 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Ozonated oil has a long tradition in medical therapy. Here, the results from previous studies regarding the antibacterial effects of ozonated oils were compared. In addition, the aim of this study was to further examine the antibacterial activity against Escherichia coli for two different ozonated oils, in regard to the ozone exposure time and amount. Using agar dilution, the minimum inhibitory concentration of the oils was studied. For all of the tested concentrations with the agar dilution method, bacterial growth was observed. Furthermore, agar dilution was combined with spread-plating to determine the inhibition zone with and without the emulsifier. The emulsifier-free and emulsifier-containing experiments with agar dilution and spread-plating did not result in an inhibition of bacterial growth by ozonated oils. Moreover, the bacteria were exposed to the oils for various intervals before being grown on either solid or liquid medium to determine the time-dependent antibacterial effects of the ozonated oil. For both media, the results were compared to non-ozonated oil and NaCl solution as a negative control. The bacteria in the solid medium were already completely inactivated after 5 min of exposure with the ozonated oil. For the liquid medium, also shorter exposition times were investigated. After 1 min, there was no inhibition in the ozonated oils with the liquid medium. After 5 min, the bacterial growth in the ozonated oils was significantly reduced in the liquid medium.
Introduction
Bacterial resistance against antibiotics has become a serious problem in the last few decades. Antibiotics against bacterial infections are increasingly losing their effectiveness, leading to severe disease progression, primarily in hospital environments. Alternative treatments with antimicrobial substances offer help in these cases. Ozone can be an effective alternative to conventional therapies based on antibiotics and anti-inflammatory drugs because of its disinfectant properties (Song et al. Citation2018). However, the application of ozone is associated with several challenges, e.g., it is difficult to store, inhalation is harmful, and applying ozonated gas to wounds is difficult (Derco et al. Citation2018). Therefore, ozone is mostly applied in clinical settings (Anzolin, da Silveira-Kaross and Bertol Citation2020). Alternatively, ozonated oils can be used, which are easier to apply and store. Ozonated oils have been used at least since World War I as an effective therapeutic agent for the treatment of chronic wounds, including ulcers, and significantly improve healing outcomes (Derco et al. Citation2018; Stoker Citation1916). Since then, ozonated oils have proven in many clinical studies to be one of the best topical disinfectants for chronic wounds, inactivating not only bacteria but also viruses and fungi (Derco et al. Citation2018).
Numerous studies have investigated the antimicrobial effect of ozonated oils with different strains, a variety of methods, and diverging results. Gram-negative strain Escherichia coli (E. coli) is used in the majority of studies (de Almeida Kogawa et al. Citation2015; Díaz et al. Citation2006; Díaz et al. Citation2012; Lezcano et al. Citation2000; Moureu et al. Citation2015; Rodrigues et al. Citation2004; Sechi et al. Citation2001; Serio et al. Citation2017; Skalska et al. Citation2009) whilst Staphylococcus aureus (S. aureus) is primarily used representing Gram-positive species (de Almeida Kogawa et al. Citation2015; Díaz et al. Citation2006; Díaz et al. Citation2012; Lezcano et al. Citation2000; Montevecchi et al. Citation2013; Moureu et al. Citation2015; Rodrigues et al. Citation2004; Sechi et al. Citation2001; Serio et al. Citation2017; Song et al. Citation2018). Other strains like Staphylococcus epidermidis (S. epidermidis) (Lezcano et al. Citation2000; Sechi et al. Citation2001) and MRSA (Song et al. Citation2018) were investigated only in a minority of studies. A systematic overview