Abstract
Chemical and isotopic data of groundwater of the Upper Cretaceous aquifer in the Orontes basin, Syria, have been used to assess the groundwater geochemistry, the origin of groundwater recharge and groundwater residence time. The chemical data indicate that dissolution of evaporite minerals is the main process controlling groundwater mineralization. The composition of stable isotopes δ18O and δ2H, together with 14C activity, reflect the existence of three different groups: (a) groundwater in the Coastal Mountains with δ18O of –6.65‰, quite similar to modern-day precipitation, and high 14C (>50 pmC); (b) groundwater in the unconfined aquifer of the Hama Uplift, which has δ18O of –5.52‰ and 14C near 20 pmC, and is recharged locally; and (c) groundwater from the confined aquifer of the Homs Depression, which is characterized by more depleted δ18O,, –8.01‰, and low 14C (<7 pmC), and might be recharged in the northern piedmont of the Anti-Lebanon Mountains. The distinctive isotope signatures of the latter two groups indicate different recharge elevations and palaeoclimatic effects. The low recharge rate of the groundwater in the Hama Uplift aquifer, and the even slower recharge rate in the Homs Depression aquifer, are reflected by groundwater 14C residence times of 5 and over 22 Ka BP, respectively.
Editor D. Koutsoyiannis
Citation Al-Charideh, A., 2013. Recharge and mineralization of groundwater of the Upper Cretaceous aquifer in Orontes basin (Syria). Hydrological Sciences Journal, 58 (2), 452–467.
Résumé
Les données chimiques et isotopiques des eaux souterraines de l'aquifère du Crétacé supérieur dans le bassin de l'Oronte, en Syrie, ont été utilisées pour déterminer les processus générant la composition chimique, l'origine de la recharge, et le temps de séjour des eaux souterraines. Les données chimiques montrent que la dissolution des minéraux évaporitiques est le processus principal contrôlant la minéralisation des eaux souterraines. La composition en isotopes stables δ18O et δ2H, ainsi que l'activité en 14C, reflètent l'existence de trois groupes différents : (a) les eaux souterraines des montagnes côtières avec δ18O de –6,65‰, assez semblable aux précipitations des temps modernes, et un 14C élevé (>50 PMC), (b) les eaux souterraines de l'aquifère libre du soulèvement de Hama, avec un δ18O de –5,52‰ et un 14C de près de 20 PMC et qui sont rechargées localement, et (c) les eaux souterraines de l'aquifère captif de la dépression de Homs, qui sont caractérisées par un appauvrissement accru en δ18O (–8,01‰) et un 14C bas (<7 PMC), et qui peuvent être rechargées dans le piémont nord des montagnes de l'Anti-Liban. Les signatures isotopiques distinctes entre les deux derniers groupes indiquent une altitude de recharge et des effets paléoclimatiques différents. La baisse du taux de recharge des eaux souterraines dans l'aquifère du soulèvement de Hama, et le taux de recharge plus prononcée dans l'aquifère de la dépression de Homs, se traduisent respectivement par des temps de résidence des eaux souterraines 14C de 5 Ka BP et e plus de 22 Ka BP.
Citation Al-Charideh, A., 2013. Recharge and mineralization of groundwater of the Upper Cretaceous aquifer in Orontes basin (Syria). Hydrological Sciences Journal, 58 (2), 1–16.
