Abstract
Aims: To evaluate the feasibility and acceptability of vibration therapy (VT) in preschool children with cerebral palsy (CP) and obtain preliminary data on its potential effectiveness.
Methods: Nine children aged 2.5–4.8 years (4 boys) with CP GMFCS levels I–III participated in a single-group feasibility study, undergoing a 12-week control period without intervention, followed by 12 weeks of home-based VT (four times/week, 9 min/day, frequency 20 Hz). We assessed adherence to VT protocol, adverse events, and family acceptability of VT. Clinical assessments included motor function (GMFM-66), body composition (DXA), mobility (10-meter walk/run test), and health-related quality of life (PedsQL).
Results: VT was well tolerated and acceptable to families, with high adherence levels reported (mean = 93%). There were no observed between-period differences (ΔControl vs ΔVT) except for an improvement in the PedsQL "Movement & Balance" dimension with VT (p = 0.044). Nonetheless, changes after the VT but not the Control period were suggestive of potential treatment benefits for mobility, gross motor function, and body composition (lean mass and legs bone mineral density).
Conclusion: Home-based VT is feasible and acceptable for preschool children with CP. Our preliminary data suggest potential health benefits from VT for these children, supporting larger randomized trials to assess its effectiveness properly. Clinical trial registration number: Australian New Zealand Clinical Trials Registry (ACTRN12618002027291)
Introduction
Cerebral palsy (CP) is the most common cause of motor disorders in childhood, affecting approximately 1.5–3 of every 1000 live births (Michael-Asalu et al., Citation2019; Rosenbaum et al., Citation2007; Sadowska et al., Citation2020). Gross motor function impairment is considered a core feature of CP, leading to difficulties with walking, coordination, and musculoskeletal function, with a consequent reduction in physical activity and quality of life (Rosenbaum et al., Citation2007). CP is usually diagnosed by 18–24 months of age (Ashwal et al., Citation2004; Hubermann et al., Citation2016), with severity most accurately assessed between 2 and 5 years of age (Morgan et al., Citation2015; Patel et al., Citation2020). The early years are critical for motor function development in children due to neural plasticity (Cech & Martin, Citation2012). Rosenbaum et al. (Citation2002) demonstrated that children with CP reach 90% of their motor development potential by the age of 5 years or younger, depending on their GMFCS level (Rosenbaum et al., Citation2002). These findings are supported by Beckung et al. (Citation2007), who reported that the median age for reaching 80-90% motor function score was 5 years of age for children with GMFCS level I-II, and 7 years of age for children with GMFCS level III (Beckung et al., Citation2007).
The importance of early intervention to maximize long-term function in children with CP and the potential for living fully has been widely acknowledged (Blackman, Citation2002; Fiss & Jeffries, Citation2020; Granild-Jensen et al., Citation2015). However, little is known about the feasibility of therapeutic approaches in young children with CP (Morgan et al., Citation2016; Spittle & Morgan, Citation2018). Recent studies on treadmill training (Mattern-Baxter et al., Citation2013), conductive education, and physiotherapy (Mohamed et al., Citation2020) have shown improvements in motor development among children with CP aged between 9 and 48 months.
Vibration therapy (VT), as a type of weight-bearing therapeutic modality, has the potential to be implemented in the rehabilitation plan for children with CP younger than 5 years old. VT research suggests that it improves neuromuscular performance via reflex activation of alpha motor neurons, leading to a reflex contraction of the homonymous muscle (Rittweger, Citation2010). In addition, it has been suggested that VT stimulates proprioception, inducing spinal and cortical reorganization and, consequently, improving motor control (van Nes et al., Citation2004).
Side-alternating VT (sVT) has been used as a therapeutic tool. To date, several studies have reported sVT to be associated with improvements in motor function (Ibrahim et al., Citation2014; Martakis et al., Citation2019), muscle strength (El-Shamy, Citation2014; Ibrahim et al., Citation2014; Stark et al., Citation2010), balance (El-Shamy, Citation2014), bone density (Gusso et al., Citation2016; Stark et al., Citation2010), mobility (Gusso et al., Citation2016; Ibrahim et al., Citation2014; Ruck et al., Citation2010), and quality of life (Gusso et al., Citation2016) in children and young adults with CP, with no serious adverse effects reported (Ritzmann et al., Citation2018). sVT is thought to be better tolerated than other vibration modalities due to smaller vibration transmissibility to the head and upper body than, for example, in synchronous vibration (Abercromby et al., Citation2007).
There is still a paucity of data on the potential effects and feasibility of sVT in children younger than 5 years of age. The existing literature comprises two studies, with conflicting results on sVT impact on motor function. Stark et al. (Citation2010) included children aged 2–5 years among participants in a trial investigating the effectiveness of sVT in individuals with CP but across a broad age range (2–25 years), reporting improvements in gross motor function, bone density, and muscle force (Stark et al., Citation2010). However, no separate analyses were conducted for the group aged less than 5 years. Subsequently, the same authors (2016) reported no effects of sVT on gross motor function in young children aged 12–24 months but observed that it was a safe therapy (Stark et al., Citation2016). Further research is required to evaluate the potential of the sVT as an early intervention tool for young children with mild to moderate CP.