of the different studies and their contradicting results is listed in Table S1 and Table S2 in Appendix A, respectively. The main culture media used were Mueller–Hinton (MH), Brain Heart Blood (BHB), or lysogeny broth (LB) (e.g., Lezcano et al. (Citation2000); Grootveld et al. (Citation2004); Serio et al. (Citation2017)). To allow for better dispersion of oil in water-based media, emulsifying agents like Tween-80 (de Almeida Kogawa et al. Citation2015; Lezcano et al. Citation2000; Moureu et al. Citation2015; Sechi et al. Citation2001) or Dimethylsulfoxide (DMSO) (Song et al. Citation2018) were added. Many studies do not report which media and if or which emulsifier were used (Díaz et al. Citation2006; Díaz et al. Citation2012; Montevecchi et al. Citation2013; Rodrigues et al. Citation2004; Serio et al. Citation2017; Skalska et al. Citation2009; Song et al. Citation2018). The oil type carrying the antimicrobial agent (ozone) used in previous studies was mainly ozonated sunflower oil (de Almeida Kogawa et al. Citation2015; Díaz et al. Citation2006; Díaz et al. Citation2012; Lezcano et al. Citation2000; Moureu et al. Citation2015; Rodrigues et al. Citation2004; Sechi et al. Citation2001; Serio et al. Citation2017; Skalska et al. Citation2009). In addition, the ozonated olive oil (Díaz et al. Citation2006; Montevecchi et al. Citation2013), ozonated linseed oil (de Almeida Kogawa et al. Citation2015), ozonated baru oil (de Almeida Kogawa et al. Citation2015), as well as the ozonated camellia oil (Song et al. Citation2018) were applied. Agar dilution (de Almeida Kogawa et al. Citation2015; Díaz et al. Citation2006; Díaz et al. Citation2012; Lezcano et al. Citation2000; Sechi et al. Citation2001) and microdilution (Moureu et al. Citation2015) were mainly applied to determine the minimum inhibitory concentration (MIC). Other studies determined the inhibition zone by conducting agar diffusion tests (Montevecchi et al. Citation2013; Rodrigues et al. Citation2004; Serio et al. Citation2017; Song et al. Citation2018). Furthermore, the macrodilution method used the minimum bactericidal concentration as the main measurement parameter (Díaz et al. Citation2006; Díaz et al. Citation2012; Lezcano et al. Citation2000). Lezcano et al. (Citation2000) determined the correlation between contact time (ozonated oil with bacteria) and live cell count, whereas the minimum peroxide value was the predominant parameter in the study conducted by Skalska et al. (Citation2009).
The contradictory information and lack of systematic analyses of the antimicrobial effect of ozonated oils were our motivation to conduct this study, where we investigated the time-dependent effects of exposing E. coli cells to ozonated oils in a kinetic analysis. This study reports a detailed experimental procedure and the results of the antibacterial effect of ozonated oils on E. coli K-12.
Materials and methods
Materials
E. coli K-12 MG1655 was used as the test strain. Growth was carried out in LB (10 g l-1 tryptone, 5 g l-1 yeast extract, 10 g l-1 NaCl) or plates supplemented with 2% agar-agar. An emulsifying agent, Tween-80 (2%, v/v), was used if applicable. Ozonated oils were obtained from Ozolife® (Valencia, Spain, Ozone Oil 600) as well as from ACTIVOZONE (Pontevedra, Spain, Ozone Oil 1200). As specified by the manufacturers, the peroxide values of the oils contained 600 meq kg−1 and 1200 meq kg−1 10%, respectively. Ozone Oil 600 consists of ozonated sunflower oil, olive oil, tea tree oil, ascorbyl palmitate, and tocopherol acetate. Ozone Oil 1200 includes ozonated sunflower oil and ozonated olive oil. Sunflower oil (Brölio, Hamm, Germany) was used as a negative control throughout all experiments. In order to determine the actual concentration of the peroxide value for both ozonated oils, an examination in an external laboratory was commissioned (my-lab International GmbH, Berlin, Germany). According to the test results, the peroxide value of Ozone Oil 1200 is 428.4 meq kg−1 and for Ozone Oil 600 it is 92.1 meq kg−1.