Key words:
Mots clefs:
1 INTRODUCTION
Both chemical and isotopic data are effective tools for solving various problems in hydrology, in particular in arid and semi-arid regions (IAEA Citation1980, Citation1983, Love et al. Citation1994, Clark and Fritz Citation1997, Cook and Herczeg Citation2000). Groundwater is an important source of drinking water for much of the population in Syria. Continuously increasing abstraction of groundwater resources to meet rising agricultural and domestic needs, leads to a growing deficit of water. The drawdown of piezometric levels and progressive degradation of water quality are the consequences of such intensive exploitation (Rasoul Agha Citation1999). In fact, this increase of groundwater abstraction from the several basins in Syria often requires exploitation of palaeo-groundwater that typically has depleted values of stable isotopes (relative to modern precipitation) and low 14C activities proving that no significant renewal of water has occurred recently (Selkhozpromexport Citation1979, Ferronsky et al. Citation1991, Wagner and Geyh Citation1999, Kattan Citation2002, Al-Charideh and Abou-Zakhem Citation2009, Al-Charideh Citation2011b). Similar isotopic patterns in groundwater are also observed in many basins of the Nubian Sandstone in northern Africa and in the arid zones of the Middle East (Gat and Issar Citation1974, Bajjali et al. Citation1997, Abd El Samie and Sadek Citation2001, Vengosh et al. Citation2007, Zouari et al. Citation2011). However, limestone and dolomitic rocks of Upper Cretaceous age constitute a highly productive aquifer in the highlands and mountain ranges of western Syria (). In the areas where they outcrop, which have a sub-humid Mediterranean-type climate, these carbonate rocks receive relatively abundant recharge and provide the principal reservoir of freshwater supply for the region. However, this aquifer extends downward and further east to the more arid parts of Syria, where it is mostly covered by a thick sequence of marls.
This work presents the primary results of a multiple tracer study focused mainly on the deep Upper Cretaceous aquifer; several samples taken from the outcropping aquifer were assumed to serve as reference data for the present-day recharge. However, preliminary radiocarbon and stable isotope determinations suggested the presence of fossil groundwater in a number of wells. Therefore, a multi-tracer approach (18O, 2H, 13C, 3H and 14C, as well as the main and some trace chemical constituents) was used to develop an initial schematic geo-hydrological model of groundwater recharge, circulation and chemical evolution. The specific objectives of this work are: (a) to identify the different recharge regimes; (b) to determine the dominant geochemical processes which influence groundwater chemical composition; (c) to determine its subsurface residence time; and, (d) to schematize groundwater flow patterns and the nature of aquifers in selected important hydrogeological regions of the basins. It is worth mentioning that the primary results from this study could be useful for other basins in Syria and in Middle East countries for understanding the recharge process and planning water resources management, in particular for the Upper Cretaceous aquifer.
2 STUDY AREA
The Orontes basin in western Syria extends over an area of about 21 600 km2 (Selkhozpromexport Citation1979). The basin is bound to the west by the Coastal Mountains that reach 1500 m a.s.l., in the south by the northern Anti-Lebanon Mountains (2000 m), in the east by the northern Palmyrides Range of mountains (1300 m) and in the north by the Aleppo Plateau (Ponikarov Citation1967). The central sector of the study basin, the so-called Hama Uplift and Homs Depression, are relatively flat and situated at 300–400 m ().
The present climate of the study area is semi-arid Mediterranean in the west and changes to arid continental in the east. The N–S trending mountain ridge along the Dead Sea Fault acts as an orographic barrier that can be clearly seen in the rainfall pattern (). Thus, the annual precipitation ranges from 800 mm/year along the narrow western slopes of the Coastal Range to less than 100 mm/year east of the Hama Uplift and Homs Depression. Due to the small precipitation values, it follows that groundwater resources in the Orontes basin are replenished at low rates and there is consequently a high risk of over exploitation by pumping from wells. Since 1980, groundwater extraction from the Upper Cretaceous and Neogene aquifers system has increased greatly from 1000 to 1900 hm3/year in 2003, as a result of the growth in water needs (GCHS Citation2003, SDWC Citation2008).
The geological formations of the Orontes basin consist of marine carbonate deposits ranging in age from Upper Jurassic to Pliocene, with terrestrial sedimentation increasing from the Miocene onwards as the area was subjected to uplift accompanied by regression of the sea ((a)).
Fig. 2 (a) Simplified geological map of the study area; (b) schematic cross-section A–A′; and distribution of the sampling sites, nos. 1–40. Key: 1: Jurassic, 2: Upper Cretaceous aquifer (limestone and dolomites), 3: Upper Cretaceous (clay and limestone of Senonian to Maestrichtian), 4: Palaeogene (chalks and marls), 5: Neogene (conglomerates and limestone aquifer), 6: Basalt (Miocene/Pliocene) aquifer, 7: Quaternary, 8: Main faults, 9: Wells, 10: Cities.