This feasibility study was designed to address this need. We aimed to assess the feasibility of 12 weeks of home-based sVT and to evaluate the associations between sVT and potential improvements in gross motor function, body composition, muscle function, and health-related quality of life in preschool children with CP.
Methods
Study Design
This feasibility study used a single-group design with all participants acting as their own controls (). After the baseline assessment (T0), participants underwent a 12-week lead-in ‘control’ period (T0-T1), followed by a pre-intervention assessment (T1). Immediately after the latter, participants started a 12-week intervention period (T1-T2), with the post-intervention assessment (T2) conducted 2–5 days after finishing the intervention. Throughout the study, participants continued with their ongoing healthcare and lifestyle (e.g., physiotherapy, conductive education, and swimming lessons) but were recommended to avoid starting any new activities.
This study followed the principles of the Declaration of Helsinki by adhering to all appropriate institutional and international guidelines and regulations for medical research. The study was approved by the Northern B Health and Disability Ethics Committee (19/NTB/1), with locality approval for recruitment granted by the Auckland and Waikato District Health Boards. The trial was prospectively registered at the Australian New Zealand Clinical Trials Registry (ACTRN12618002027291) on the 18 December 2018. Before entering the study, parents/caregivers provided written informed consent.
As this was a feasibility study, a sample size calculation was not conducted. We aimed to recruit approximately 20 participants between May 2019 and December 2020.
Participants
Nine children (mean age 3.6 ± 0.9 years; 4 boys) participated in the study, including three with GMFCS I, five with GMFCS II, and one with GMFCS III. The demographic and clinical characteristics of the study population at baseline are presented in .
Table 1. Demographic and clinical characteristics of participants.
Inclusion criteria were: (1) age 2 to 4 years 11 months; (2) CP diagnosis or an indication of having CP as per neurologist report, GMFCS level I—III (Palisano et al., Citation1997); (3) ability to understand and follow researcher’s instructions; (4) ability to safely stand on a vibration plate with or without support; (5) have no planned surgery within 5 months before/after entering the study.
The exclusion criteria were as follows: (1) a bone fracture within 12 weeks of enrollment; (2) history of using anabolic agents, glucocorticoids (excluding inhaled), or growth hormone (regardless of dose) for at least 1 month, within the 3 months prior to enrollment; (3) history of botulinum toxin injection into the lower limb(s) within the 3 months before enrollment; (4) history of an illness or findings on physical examination that might prevent the child from completing the study (e.g., acute thrombosis, tendinitis).
Recruitment
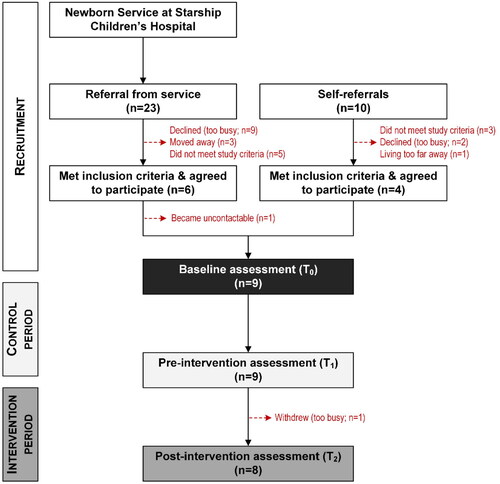
The recruitment and retention of participants throughout the study are shown in . Clinical data on potential participants recorded in the Auckland City Hospital neonatal database were screened by a neurodevelopmental psychologist, identifying infants at high risk of CP (Newborn Services at Starship Hospital). Records were processed chronologically, beginning with those born on the 1 January 2015, to identify children who appeared to meet the inclusion criteria. The contact details of families interested in participating in the study were provided to the research team, who screened their medical records and contacted those who met the inclusion criteria. Our research team was not provided with information on the number of records screened or families contacted by the neurodevelopmental psychologist, but 23 interested families were referred to the research team for eligibility. Five children who did not meet the inclusion criteria and three families who moved cities were excluded. The remaining 15 families were contacted, with nine declining participation due to busy family schedules. The remaining six children were enrolled in the study (). In addition, we were contacted by ten families who received information about the study through word-of-mouth; four were excluded for not meeting the study criteria, and two declined to participate after further information was provided. Thus, four children whose caregivers self-referred to the study were enrolled (). One family from the group referred from the service withdrew prior to the first assessment. Therefore, we assessed the data of 9 participants, including a child who withdrew two weeks after starting VT.
It is important to note that the study was conducted during the COVID-19 pandemic, which impacted the number of enrolled participants. We had to stop the recruitment earlier than the planned date due to government-imposed lockdowns and the consequent movement restrictions in the region (Baker et al., Citation2020).