Plating and CFU counts
The cultures of E. coli were grown overnight and diluted to an optical density at a wavelength of 600 nm (OD600) of 0.1, corresponding to 1.5 × CFU ml−1 (Trau Citation2019). To prevent heat-based ozone decomposition, plate casting was carried out at the lowest temperature possible (55 °C). Oil was mixed with media in the following concentrations: 1 mg ml−1, 15 mg ml−1, and 30 mg ml−1 and subsequently vortexed for 60 s. 100 µl standardized bacteria dilution was added to each plate, and plates were incubated for 18 h at 37 °C. The colony forming unit (CFU) count was evaluated via manual counting and different automatic cell counters (Promega Colony Counter by Promega Cooperation, APD Colony Counter by APD Lab, and Microbial Colony Counter by MLTool Technologies).
Minimum inhibitory concentration determination
The MIC determination was carried out as described in the previous work (de Almeida Kogawa et al. Citation2015; Díaz et al. Citation2006; Díaz et al. Citation2012; Lezcano et al. Citation2000; Sechi et al. Citation2001), based on the agar dilution method without an emulsifier, according to the Clinical and Laboratory Standard Institute (CLSI). All experiments were carried out in triplicate with Ozone Oil 1200 at concentrations of 30 mg ml−1 and 45 mg ml−1. A bacterial suspension with an OD600 of 0.01 was prepared. Two microliters of the suspension were placed as a spot on agar plates. The inoculated plates were incubated at room temperature until the spots were absorbed. Afterward, they were incubated at 37 °C for 18 h.
Inhibition zone determination
In addition to the CLSI method, spread-plating CFU counts were conducted, whereby bacteria were spread evenly onto the plate instead of placing spots. Oil concentrations of 1 mg ml−1, 15 mg ml−1, and 30 mg ml−1 with Ozone Oil 600 were used. Plates were incubated at 37 °C for 18 h, and the experiments were carried out in triplicate. For CFU counts without an emulsifier, oil and medium were mixed by vortexing. A bacterial suspension with LB medium as diluent and an OD600 of 0.1 was prepared in 2 ml eppis. These were diluted with LB medium at a total dilution of 1/3 × . Emulsifier-containing plates were supplemented with 2% Tween-80 according to Sechi et al. (Citation2001) and de Almeida Kogawa et al. (Citation2015). Inoculation was carried out with 100 µl. Mixing of medium and emulsifier: since the optimal dispersion of oil in agar-medium depends on proper detergent distribution, two possible scenarios were tested. First, 500 µl of Tween-80 were mixed with 25 ml agar in a falcon tube and mixed for 10 s before oil was added. After a second 10 s vortexing, plates were casted. In the second scenario, 500 µl Tween-80 and oil were placed together in a falcon tube and vortexed for 10 s before agar-medium (25 ml) was added and vortexed again. Here, three different mixing times of 20 s, 40 s, and 60 s were tested.
Kinetic analysis with liquid and solid medium
Kinetic analyses with liquid medium were conducted according to Song et al. (Citation2018) with minor modifications. Briefly, 900 µl ozonated oils were mixed with 100 µl bacteria suspension (cell count/ml in 0.9% NaCl), vortexed for 10 s, and incubated in Eppendorf tubes for 5 min, 10 min, 20 min, and 60 min, respectively. Fifty microliters of the oil/bacteria suspension were added to 10 ml of liquid LB in culture tubes. The incubation was carried out at 37 °C on a shaker (180 rpm) for 20 min, 60 min, and 120 min. The ozone exposure times were between 1 min and 60 min and the OD600 was measured after 0 min, 20 min, 60 min, and 90 min. The final OD600 was determined after 18 h of growth.
In addition to the liquid medium, kinetic analyses were also conducted using the solid medium. Following the procedure described for the liquid medium, 50 µl of this mixture was then transferred to the agar plates and spread evenly. Plates were incubated for 18 h at 37 °C before CFU were counted.
Results
Minimum inhibitory concentration determination
This experiment investigates the MIC of the ozonated oil against E. coli based on agar dilution without emulsifier. Despite very high concentrations of the ozonated oil, bacterial growth is observed at all selected concentrations. Therefore, no MIC can be determined at this point. Testing at even higher concentrations is not performed, as the production of a stable emulsion of oil and nutrient medium becomes increasingly challenging at higher concentrations.