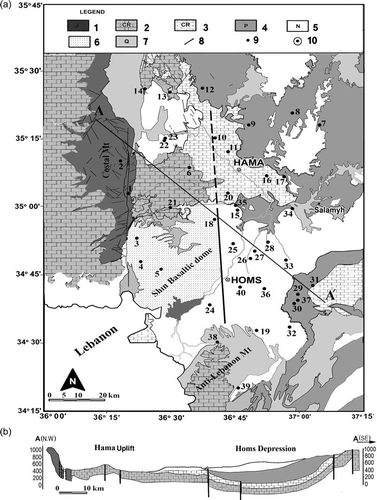
The rock formations constituting the major aquifers in the area include the dolomite and limestone of the Upper Cretaceous, in particular those of Cenomanian and Turonian age (thickness 350–500 m). Palaeogene deposits, composed of chalks, limestone and marls occur but do not constitute important aquifers ((b)). The Neogene and Quaternary deposits are composed of sandstone, conglomerate, gravel and basalt and provide important aquifers.
The main aquifer in the study area comprises the strongly fissured and karstified massive strata of the limestone and dolomite of Cenomanian to Turonian ages, with marl, sandstone and gypsum intercalation in the lower part (Brew et al. Citation2001, SDWC 2008). The upper part of this aquifer is exposed at the surface in the vicinity of Hama and along a big anticlinal structure of the northernmost limb of the northern Palmyrides ((b)). However, the major part of this aquifer, in the Homs Depression, is confined and located between two aquitards of clays and marls: Aptian-Albian deposits serve as the regional bottom, and Senonian, Palaeogene and Pliocene deposits, of up to 250 m thickness, form the top east and southeast of the Homs Depression and Hama Uplift ((b)). The overlying Senonian to Maestrichtian marly and chalky limestone and dolomite, partly containing flints and gypsum, may form a combined aquifer system with the Cenomanian-Turonian rocks of high to medium productivity in the Homs Depression. The Cretaceous aquifer is known for its great hydraulic conductivity, and it is the most reliable aquifer not only in the Orontes basin, but also throughout Syria, in terms of storage capacity and the huge discharge of issuing springs (Al-Charideh Citation2011a).
Groundwater recharge is concentrated in the high rainfall areas along the narrow western slopes of the Coastal Range, and the northeast part of the Anti-Lebanon Mountains, where the rainfall is between 500 and 800 mm/year; roughly half of the rainfall may recharge the mountain aquifer systems (Selkhozpromexport Citation1979). According to rather poorly founded information on the piezometric surface, the unconfined groundwater of the Hama Uplift flows from the west towards the Al-Ghab rift valley, where it feeds the major karstic springs. However, the groundwater in the deep confined aquifer of the Homs Depression flows from the southwest, where the recharge could be related to the Anti-Lebanon Mountains, to the northeast. In addition, the main fault direction, S–N, might act as barrier to groundwater flow between the unconfined and confined aquifer systems, and cause the lowering of piezometric head by 40 m in the confined aquifer (Hatem Citation1995).
Finally, the main and the only drainage in this area is the Orontes River, which starts in the Bekaa Valley of Lebanon and flows in a northerly direction along the east side of the mountain ridge across the Al-Ghab graben structure, where it finally makes a sharp turn west to the Mediterranean. Major karstic springs are located in the Bekaa Valley at altitudes of 690 m, and discharge into the Orontes, maintaining a baseflow even in summer. The average flow of the Orontes is reported by the Ministry of Irrigation as 6.9 m3/s, or 217 hm3/year.
3 SAMPLING AND ANALYTICAL METHODS
A total of 40 groundwater samples were collected from active pumping wells (200 to 1650 m in depth) during September 2007. The sampling event was repeated in May 2008 ((a)). No significant difference was observed in either the chemical or the stable isotopes analyses of the first and the second sampling events.
Physical and chemical parameters were measured in the field, including: temperature (T, °C), pH, electrical conductivity (EC) and total alkalinity (i.e. HCO3 −). The total dissolved inorganic carbon (TDIC) for 16 groundwater samples was precipitated in the field from about 120 L of groundwater using the well-known procedure of IAEA (Tamers and Scharpensel Citation1970). The chemical analyses of major ions such as Cl−, SO4 2-, NO3 −, Ca2+, Mg2+, Na+, K+ were conducted by liquid-ion chromatography. The charge balance was less than ±5% for all the analysed samples.