Measures
All assessment visits were conducted at the Maurice and Agnes Paykel Clinical Research Unit at the Liggins Institute (University of Auckland) by the same study investigator who was unblinded to participants’ assessment visits (i.e., T0, T1, or T2). Data was saved on separate files and not reviewed after it was collected. All data was viewed and analyzed at the end of the study. The order of the assessment tests was the same at all three visits. Before each assessment, participants’ anthropometric data (i.e., height and weight) were measured and their body mass index (BMI) was subsequently calculated. Height, weight, and BMI were transformed into sex- and age-adjusted z-scores derived as per World Health Organization (WHO) standards (Group & de Onis, Citation2006).
Primary outcome measure
The primary outcome measure was the feasibility of using home-based VT in the study population group. To establish feasibility, we assessed adherence, side effects (e.g., transient pain, skin redness, itchiness, and warmness), and the acceptability of home-based VT by families. Adherence to the VT protocol was measured as a percentage rate (100% indicates all 48 sessions were completed) (Abbott et al., Citation1994). The number of sessions completed was extracted from the VT diary filled in by the caregivers, with a given VT session deemed to have been completed if the participant had performed all three VT sets. VT diaries were also used to collect information on side effects if participants had any; caregivers were asked to add notes at the end of each VT session. The data on the acceptability of home-based VT was gathered from caregivers at the third assessment visit (i.e., after the VT period). The criteria for recognizing home-based VT as feasible was agreed upon as follows: (1) average adherence level of 70% and more; and (2) no severe side effects reported (i.e., any effects likely caused by VT and leading to its termination, such as recurrent long-lasting pain of any localization, dizziness, worsening balance, hypotonia, and hypoglycaemia); and (3) more than 70% of participating families indicate that home-based VT is acceptable for their family lifestyle.
Secondary outcome measures
Secondary outcome measures included gross motor function, mobility, body composition, muscle strength, balance, and health-related quality of life.
Gross motor function was assessed using the Gross Motor Function Measure-66 (GMFM-66), a reliable and valid scale for applied research in children with CP (Russell et al., Citation2000; Wei et al., Citation2006). The GMFM-66 consists of five dimensions with a total of 66 items: (A) lying/rolling, (B) sitting, (C) crawling/kneeling, (D) standing, and (E) walking/running/jumping (Russell et al., Citation2002). The outcome of interest was the total score obtained by summing the scores from all five dimensions and the total score calculated by GMAE (i.e., GMAE score) (Russell et al., Citation2002).
Muscle strength of the knee extension was assessed with a hand-held dynamometer (HHD) (MicroFET2, Hoggan Scientific, USA) using a “make” technique (Burns et al., Citation2005). The average of three measurements on each leg was used for analyses.
Double legs balance was estimated using the LeonardoTM Mechanography Ground Reaction Force Plate (Novotec Medical, Pforzheim, Germany). During the test, the child was asked to stand for 10 seconds while barefoot and with freely moving arms. The best result of three trials (i.e., the smallest elliptical area) was used for analyses.
Mobility was assessed using the 10-meter walk/run test. Children were asked to walk/run as fast as possible along a corridor with four lines marked at 0, 2, 8, and 10 meters. Test mode (i.e., walk or run) was chosen depending on the participant’s ability and was unchanged for that participant for the duration of the study. The test was performed three times while barefoot. The time taken to run between the 2- and 8-meter marks was recorded, and the average time in seconds was used for analyses. Participants were allowed to use a mobility device (i.e., a walker) if needed.
Body composition was measured using whole-body dual-energy X-ray absorptiometry (DXA) scans (Lunar iDXA, GE Healthcare, Madison, WI, USA). Parameters of interest were total body less head (TBLH) areal bone mineral density (aBMD), bone mineral content (BMC), lean mass, and fat mass.
Health-related quality of life was measured using the Pediatric Quality of Life Inventory Cerebral Palsy Module (PedsQL-CPM), a validated and sensitive measure (Varni et al., Citation2006). The parent-proxy report for children aged 2 to 4 years encompasses 22 items divided into five dimensions: daily activities (5 items), movement and balance (5 items), pain (4 items), fatigue (4 items), and eating activities (4 items) (Varni et al., Citation2006). The total scores for each of the five dimensions were calculated and analyzed. Given the varying perceptions of a child’s well-being, the same parent/caregiver filled out the questionnaire at each visit to avoid possible reporting discrepancies.
Procedures
During the intervention period, children underwent sVT using a Galileo Basic vibration plate (Novotec Medical GmbH, Pforzheim, Germany). Home-based VT sessions were completed standing barefoot on the plate four times a week, 9 minutes a day, at a frequency of 20 Hz and amplitude of 1.5–2.0 mm; a metal frame was used for balance support if needed. Each VT session was composed of three sets of VT (1 to 3 min) with 3-minute breaks in-between (). The vibration frequency and duration of VT sessions were increased from 12 Hz and 3 minutes a day, respectively, to the target parameters (20 Hz and 9 min) according to the intervention protocol (). The current VT protocol was chosen based on our knowledge and experience from previous studies on older children and adolescents with CP and other musculoskeletal conditions, which have demonstrated good adherence with no adverse effects (Gusso et al., Citation2016; Vesey et al., Citation2020).
Table 2. Vibration therapy (VT) treatment protocol.