Inhibition zone determination
This experiment is performed in two variants, the emulsifier-containing variant and the emulsifier-free variant. The results for the emulsifier-free variant show no inhibition of bacterial growth by the ozonated oil compared to the control plates with the non-ozonated oil. The CFUs per plate are counted by hand, and the results are shown in . In summary, all values, including those of the control groups, are in the same order of magnitude, and the correlation between CFUs and ozone quantity is not discernible. A statistical evaluation is not carried out because only a sample could be counted for some groups due to colonies that could not be identified individually.
Table 1. Counting of CFUs grown in the inhibition zone determination experiments for the emulsifier-free variant with E. coli as test strain and LB as culture medium. Ozone Oil 600 was applied as an antimicrobial agent, and non-ozonated oil and pure LB medium were employed as negative control agents. All experiments were conducted in triplicate (n = 3) and the oils (ozonated and non-ozonated) were used in three difference concentrations c1, c15, and c30.
Furthermore, the experiments are conducted for the emulsifier-containing variant. The experiment with Tween-80 as an emulsifier is carried out in two ways, which differ in regard to the mixing order of Tween-80, oil, and culture medium. The plates for the first mixing alternative are prepared by first mixing Tween-80 with LB medium, adding the oil in the respective concentrations in the next step, and then homogenizing the whole mixture. The control plates are prepared with non-ozonated oil at concentrations of 1 mg ml−1, 15 mg ml−1, and 30 mg ml−1, respectively. The experimental plates are made with Ozone Oil 600 at the same concentrations. The size and number of individual colonies do not differ significantly, irrespective of the concentration, both for the experimental plates treated with ozonated oil and for the control plates. On closer inspection, Tween-80 clumps can be seen in the medium on most plates. Due to irregularities, such as partially uncountable cell clusters and air bubbles impeding quantitative counts, a quantitative evaluation in the form of CFU counts was not conducted.
The emulsifier-containing variant is also performed with a second mixing alternative. The experimental plates of the second mixing alternative are prepared by first mixing Tween-80 with ozonated oil, adding LB medium in the next step, and then mixing the entire medium. The mixing time for homogenizing LB medium and Tween-80 oil mixtures varied between 20 s, 40 s, and 60 s. All plates were made with Ozone Oil 600 at concentrations of 15 mg ml−1 and 30 mg ml−1. The plates with concentrations of 30 mg ml−1 are similar in terms of number, morphology, and size of colonies. Plates with a concentration of 15 mg ml−1 are difficult to assess due to the presence of some cell clusters. The control plates prepared with agar medium and Tween-80 as well as with agar medium alone are comparable to those of the emulsifier-free mixing variant. For the reasons already mentioned above, the CFUs are not counted here either.
The plates made according to the first mixing alternative show significantly more foam and air bubbles, as well as Tween-80 clumps in the medium. Therefore, the second mixing alternative is better suited than the first mixing alternative. The results for the emulsifier-free and the emulsifier-containing variants both show no inhibition of bacterial growth by the ozonated oil compared to the control plates with the non-ozonated oil.
Kinetic analysis with liquid and solid media
For the kinetic analysis with liquid medium, OD600 measurements are conducted. The initial OD600 values at 0 min are distinctly higher for the ozonated oils than for the non-ozonated oil and NaCL treated tubes. The OD600 decreases with increasing time in the incubator for most groups tested. A greater decrease in values is seen for the experimental groups with ozonated oil. shows the results for the Ozone Oil 1200 and for the Ozone Oil 600. The ozone oil exposure times from left-to-right were 1 min, 5 min, 20 min, and 60 min. For both oils, the liquid is very turbid after 1 min. Starting at 5 min, it becomes much clearer and remains unchanged from this time interval onwards for higher exposure times. For the Ozone Oil 1200 for 5 min and 20 min, small and large clumps are visible. In the remaining tube, only small particles are visible. shows the control groups. There, the left tubes are treated with an oil or NaCl suspension and incubated for 1 min, and the right tubes for 60 min. Visible are turbid liquids containing a small number of small particles. The appearance of all four tubes does not differ significantly.