Stable isotope contents of the water samples were measured by isotope ratio mass-spectrometry, using the CO2 equilibration technique for δ18O (Epstein and Mayeda Citation1953) and isotopic exchange with H2 gas for δ2H (Coplen et al. Citation1991). The isotope contents are reported in the usual δ notation relative to the Vienna Standard Mean Ocean Water (V-SMOW) standard. Typical analytical uncertainty of the reported values is about ±0.1‰ for δ18O and ±1.0‰ for δ2H. The δ13C contents of total dissolved inorganic carbon were measured using isotope ratio mass spectrometry and reported in ‰ versus V-PDB (Vienna Pee Dee formation, North California, USA) (Coplen Citation1996) with a typical analytical uncertainty of ±0.1‰.
The analyses of tritium (3H) were determined by scintillation counter (Quantulus 1220) after electrolytic enrichment. The results are expressed in Tritium Units (TU) (one TU is equivalent to one 3H atom per 1018 atoms of hydrogen) with the analytical uncertainty in the order of ±0.3 TU at 2σ.
The 14C results are reported as per cent modern carbon (pmC); the degree of uncertainty depends on the quantity of analysed material, the count time and the activity of the sample. All chemical and environmental isotopes were analysed at the laboratories of the Atomic Energy Commission of Syria.
4 RESULTS AND DISCUSSION
4.1 Chemistry of groundwater
Physical and chemical characteristics of groundwater from the Upper Cretaceous aquifer systems (), show important variations with respect to increasing depth, progressive confinement from west to east and the geological facies variation of the aquifer. Groundwater temperature varies from 16 to 47°C in the study area. The total dissolved solids (TDS) values of groundwater samples range from 300 to 4000 mg/L (). The waters are weakly mineralized in the western part and highly mineralized in the deeper part of the aquifer in the northeast. The increase in TDS values of the groundwater can be explained by the chemical reactions that take place during water–rock interaction, resulting in the dissolution and precipitation of mineral phases. The majority of the groundwater samples have pH values of between 6.76 and 8.47, with a mean value of 7.3, indicating that the waters are generally neutral to slightly alkaline.
Table 1 Mean chemical composition and field data of groundwater samples from the Upper Cretaceous aquifer in the Orontes basin. See for location of sites
The differences in ionic composition indicate that there are three major water types, and the dominant anion species of the water change systematically from HCO3 − to SO4 2- to Cl− (). Groundwaters from the Coastal Mountains aquifer have a Ca-Mg-HCO3 geochemical facies typical of karst environments, indicating limestone and dolomite dissolution during infiltration.
Fig. 3 Average concentration and geochemical facies of the dominant groundwater groups in the study area.
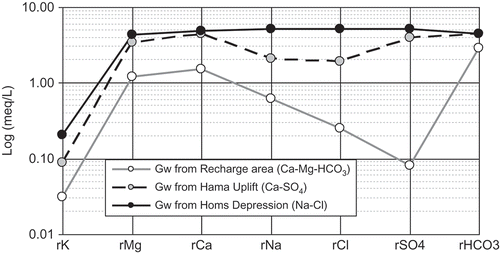
The groundwater samples from the unconfined Hama Uplift also present Ca-Mg-HCO3 geochemical facies. However, the higher Mg2+ and SO4 2- content of these waters compared with the Coastal Mountains area indicates enhanced dolomite and evaporite dissolution. The salinity of these waters ranges between 400 and 1800 mg/L. The groundwater samples from the more confined section in the Homs Depression mainly have Ca-Mg-HCO3 geochemical facies with very variable Na+ and Cl− concentrations where the salinity ranges between 400 and 3880 mg/L.
One groundwater sample (well 32, 200 m depth) is of interest due to its high salinity, dominated by magnesium, sodium, chloride and high sulphate concentrations. Due to the shallow depth of this well, the groundwater may be fed by a significant contribution from surface water of Wadi Hbeishoun during flood periods. The isotopic data, showing relative enrichment and a trace of tritium content support this suggestion.