At the end of the second assessment visit, parents/caregivers had an educational session for 20 minutes on how to use the vibration plate, fill the VT diary, and supervise their child during sessions. Throughout the VT period, consultation support was provided to families via researcher-supervised sessions at home and/or by phone/email. All Auckland-based participants (n = 6) received three home visits (week 1, weeks 5–6, and 10–11). Participants living outside of Auckland (n = 3) received support from study investigators by phone and/or email on week 1, weeks 5–6 and 10–11. They were also asked to send recorded VT sessions so that we could assess the child’s position on the vibration plate and provide further instructions if needed.
Parents/caregivers were asked to complete a VT diary, recording completed/uncompleted sessions, reasons for missing sessions, and perceived side effects (if any). In particular, they were also instructed to immediately contact the research team if they had any concerns about adverse effects potentially associated with VT, including tiredness or pain.
Data analyses
The potential effectiveness of VT treatment on clinical outcomes was assessed using generalized linear mixed models with a repeated measures design, with the change (Δ) from the previous baseline as the outcome. Thus, Δ Control was calculated as T1-T0, and Δ VT as T2-T1. In addition to the main treatment-period parameter (Control/Intervention), all models included as covariates the child’s GMFCs level, their age at a given assessment, and the value of the outcome at the respective baseline (T0 for Control and T1 for VT). The participant’s study ID was also included as a random factor to account for the non-independence of multiple measurements on the same participant.
Statistical analyses were carried out based on the principle of intention-to-treat, so all data recorded in clinical assessments were included in the analyses. Data for the Control and VT periods are provided as means and standard deviations (SD), with effectiveness assessed on the adjusted mean differences (aMD) and respective 95% confidence intervals (CI) for their respective Δ. The within-period Δ are similarly reported as aMD and 95% CI. Data were analyzed using the “proc mixed” procedure in SAS v9.4 (SAS Institute, Cary, NC, USA). All statistical tests were two-sided, with statistical significance maintained at p < 0.05, and without adjustment for multiple comparisons as per Rothman (Rothman, Citation1990).
Results
Feasibility of VT protocol
Adherence to the VT treatment program was high at 93% on average (quartile 1 = 93%; median = 99%; quartile 3 = 100%). The main reported reason for missing sessions was a lack of time. No parents/caregivers contacted us with any concerns regarding VT sessions. There were also no reported adverse effects, except for two participants who reported occasional’ tickling sensations’ along their legs and ears during VT sessions (). However, these were considered a simply minor nuisance, as they resolved within "half a minute or so" after VT session completion and did not affect the children’s ability to follow the treatment protocol ().
Table 3. Feasibility and acceptability data on vibration therapy sessions, and caregivers’ feedback.
All but one family found home-based VT acceptable. The exception was a family with the lowest adherence rate (#2, 54%; 27/48 sessions), who reported a lack of time to follow the study protocol and their child’s frequent reluctance to undergo VT (). They admitted that clinic- or daycare-based VT would be preferable for them for the reasons mentioned above. Importantly, one family who withdrew two weeks after starting VT declared that home-based VT was acceptable for them, but they had to withdraw because they had a newborn baby (#6, ). Of note, those two families (i.e., #2 and #6) and the family that withdrew before the baseline assessment (#9) were referred to the study by the hospital service. Conversely, all self-referred participants (n = 4) completed the study with high levels of adherence (92-100%), and reported home-based VT as suitable for them ().
Caregivers reported greater involvement in the VT sessions during the first three to four weeks, as the children were training to maintain the correct position on the plate with knees apart and slightly bent, with children requiring less support and encouragement for the remaining weeks of VT. Notably, the caregivers of the four youngest participants reported that their biggest challenge was convincing their child to perform VT sessions (). Caregivers reported different techniques for encouragement, such as watching videos, reading favorite books, viewing picture books, or promising their children a treat afterwards. Two children with GMFCS levels II and III used an adjustable metal frame for support over the study duration (#5 and #1, respectively), while another with GMFCS level II used it during the first three weeks of training but not thereafter (#10).
Overall, families provided rather positive feedback on VT, for example:
"My child enjoyed VT. I feel he is more stable."
"I feel my child is stronger and is more balanced."
"Doing VT is a great experience with changes being noticed after the 2nd week of treatment with improvements in gait and walking speed."
Secondary outcomes
Direct comparisons between Control and VT periods yielded no differences in functional tests (i.e., GMFM-66, 10-meter walk/run tests, dynamometry, and balance; ) or body composition (), but there was an observed 32% improvement in the Movement and Balance module of the PedsQL-CPM (p = 0.044; ). However, there were several within-period improvements in functional and body composition outcomes after 12 weeks of VT but not after the Control period, including gross motor function (raw score, +9 points; p = 0.005), 10-meter run/walk test (–1.0 second; p = 0.031), TBLH lean mass (+0.6 kg; p = 0.042), and legs aBMD (+0.021 g/cm2; p = 0.003) ( and ). The exception was legs BMC, with participants showing improvements after both Control (p = 0.006) and VT (p = 0.005) periods of similar magnitudes (+10 g/cm2; ).
Table 4. Functional and health-related quality of life outcomes.