Figure 1. Resulting tubes with E. coli as test strain for liquid medium kinetic analysis. Ozone Oil 1200 and Ozone Oil 600 are used as antimicrobial agent, non-ozonated oil and NaCl are applied as negative control agent, and LB is employed as culture medium. The exposure times are for all agents between 1 min and 60 min. As with the solid medium kinetic analysis, cloudy spots are observed for the Ozone Oil 1200.
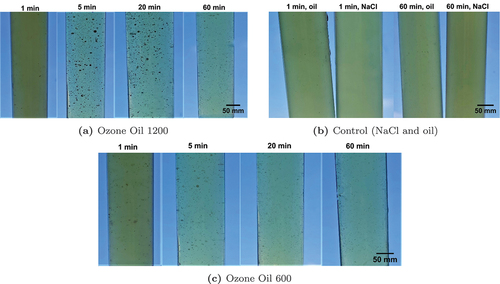
When comparing an exposure time of 1 min for the Ozone Oil 1200 and the non-ozonated oil, the two tubes look very similar, except for the liquid of the experimental group is clearer compared to the control group. In general, the number of particles in the experimental group is significantly higher than in the control group. The OD600 is not determined after 18 h incubation, as the particles and clumps interfere with the measurements and therefore bias the result. Since there is no difference between the experimental group and the control group after 1 min, the short exposure time is not sufficient to noticeably influence growth. From an exposure time of 5 min, bacterial growth is reduced for both ozonated oils. The results confirm the conclusion drawn for solid medium experiments that bacterial growth is considerably too completely inhibited for both oils already from an exposure time of 5 min. The use of an emulsifier in the experiments is not necessary.
The results of the kinetic analysis with solid medium are shown in The plates of the upper row are treated with Ozone Oil 600 and the plates of the second row with Ozone Oil 1200. The two lower rows show the control plates on which non-ozonated oil or NaCl solution is spread. The replicates of all plates show comparable results.
Figure 2. Resulting Petri dishes with E. coli as test strain for solid medium kinetic analysis and different exposure times and antimicrobial agents or negative control agents. Ozone Oil 600 and Ozone Oil 1200 are compared as antimicrobial agents and non-ozonated oil and NaCl as negative control agents for exposure times of 5 min, 10 min, 20 min, 60 min, and 90 min, respectively. As culture medium 0.9% NaCl is used.

The exposure time is 5 min, 10 min, 20 min, 1 h, and 1.5 h from left to right. The control groups in the two lower rows show pronounced growth of colonies. The Ozone Oil 600 completely inhibited bacterial growth already after 5 min of exposure. In the case of the Ozone Oil 1200 plates, individual circular colonies cannot be seen on any of the plates, but small spots can be seen on each plate, which appear milky and cloudy in daylight. Therefore, the plates are also investigated with a microscope. On the control plates, a large number of rod-shaped E. coli bacteria are visible with the microscope. On the experimental plates treated with the Ozone Oil 1200 and an exposure time of 90 min, cloudy spots are visible with the microscope. The microscopic examinations reveal that the cloudy spots on the experimental plates are not E. coli, but another substance that may have been caused by additives or impurities in the oil. No growth was visible after 5 min of ozone incubation. Therefore, counting the CFU of the control plates with NaCl solution or non-ozonated oil is omitted. The kinetic analysis with solid medium is carried out according to the procedure from Song et al. (Citation2018), except for omitting an additive for mixing. The results can be compared with the present work, but with respect to the different cell types. According to Song et al. (Citation2018), the inactivation rate of S. aureus and S. epidermidis with camellia oil is 100% after 5 min. Thereby, the results are comparable to those of the present work.
