade dos Açores.
- Campbell C, Evans R, Morgan D, Dickinson TA, Arsenault MP. 2007. Phylogeny of subtribe Pyrinae (formerly the Maloideae, Rosaceae): limited resolution of a complex evolutionary history. Plant Systematics and Evolution. 266:119–145.
- Carvalho M. 2011a. Sinapidendron angustifolium. The IUCN Red List of Threatened Species 2011: e.T180554A7646995.
- Carvalho M. 2011b. Sinapidendron frutescens. The IUCN Red List of Threatened Species 2011: e.T180555A7647204.
- Dogan Guner E, Duman H, Pinar NM. 2011. Pollen morphology of the genus Seseli L. (Umbelliferae) in Turkey. Turkish Journal of Botany. 35:175–182.
- Dunbar A. 1975a. On pollen of Campanulaceae and related families with special reference to the surface ultrastructure, I. Campanulaceae subfam. Campanuloidae. Botaniska Notiser. 128:73–101.
- Dunbar A. 1975b. Pollen of Campanulaceae and related families with special reference to the surface ultrastructure. II. Campanulaceae subfam. Cyphioideae and subfam, Lobelioideae, Goodeniaceae, Sphenocleaceae. Botaniska Notiser. 128:102–118.
- El Ghazali GE. 1993. A study on the pollen flora of Sudan. Review of Palaeobotany and Palynology. 76:99–345.
- Erdtman G. 1952. Pollen morphology and plant taxonomy. Angiosperms. Stockholm: Almqvist and Wiksell. p. 133–134.
- Erdtman G. 1966. Pollen morphology and plant taxonomy – Angiosperms. Stockolm: Almqvist and Wiksell. p. 553.
- Fernandes F, Carvalho JA. 2014. An historical review and new taxa in the Madeiran endemic genus Monizia (Apiaceae, Apioideae). Webbia. 69:13–37.
- Fernández-Palacios JM. 2010. The islands of Macaronesia. In: Serrano ARM, Borges PAV, Boieiro M, Oromí P, editors. Terrestrial arthropods of Macaronesia – biodiversity, ecology and evolution. Lisboa: Sociedade Portuguesa de Entomologia, p. 1–30.
- Frankiewicz KE, Oskolski A, Banasiak Ł, Fernandes F, Reduron JP, Reyes Betancort JA, Szczeparska L, Alsarraf M, Baczyński J, Spalik K. 2020. Parallel evolution of arborescent carrots (Daucus) in Macaronesia. American Journal of Botany. 107(3):394–412.
- Halbritter H. 2016. Canarina canariensis. In: PalDat – a palynological database. https://www.paldat.org/pub/Canarina_canariensis/300968;jsessionid=F4CB91B95C2F75A930DEBCFA50087B98.
- Hebda RJ, Chinnappa CC. 1990. Studies on pollen morphology of Rosaceae in Canada. Review of Palaeobotany and Palynology. 64:103–108.
- Hebda RJ, Chinnappa CC. 1994. Studies on pollen morphology of Rosaceae. Acta Botanica Gallica. 141:183–193.
- Jardim R, Francisco D. 2000. Endemic flora of Madeira. Funchal: Múchia Publicações.
- Jardim R, Sequeira MM. 2008. The vascular plants (Pteridophyta and Spermatophyta) of the Madeira and Selvagens archipelagos. In: Borges PAV, Abreu C, Aguiar AMF, Carvalho P, Jardim, R, Melo I, Oliveira P, Sérgio C, Serrano ARM, Vieira P, editors. A list of the terrestrial fungi, flora and fauna of Madeira and Selvagens archipelagos. Funchal and Angra do Heroísmo: Direcção Regional do Ambiente da Madeira and Universidade dos Açores, p. 157–207.
- Kell SP. 2011a. Sinapidendron rupestre. The IUCN Red List of Threatened Species 2011: e.T162258A5565500.
- Kell SP. 2011b. Sinapidendron sempervivifolium. The IUCN Red List of Threatened Species 2011: e.T165227A115870710.
- Khalik KA, van den Berg RG, van der Maesen LJG, El Hadidi MN. 2002. Pollen morphology of some tribes of Brassicaceae from Egypt and its systematic implications. Feddes Repertorium. 113:211–223.
- Khansari E, Zarre S, Alizadeh K, Attar F, Aghabeigi F, Salmaki Y. 2012. Pollen morphology of Campanula (Campanulaceae) and allied genera in Iran with special focus on its systematic implication. Flora. 207:203–211.
- La Serna-Ramos IE. 1996. Pollen characters of Canary Resedaceae with special reference to endemic taxa. Grana. 35:16–23.
- La Serna-Ramos IE, Padron-Mederos MA. 2008. Pollen morphology of endemic species of the Gonospermum Less., Lugoa DC. and Tanacetum L. complex (Asteraceae: Anthemideae) in the Canary Islands (Spain), and its taxonomical implications. Grana. 47:247–261.
- La Serna-Ramos IE, Sosa LN, Perez de Paz PL. 1994. A palynological study of the genus Sideritis subgenus Marrubiastrum (Lamiaceae): Macaronesian endemism. Grana. 33:21–37.