Table 5. Anthropometric and body composition outcomes.
It should be noted that not all participants performed all the assessments, and the number of assessments completed is provided in and . The DXA scan was the clinical assessment with the lowest acceptability by our young participants, as three out of nine children refused such scans (#2, 5, and 6; ). However, the anxiety of some young children and their consequent unwillingness to undergo DXA scans was expected, given the participants’ ages and that such scans are not routine procedures for children with CP (Bianchi et al., Citation2010). Therefore, we attempted to prevent such anxiety by sending all families a video beforehand showing how DXA scans are performed at our clinical research facilities. During the scanning, participants were wrapped in a cloth to limit their movements and watched their favorite video for distraction. The same participants (i.e., #2, 5, and 6) could not perform dynamometry testing as they misunderstood the instructions.
Seven participants completed the 10-meter walk/run tests; two children refused to do so (#2 and 6). One participant used a walker during the tests (#1). To maximize success in conducting the test, we asked parents to wait for their children at the finish 'line’ (i.e., 10-m mark) and encourage their children to walk/run toward them, and all but one caregiver (#2) was willing to assist. Six participants performed a balance test; one child could not stand without support (#1), and two others could not follow the instructions (#2 and 6). The GMFM-66 test was performed with all the children except participant #2, who refused. Notably, many caregivers were actively involved in the testing, encouraging their children to follow the investigator’s instructions, which made it markedly easier to complete the clinical assessments. All parents/caregivers returned completed questionaries (PedsQL-CPM); no issues were reported.
Discussion
The results of this study demonstrate that the proposed protocol of 12 weeks of home-based VT (4 times/week, 9 min/day, at 20 Hz, and in a standing position) is feasible in children with CP aged 2–4 years. Adherence was high (93% on average); there were no severe adverse effects reported, and VT was well-tolerated with only minor nuisance complaints of occasional short-term itchiness in the legs and ears in two participants that disappeared half a minute after VT sessions ended. Caregivers had high levels of engagement with VT sessions, reporting that the greatest challenge to comply with the study protocol was keeping their children motivated, especially among those 3-year-olds and younger. Therefore, caregivers’ motivation is essential for adherence to home-based VT in children younger than five. It should be considered while designing future studies in young children and discussing the study procedures with parents/caregivers. It is also worth noting that the recruitment way (i.e., hospital- or self-referred) may contribute to the caregivers’ adherence to the study protocol and its results. In the current study, families which withdrew and demonstrated the lowest adherence level and non-acceptance of home-based VT were referred to the study by the hospital service. In contrast, self-referred families reported high acceptance of the home-based VT. This could be a subject of further investigation in future studies.
During the assessment visits, the youngest children were the most challenging to collect data due to their relatively short attention spans. Therefore, we recommend assessment visits be short, no more than 1.5 hours, to minimize the burden on participants. It is important to provide families and participants time to familiarize themselves with the research facilities. We also observed that a pre-assessment visit would be valuable for children who may experience high-stress levels in unfamiliar surroundings. We recommend considering GMFM-66 for the assessment of gross motor function to be utilized in future studies on children between 2 and 5 years old.
The findings from the other assessments adopted in this study should be interpreted with caution, given the participants’ young age and lack of data on their responsiveness to change, and challenges in getting the children to follow the assessment protocols accurately. Our experience with the 10-meter walk/run test cannot be generalized for all children with CP. In our study, children could walk or run with or without assistive devices, but this might not apply to all children with CP. Therefore, we do not recommend using the 10-meter walk/run test in studies involving children younger than five years old and GMFCS level II and higher. Similarly, the balance and dynamometry tests would likely be inappropriate for this age group as well, given the children’s relatively short attention span and difficulty following assessment instructions.
Based on our data collection, DXA scanning was feasible only on those older than three years. In future study designs, this must be considered to avoid incomplete data collection due to a lack of cooperation and difficulties with positioning during scanning in younger participants.
Likely for VT sessions, the caregiver’s positive attitude and motivation to participate in the study and high involvement with the child’s VT sessions are essential to achieve high adherence to the treatment protocol. This aspect is vital for future studies, and researchers should be trained in communication with the families and work to create a positive atmosphere during VT sessions and clinical assessments.
Based on our previous experience, we suggest a randomized cross-over design would be the most appropriate for children with CP, given the high motivation of the families to participate in the intervention group with a reluctance to be a part of the control group. While our study design and small sample limited evaluation of treatment effectiveness, there were observed within-period improvements in motor function and body composition associated with VT but not the Control period, suggesting that VT might benefit children 2–4 years of age with CP.
The main limitation of this study was the small number of participants compared to our original target of 20. Unfortunately, recruitment was markedly impaired by the COVID-19 pandemic, when restrictive measures to halt the spread of the SARS-CoV-2 virus were imposed in New Zealand (Baker et al., Citation2020). Nonetheless, we achieved our primary aim of ascertaining the feasibility of home-based VT in preschool children with CP, observing high adherence and good acceptability of the VT protocol. The assessor was not blinded to the assessment’s time-point (i.e., baseline, Control, or VT). Future studies may consider involving an extended research team to allow for assessment blinding to minimize the likelihood of bias. Also, no data were collected on other potential activities the children might have been doing during the study (e.g., physiotherapy or swimming lessons), and we recommend that these data should be prospectively recorded. Lastly, our simplified study design meant we could not assess treatment effectiveness. However, we obtained preliminary data suggesting potential beneficial effects of sVT on gross motor function and body composition.