Discussion
For the performed agar dilution for determining the MIC, the tested Ozone Oil 1200 induces a reduction in growth of the E. coli bacteria. However, a comparison with the literature shows that the here tested oil, despite comparatively quite high peroxide numbers, according to the manufacturer, has a significantly lower antimicrobial effect. This may be because of an unsuitable process for mixing and casting the plates, as there is little information about this provided in the previous work. In most cases, no precise information is given on how to carry out the experiment, but reference is made to the guideline. In particular, the procedure for mixing the individual components is not described. Here, too, the guideline cannot be followed, as it was designed for studies of antibiotic agents, which have a different mixing behavior than oil. Unlike the studies presented in previous works, no emulsifier was used. Instead of using an emulsifier, the ozonated oil was mixed with the LB medium by using a vortexer due to the results of preliminary experiments. Although according to Iten (Citation2010) and Remmal et al. (Citation1993), emulsifiers such as Tween-80 negatively influence the antibacterial effect of the oils, the oils nevertheless performed better in the studies presented. Even for oils with similar peroxide values, a strong deviation of the MIC in the previous research is observed. This is also the case for the current results compared to the previous research results. Furthermore, the antibacterial activity of an oil against bacteria can also vary within a bacterial species, as Sechi et al. (Citation2001) shows. Two different E. coli strains tested gave a MIC of 4.75 mg ml−1 and 1.18 mg ml−1, respectively. Thus, only studies testing the same bacterial strain are comparable. The large discrepancy between the previous research results and between the results here compared to the previous results shows again that standardized norms for the production and evaluation of ozonated oils are necessary. Furthermore, a more precise documentation of the mixing process and the casting of the plates is indispensable.
Furthermore, experiments with the agar dilution method combined with spread-plating were conducted to determine the inhibition zone. Here, the antibacterial activity of the examined oils cannot be determined. For the comparison with previous studies (de Almeida Kogawa et al. Citation2015; Lezcano et al. Citation2000; Sechi et al. Citation2001), which used Tween-80 as emulsifier, information on the mixing process is missing. The only exception is the experiment by Lezcano et al. (Citation2000), for which it was stated that the mixing process was supported by ultrasound. However, exact experimental parameters are also missing, which makes reproduction impossible. It is therefore possible that Tween-80 showed only moderate success as an emulsifier in the present investigations, as it was applied differently. According to Iten (Citation2010) and Donaldson et al. (Citation2005), Tween-80 inhibits the antibacterial effect of oils. Since no antibacterial effect of the oil is observed for both, the emulsifier-free and emulsifier-containing variant, this statement cannot be assessed. When spreading the bacterial suspension, the plates made with the emulsifier-containing variant behaved similarly to those made with the emulsifier-free variant. The changes in bacterial growth compared to the different control groups are probably not triggered by the ozone in the oil, but by other factors, such as the oil itself as well as the addition of an emulsifier. Too high temperatures of the medium may be a possible reason for the lack of antibacterial effect. As observed in a preliminary experiment with potassium iodide starch paper, ozone outgases from the oil at the temperatures needed to cast the agar plates. It is therefore possible that a certain amount of ozone is lost even before the plates are cast and solidified. Agar dilution combined with spread-plating proved to be less practicable, as the oil on the agar surface impeded adequate spreading of the bacteria.
For the kinetic analysis with solid medium, the inhibition rate is considered to be 100% for both ozonated oils, due to no detectable growth. The Ozone Oil 1200, despite complete E. coli bacterial growth inhibition, left cloudy spots on the plates, which cannot be assigned to a typical bacterial culture. For the liquid medium, the oils show comparable activity, except for the cloudy spots.
The quality of ozonated oil is a crucial factor in its effectiveness in medical therapy. The quality of ozonated oil depends on several parameters, such as quality and efficacy of the ozone generator, ozonation conditions, species contents, and reaction kinetics. Three indicators of the quality of ozonated oil are the iodine value, the acid value, and the peroxide value, which represent essential parameters for the therapeutic application of this form of ozone therapy. In this study, we used ozonated oil from a commercially available source, and while the iodine value and the acid value were not available on the manufacturer’s website, we provided the peroxide value in this work. It is important to note that recently, the OZONIA 3000 sunflower oil (Innovares Srl, Sant’Ilario d’Enza, Italy) was approved by the European Chemical Agency (ECHA) in accordance with REACH regulation. Further investigation regarding the antibacterial effect in medical therapy is especially crucial for this oil, given its certification.