- León-Arencibia MCL, Serna-Ramos IE. 1992. Palynological study of Lavandula (sect. Pterostoechas, Labiatae): Canario-maderiense endemics. Grana. 31:187–195.
- Médail F, Quézel P. 1999. Biodiversity hotspots in the Mediterranean Basin: setting global conservation priorities. Conservation Biology. 13:1510–1513.
- Menezes de Sequeira M, Jardim R, Gouveia M, Góis-Marques CA, Eddie WMM. 2021. Population decline in the Critically Endangered Musschia isambertoi (Campanulaceae) endemic to Desertas Islands (Madeira Archipelago) calls for urgent conservation management. Journal for Nature Conservation. 60:125955.
- Menezes de Sequeira M, Jardim R, Silva M, Carvalho L. 2007. Musschia isambertoi M. Seq., R. Jardim, M. Silva & L. Carvalho (Campanulaceae), a new species from the Madeira Archipelago (Portugal). Anales del Jardín Botánico de Madrid. 64:135–146.
- Menezes T, Romeiras MM, de Sequeira MM, Moura M. 2018. Phylogenetic relationships and phylogeography of relevant lineages within the complex Campanulaceae family in Macaronesia. Ecology and Evolution. 8(1):88–108.
- Morgado LN, Gonçalves-Esteves V, Resendes R, Mateus Ventura MAM. 2017. A pollen inventory of endemic species from the Azores archipelago, Portugal. Palynology. 42:273–289.
- Morgado LN, Gonçalves-Esteves V, Resendes R, Ventura MAM. 2015. Pollen morphology of Poaceae (Poales) in the Azores, Portugal. Grana. 54:282–293.
- Morgado LN, Resendes R, Moura M, Ventura MAM. 2014. Pollen resources used by Chrysoperla agilis (Neuroptera: Chrysopidae) in the Azores, Portugal. European Journal of Entomology. 111:143–146.
- Myers N, Mittermeier R, Mittermeier C, Fonseca G, Kent J. 2000. Biodiversity hotspots for conservation priorities. Nature. 403(6772):853–858.
- Padrón-Mederos MA, La Serna-Ramos IE. 2011. Pollen morphology of genus Allagopappus Cass. (Asteraceae: Inuleae), endemic to the Canary Islands, Spain. Plant Biosystems. 145:809–817.
- Perveen A, Qaiser M. 1999. Pollen flora of Pakistan. XIII. Campanulaceae. Turkish Journal of Botany. 2:45–51.
- Pinheiro de Carvalho MMA, Câmara IG, dos Santos TMM, Correia RS. 2001. Palynological study of the endemic woody Sonchus from the Flora of Madeira. A morphological and molecular approach. Polen. 11:67–77.
- Pinheiro de Carvalho MMA, Ornelas IR, Santos Dias J, dos Santos TMM, Paiva JA, Câmara IG. 2003. Palynological characterization of the endemic Asteroideae from the archipelago of Madeira. Boletim do Museu Municipal do Funchal. 54:5–23.
- Potter D, Eriksson T, Evans R, Oh S, Smedmark JEE, Morgan DR, Kerr M, Robertson KR, Arsenault M, Dickinson TA, et al. 2007. Phylogeny and classification of Rosaceae. Plant Systematics and Evolution. 266:5–43.
- Press JR, Short MJ. 1994. Flora of Madeira. London: HMSO.
- Punt W, Hoen PP, Blackmore S, Nilsson S, Le Thomas A. 2007. Glossary of pollen and spore terminology. Review of Palaeobotany and Palynology. 143:1–81.
- Spalik K, Downie SR. 2007. Intercontinental disjunctions in Cryptotaenia (Apiaceae, Oenantheae): an appraisal using molecular data. Journal of Biogeography. 34:2039–2054.
- Spínola V, Castilho PC. 2016. Phytochemical profile, chemotaxonomic studies, and in vitro antioxidant activities of two endemisms from Madeira Archipelago: Melanoselinum decipiens and Monizia edulis (Apiaceae). Chemistry & Biodiversity. 13(10):1290–1306.
- Sun J, Shi S, Li J, Yu J, Wang L, Yang X, Guo L, Zhou S. 2018. Phylogeny of Maleae (Rosaceae) based on multiple chloroplast regions: implications to genera circumscription. BioMed Research International. 2018:7627191.
- Takahata Y, Hinata K. 1986. A consideration of the species relationships in subtribe Brassicinae (Cruciferae) in view of cluster analysis of morphological characters. Plant Species Biology. 1:79–88.
- Warwick SI, Black LD. 1993. Molecular relationships in subtribe Brassicinae (Cruciferae, tribe Brassiceae). Canadian Journal of Botany. 71:906–918.
- Warwick SI, Sauder C. 2005. Phylogeny of Tribe Brassiceae (Brassicaceae) based on chloroplast restriction site polymorphisms and nuclear ribosomal internal transcribed spacer and chloroplast trnL intron sequences. Canadian Journal of Botany. 83:467–483.