Conclusion
Our study showed that home-based VT is a feasible and accepted therapy for preschool children with CP. While there were no between-period differences (Δ Control vs Δ VT), changes after the VT but not the Control period were suggestive of potential treatment benefits for mobility, gross motor function, and body composition, which indicate the need for larger randomized trials to assess VT effectiveness appropriately.
Author contributions
SG, PLH, and AA designed the study and protocol submission. AA and JT were involved in participants’ recruitment. AA carried out assessments and managed the study database. JGBD analyzed the data with the input of AA, SG, PLH, and GOG. AA drafted the manuscript with input from SG and JGBD. All authors critically revised the manuscript and approved the final submitted version.
Acknowledgments
We would like to express our gratitude to the participants and their families for their invaluable assistance with this study. We thank Janene McMillan for her support during the participants’ visits.
Disclosure statement
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Additional information
Funding
Notes on contributors
Alena Adaikina
Dr Alena Adaikina is a post-doctoral research fellow at the Liggins Institute at the University of Auckland. Her research focuses on rehabilitation approaches to children and adolescents with disabilities.
José G. B. Derraik
Dr José G. B. Derraik is a clinical and public health researcher with academic appointments in New Zealand, Thailand, Sweden, and China. His research focus primarily on paediatrics (particularly childhood obesity), intervention studies, and the long-term effects of early life events (i.e., Developmental Origins of Health and Disease – DOHaD)
Janice Taylor
Janice Taylor is a senior registered psychologist at the Child Development Unit at Starship Children’s Health, Te Whatu Ora. The main focus of our monitoring and research is with premature babies, and additionally those with neonatal HIE and those babies who have undergone significant cardiac surgery early in life.
Gina L. O’Grady
Dr Gina L. O’Grady is a Paediatric Neurologist at Starship Children’s Health, Te Whātu Ora – Health New Zealand. She has an interest in neuromuscular disease investigation, management and clinical trials.
Paul L. Hofman
Professor Paul L. Hofman is a paediatric endocrinologist who divides time between clinical and research work. He has a focus on clinical research in the areas of child and adolescent diabetes, new born screening, obesity, iodine and fertility and developmental origins of adult disease.
Silmara Gusso
Dr Silmara Gusso is a Senior Lecturer in the Department of Exercise Sciences at the University of Auckland. Her research interests focuses on the effects of chronic conditions on the health of paediatric populations and the role of exercise on their prevention and treatment.
References
- Abbott, J., Dodd, M., Bilton, D., & Webb, A. (1994). Treatment compliance in adults with cystic fibrosis. Thorax, 49(2), 115–120. https://doi.org/10.1136/thx.49.2.115
- Abercromby, A. F., Amonette, W. E., Layne, C. S., McFarlin, B. K., Hinman, M. R., & Paloski, W. H. (2007). Vibration exposure and biodynamic responses during whole-body vibration training. Medicine and Science in Sports and Exercise, 39(10), 1794–1800. https://doi.org/10.1249/mss.0b013e3181238a0f
- Ashwal, S., Russman, B. S., Blasco, P. A., Miller, G., Sandler, A., Shevell, M., & Stevenson, R, (2004). Practice parameter: diagnostic assessment of the child with cerebral palsy. Report of the quality standards subcommittee of the American academy of neurology and the practice committee of the child neurology society. Neurology, 62(6), 851–863. https://doi.org/10.1212/01.Wnl.0000117981.35364.1b
- Baker, M. G., Wilson, N., & Anglemyer, A. (2020). Successful elimination of Covid-19 transmission in New Zealand. The New England Journal of Medicine, 383(8), e56. https://doi.org/10.1056/NEJMc2025203
- Beckung, E., Carlsson, G., Carlsdotter, S., & Uvebrant, P. (2007). The natural history of gross motor development in children with cerebral palsy aged 1 to 15 years. Developmental Medicine and Child Neurology, 49(10), 751–756. https://doi.org/10.1111/j.1469-8749.2007.00751.x
- Bianchi, M. L., Baim, S., Bishop, N. J., Gordon, C. M., Hans, D. B., Langman, C. B., Leonard, M. B., & Kalkwarf, H. J. (2010). Official positions of the International Society for Clinical Densitometry (ISCD) on DXA evaluation in children and adolescents. Pediatric Nephrology (Berlin, Germany), 25(1), 37–47. https://doi.org/10.1007/s00467-009-1249-z
- Blackman, J. A. (2002). Early intervention: a global perspective. Infants & Young Children, 15(2), 11–19. https://doi.org/10.1097/00001163-200210000-00004
- Burns, S. P., Breuninger, A., Kaplan, C., & Marin, H. (2005). Hand-held dynamometry in persons with tetraplegia: comparison of make-versus break-testing techniques. American Journal of Physical Medicine & Rehabilitation, 84(1), 22–29. https://doi.org/10.1097/01.phm.0000150790.99514.c6
- Cech, D. J., & Martin, S. T. (2012). Nervous system changes. Functional Movement Development Across the Life Span (Third Edition. (pp. 174–212). W.B. Saunders. https://doi.org/10.1016/B978-1-4160-4978-4.00009-0
- El-Shamy, S. M. (2014). Effect of whole-body vibration on muscle strength and balance in diplegic cerebral palsy: a randomized controlled trial. American Journal of Physical Medicine & Rehabilitation, 93(2), 114–121. https://doi.org/10.1097/PHM.0b013e3182a541a4
- Fiss, A. L., & Jeffries,L. F. (2020). Early intervention services for young children with cerebral palsy. In: Miller Bachrach, S., Lennon, N., O'Neil, M.E. (Eds.), Cerebral palsy (pp. 2455–2472). Springer.