However, the results strengthen the statement that ozonated oils have a destructive effect on bacteria and can therefore be used therapeutically to treat wounds infected with E. coli bacteria.
Summary and conclusion
Studies have shown the effectiveness of ozonated oils for treating chronic wounds when antibiotics are ineffective. However, there are inconsistencies in detection methods and a lack of standardized procedures in the previous work. We investigated the antibacterial effect of two ozonated oils on E. coli using agar dilution and spread plating. Unlike previous studies, we found no complete growth inhibition of E. coli despite high concentrations using the agar dilution method. Control experiments without oil but with Tween-80 as an emulsifier showed inhibition of bacterial colonies. The influence of ozone exposure time on bacteria was investigated in a kinetic analysis (solid and liquid media), and both oils showed complete inactivation of bacteria after 5 min. However, there are inconsistencies in the previous research due to different methods and parameters, as well as variations in ozonated oil production. The peroxide number can differ greatly from what the manufacturer has stated, as tests on our own oils have shown, which affects their effectiveness. Further studies and standardization of oils and test procedures are needed to investigate the safety and effectiveness of ozonated oils in treating chronic wounds.
Author on contributors
Conceptualization and methodology, L.P., A.-M.J., G.S., A.-K.K., and C.P.; validation, formal analysis and investigation, A.-M.J.; resources, A.-K.K.; writing – original draft preparation, L.P.; writing – review and editing, L.P., A.-M.J., R.-R.F.M., G.S., A.-K.K., and C.P.; visualization, A.-M.J. and R.-R.F.M.; supervision, L.P., G.S., A.-K.K., and C.P.; funding acquisition and project administration, C.P. All authors have read and agreed to the published version of the manuscript.
Supplemental Material
Download PDF (3.6 MB)Acknowledgments
We thank David Thiele for the laboratory briefing and the support during the experiments.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/01919512.2023.2233746.
Additional information
Funding
References
- Anzolin, A. P., N. L. da Silveira-Kaross, and C. D. Bertol. 2020. “Ozonated Oil in Wound Healing: What Has Already Been Proven?” Medical Gas Research 10 (1): 54–59. https://doi.org/10.4103/2045-9912.279985.
- de Almeida Kogawa, N. R., E. J. de Arruda, A. C. Micheletti, M. F. C. Matos, L. C. S. de Oliveira, D. P. de Lima, Nadia C. P. Carvalho, et al. 2015. “Synthesis, Characterization, Thermal Behavior, and Biological Activity of Ozonides from Vegetable Oils.” RSC Advances 5 (80): 65427–36. https://doi.org/10.1039/C5RA02798E.
- Derco, J., M. Koman, J. Derco, and M. Koman. 2018. Ozone in Nature and Practice. London: IntechOpen. https://doi.org/10.5772/intechopen.68925.
- Díaz, M. F., R. Hernández, G. Martnez, G. Vidal, M. Gómez, H. Fernández, and R. Garcés. 2006. “Comparative Study of Ozonized Olive Oil and Ozonized Sunflower Oil.” Journal of the Brazilian Chemical Society 17 (2): 403–07. https://doi.org/10.1590/S0103-50532006000200026.
- Díaz, M. F., Y. Sánchez, M. Gómez, F. Hernández, and M. D. Veloso. 2012. “Physicochemical Characteristics of Ozonated Sunflower Oils Obtained by Different Procedures.” Grasas y Aceites 63 (4): 466–74. https://doi.org/10.3989/gya.073212.
- Donaldson, J. R., S. L. Warner, R. G. Cates, and D. Gary Young. 2005. “Assessment of Antimicrobial Activity of Fourteen Essential Oils When Using Dilution and Diffusion Methods.” Pharmaceutical Biology 43 (8): 687–95. https://doi.org/10.1080/13880200500384932.
- Grootveld, M., A. Baysan, N. Sidiiqui, J. Sim, C. Silwood, and E. Lynch. 2004. “History of the Clinical Applications of Ozone.” In Ozone: The Revolution in Dentistry, edited by L. Abu-Naba'a, and E. Lynch, 23–30. London: Quintessence Publishing Company.
- Iten, F. 2010. “In-vitro Vergleiche der antimikrobiellen Wirkung von pflanzlichen Vielstoffgemischen, artifiziellen Stoffkombinationen und phytogenen Monosubstanzen am Beispiel des ätherischen Öls von Thymus vulgaris.” PhD diss., University of Zurich.
- Lezcano, I., N. Nuñez, M. Espino, and M. Gómez. 2000. “Antibacterial Activity of Ozonized Sunflower Oil, Oleozón, Against Staphylococcus Aureus and Staphylococcus epidermidis.” Ozone: Science & Engineering 22 (2): 207–14. https://doi.org/10.1080/01919510008547221.
- Montevecchi, M., A. Dorigo, M. Cricca, and L. Checchi. 2013. “Comparison of the Antibacterial Activity of an Ozonated Oil with Chlorhexidine Digluconate and Povidone-Iodine. A Disk Diffusion Test.” The New Microbiologica 36 (3): 289–302.
- Moureu, S., F. Violleau, D. A. Haimoud-Lekhal, and A. Calmon. 2015. “Ozonation of Sunflower Oils: Impact of Experimental Conditions on the Composition and the Antibacterial Activity of Ozonized Oils.” Chemistry and Physics of Lipids 186:79–85. https://doi.org/10.1016/j.chemphyslip.2015.01.004.
- Remmal, A., T. Bouchikhi, K. Rhayour, M. Ettayebi, and A. Tantaoui-Elaraki. 1993. “Improved Method for the Determination of Antimicrobial Activity of Essential Oils in Agar Medium.” Journal of Essential Oil Research 5 (2): 179–84. https://doi.org/10.1080/10412905.1993.9698197.
- Rodrigues, K. L., C. C. Cardoso, L. R. Caputo, J. C. T. Carvalho, J. E. Fiorini, and J. M. Schneedorf. 2004. “Cicatrizing and Antimicrobial Properties of an Ozonised Oil from Sunflower Seeds.” Inflammopharmacology 12 (3): 261–70. https://doi.org/10.1163/1568560042342275.
- Sechi, L. A., I. Lezcano, N. Nunez, M. Espim, I. Duprè, A. Pinna, P. Molicotti, et al. 2001. “Antibacterial Activity of Ozonized Sunflower Oil (Oleozon).” Journal of Applied Microbiology 90 (2): 279–84. https://doi.org/10.1046/j.1365-2672.2001.01235.x.
- Serio, F., G. Pizzolante, G. Cozzolino, M. D’Alba, F. Bagordo, M. De Giorgi, T. Grassi, et al. 2017. “A New Formulation Based on Ozonated Sunflower Seed Oil: In vitro Antibacterial and Safety Evaluation.” Ozone: Science & Engineering 39 (3): 139–47. https://doi.org/10.1080/01919512.2016.1272405.
- Skalska, K., S. Ledakowicz, J. Perkowski, and B. Sencio. 2009. “Germicidal Properties of Ozonated Sunflower Oil.” Ozone: Science & Engineering 31 (3): 232–37. https://doi.org/10.1080/01919510902838669.
- Song, M., Q. Zeng, Y. Xiang, L. Gao, J. Huang, J. Huang, K. Wu, and J. Lu. 2018. “The Antibacterial Effect of Topical Ozone on the Treatment of MRSA Skin Infection.” Molecular Medicine Reports 17 (2): 2449–55. https://doi.org/10.3892/mmr.2017.8148.
- Stoker, G. 1916. “The Surgical Uses of Ozone.” The Lancet 188 (4860): 712. https://doi.org/10.1016/S0140-67360131717-8.
- Trau, Y. 2019. “Direct E. Coli Cell Count at OD600.” Accessed June 1, 2022. https://tipbiosystems.com/wp-content/uploads/2020/05/AN102-E.coli-Cell-Count_2019_04_25.pdf.