- Granild-Jensen, J. B., Rackauskaite, G., Flachs, E. M., & Uldall, P. (2015). Predictors for early diagnosis of cerebral palsy from national registry data. Developmental Medicine and Child Neurology, 57(10), 931–935. https://doi.org/10.1111/dmcn.12760
- WHO Multicentre Growth Reference Study Group. (2006). WHO Child Growth Standards Based on Length/Height, Weight and Age. Acta Paediatrica, 95, 76–85.
- Gusso, S., Munns, C., Colle, P., Derraik, J. G. B., Biggs, J., Cutfield, W. S., & Hofman, P. L. (2016). Effects of whole-body vibration training on physical function, bone and muscle mass in adolescents and young adults with cerebral palsy. Scientific Reports, 6, 22518. https://doi.org/10.1038/srep22518
- Hubermann, L., Boychuck, Z., Shevell, M., & Majnemer, A. (2016). Age at referral of children for initial diagnosis of cerebral palsy and rRehabilitation: current practices. Journal of Child Neurology, 31(3), 364–369. https://doi.org/10.1177/0883073815596610
- Ibrahim, M. M., Eid, M. A., & Moawd, S. A. (2014). Effect of whole-body vibration on muscle strength, spasticity, and motor performance in spastic diplegic cerebral palsy children. Effect of whole-body vibration. Egyptian Journal of Medical Human Genetics, 15(2), 173–179. https://doi.org/10.1016/j.ejmhg.2014.02.007
- Martakis, K., Stark, C., Alberg, E., Bossier, C., Semler, O., Schönau, E., & Duran, I. (2019). Motor function improvement in children with ataxia receiving interval rehabilitation, including vibration-assisted hometraining: a retrospective study. Klinische Padiatrie, 231(6), 304–312. https://doi.org/10.1055/a-1001-2284
- Mattern-Baxter, K., McNeil, S., & Mansoor, J. K. (2013). Effects of home-based locomotor treadmill training on gross motor function in young children with cerebral palsy: a quasi-randomized controlled trial. Archives of Physical Medicine and Rehabilitation, 94(11), 2061–2067. https://doi.org/10.1016/j.apmr.2013.05.012
- Michael-Asalu, A., Taylor, G., Campbell, H., Lelea, L. L., & Kirby, R. S. (2019). Cerebral palsy: diagnosis, epidemiology, genetics, and clinical update. Advances in Pediatrics, 66, 189–208. https://doi.org/10.1016/j.yapd.2019.04.002
- Mohamed, S. H., Fahmy, S. A. E. H., Mohamed, A. G., & El-Sabbagh, M. H. (2020). Efficacy of physiotherapy and conductive education in improving motor skills and mental function in children with cerebral palsy. Open Journal of Pediatrics, 10(2), 369–380. https://doi.org/10.4236/ojped.2020.102038
- Morgan, C., Darrah, J., Gordon, A. M., Harbourne, R., Spittle, A., Johnson, R., & Fetters, L. (2016). Effectiveness of motor interventions in infants with cerebral palsy: a systematic review. Developmental Medicine and Child Neurology, 58(9), 900–909. https://doi.org/10.1111/dmcn.13105
- Morgan, C., Novak, I., Dale, R. C., & Badawi, N. (2015). Optimising motor learning in infants at high risk of cerebral palsy: a pilot study. BMC Pediatrics, 15(1), 30. https://doi.org/10.1186/s12887-015-0347-2
- Palisano, R., Rosenbaum, P., Walter, S., Russell, D., Wood, E., & Galuppi, B. (1997). Gross motor function classification system for cerebral palsy. Developmental Medicine and Child Neurology, 39(4), 214–223. https://doi.org/10.1111/j.1469-8749.1997.tb07414.x
- Patel, D. R., Neelakantan, M., Pandher, K., & Merrick, J. (2020). Cerebral palsy in children: a clinical overview. Translational Pediatrics, 9(Suppl 1), S125–S135. https://doi.org/10.21037/tp.2020.01.01
- Pierce, S. R., Skorup, J., Miller, A., Paremski, A. C., & Prosser, L. A. (2019). The responsiveness and validity of the early clinical assessment of balance in toddlers with cerebral palsy: brief report. Developmental Neurorehabilitation, 22(7), 496–498. https://doi.org/10.1080/17518423.2018.1523244
- Rittweger, J. (2010). Vibration as an exercise modality: how it may work, and what its potential might be. European Journal of Applied Physiology, 108(5), 877–904. https://doi.org/10.1007/s00421-009-1303-3
- Ritzmann, R., Stark, C., & Krause, A. (2018). Vibration therapy in patients with cerebral palsy: a systematic review. Neuropsychiatric Disease and Treatment, 14, 1607–1625. https://doi.org/10.2147/NDT.S152543
- Rosenbaum, P., Paneth, N., Leviton, A., Goldstein, M., Bax, M., Damiano, D., Dan, B., & Jacobsson, B. (2007). A report: the definition and classification of cerebral palsy April 2006. Developmental Medicine and Child Neurology. Supplement, 109, 8–14.
- Rosenbaum, P. L., Walter, S. D., Hanna, S. E., Palisano, R. J., Russell, D. J., Raina, P., Wood, E., Bartlett, D. J., & Galuppi, B. E. (2002). Prognosis for gross motor function in cerebral palsy: creation of motor development curves. JAMA, 288(11), 1357–1363. https://doi.org/10.1001/jama.288.11.1357
- Rothman, K. J. (1990). No adjustments are needed for multiple comparisons. Epidemiology, 1(1), 43–46.
- Ruck, J., Chabot, G., & Rauch, F. (2010). Vibration treatment in cerebral palsy: A randomized controlled pilot study. Journal of Musculoskeletal and Neuronal Interactions, 10(1), 77–83.
- Russell, D. J., Avery, L. M., Rosenbaum, P. L., Raina, P. S., Walter, S. D., & Palisano, R. J. (2000). Improved scaling of the gross motor function measure for children with cerebral palsy: evidence of reliability and validity. Physical Therapy, 80(9), 873–885. https://doi.org/10.1093/ptj/80.9.873
- Russell, D. J., Rosenbaum, P., Wright, M., & Avery, L. M. (2002). Gross Motor Function Measure (GMFM-66 & GMFM-88) User’s Manual. Mac Keith press.
- Sadowska, M., Sarecka-Hujar, B., & Kopyta, I. (2020). Cerebral palsy: current opinions on definition, epidemiology, risk factors, classification and treatment options. Neuropsychiatric Disease and Treatment, 16, 1505–1518. https://doi.org/10.2147/NDT.S235165
- Spittle, A. J., & Morgan, C. (2018). Early intervention for children with cerebral palsy. In: Miller, F., Bachrach, S., Lennon, N., O'Neil, M.E(eds) Cerebral Palsy. (pp. 193–200). Springer.
- Stark, C., Herkenrath, P., Hollmann, H., Waltz, S., Becker, I., Hoebing, L., Semler, O., Hoyer-Kuhn, H., Duran, I., Hero, B., Hadders-Algra, M., & Schoenau, E. (2016). Early vibration assisted physiotherapy in toddlers with cerebral palsy – a randomized controlled pilot trial. Journal of Musculoskeletal & Neuronal Interactions, 16(3), 183–192.
- Stark, C., Nikopoulou-Smyrni, P., Stabrey, A., Semler, O., & Schoenau, E. (2010). Effect of a new physiotherapy concept on bone mineral density, muscle force and gross motor function in children with bilateral cerebral palsy. Journal of Musculoskeletal & Neuronal Interactions, 10(2), 151–158.
- van Nes, I. J., Geurts, A. C., Hendricks, H. T., & Duysens, J. (2004). Short-term effects of whole-body vibration on postural control in unilateral chronic stroke patients: preliminary evidence. American Journal of Physical Medicine & Rehabilitation, 83(11), 867–873. https://doi.org/10.1097/01.phm.0000140801.23135.09
- Varni, J. W., Burwinkle, T. M., Berrin, S. J., Sherman, S. A., Ba, K. A., Malcarne, V. L., & Chambers, H. G. (2006). The PedsQL in pediatric cerebral palsy: reliability, validity, and sensitivity of the generic core scales and cerebral palsy module. Developmental Medicine & Child Neurology, 48(6), 442–449. https://doi.org/10.1111/j.1469-8749.2006.tb01293.x
- Vesey, R. M., Hofman, P. L., Derraik, J. G. B., Colle, P., Biggs, J. B., Munns, C. F., Cutfield, W. S., & Gusso, S. (2020). Safety, feasibility and efficacy of side‐alternating vibration therapy on bone and muscle health in children and adolescents with musculoskeletal disorders: A pilot trial. Journal of Paediatrics and Child Health, 56(8), 1257–1262. https://doi.org/10.1111/jpc.14913
- Wei, S., Su-Juan, W., Yuan-Gui, L., Hong, Y., Xiu-Juan, X., & Xiao-Mei, S. (2006). Reliability and validity of the GMFM-66 in 0-to 3-year-old children with cerebral palsy. American Journal of Physical Medicine & Rehabilitation, 85(2), 141–147.