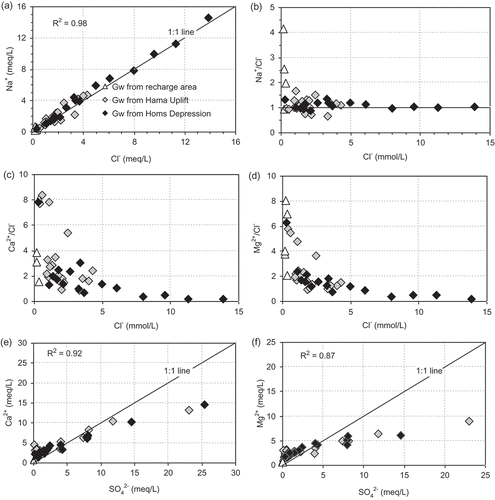
The groundwater samples have concentrations of Na+ between 5 and 571 mg/L and of Cl− between 6 and 990 mg/L (). The high concentrations of Na+ and Cl− detected in the southeast of the Homs Depression, where the aquifer is more confined (well depths > 900 m) may suggest the dissolution of chloride salts formed as very thin salt layers during evaporation of a closed or restricted marine basin several million years ago (El Naka and Al Kuisi Citation2004) ((a)). The molar ratios of Na+/Cl− for groundwater samples of the study area generally range from 0.65 to 4.14, with a median value of 1.09 ((b)). Most of the Na/Cl molar ratios of groundwater samples with Cl− <5 mmol/L exhibit higher values (0.9–4.1) than water in the hydrological cycle (0.86–1) suggesting an additional source of Na+, perhaps released from water–rock interaction, such as plagioclase dissolution of the volcanic basalt abundant in the study area (Magaritz et al. Citation1981, Sami Citation1992). Many of the groundwater samples of the unconfined aquifer of the Hama Uplift have lower Na/Cl ratios that are more similar to those of modern rainfall, reflecting recharge directly to the aquifer. The major part of the deep groundwater samples of the confined aquifer in the Homs Depression has Na+/Cl− molar ratios of approximately 1, suggesting halite dissolution (Jankowski and Acworth Citation1997). The trend of Ca2+/Cl− and Mg2+/Cl− ratios versus Cl− for the groundwater samples is similar to Na+/Cl− versus Cl− concentration ((c,d)). These data support a model whereby the chemistry of the low-salinity groundwater with Cl− <5 mmol/L is controlled by different processes (mixing, evaporation and water–rock interaction). However, halite dissolution in the more confined aquifer, with Cl− concentrations of >5 mmol/L, produces groundwater with lower, less variable cation/Cl ratios, consistent with homogenization and dissolution of evaporite sediment that produces the high groundwater salinity.
The groundwater samples have concentrations of SO4 2- of between 2 and 1200 mg/L, and of Ca2+ between 10 and 300 mg/L. The plot of Ca2+ and SO4 2- ((e) shows a clustering of data at low SO4 2- values, which may indicate changes in the Ca2+ concentrations that are associated with relatively minor change in SO4 2- (). However, for some groundwater samples, there is a linear increase between Ca2+ and SO4 2- that is close to the theoretical regression line for gypsum dissolution in equilibrium (slope = 1) suggesting that both ions are controlled by gypsum dissolution. The computation of saturation indices shows a progressive saturation in gypsum with an increase in SO4 2- concentration (). Also, the strong observed relationship (R2 = 0.87) between SO4 2- and Mg2+ ((f)) indicates that these are most likely derived from the same evaporite dissolution. Water samples that are placed below the 1:1 line indicate a deficit of Ca2+ and Mg2+ relative to SO4 2-. This depletion of Ca2+ and Mg2+ indicates the contribution of another geochemical process that effects cation contents and may be related to the mechanism of base exchange, by which Ca2+ and Mg2+ are adsorbed on the surface of clay minerals with the release of Na+, causing more saline water (Chapelle and Knobel Citation1983, Plummer et al. Citation1990). In addition, some sulphate reduction is detectable in several wells (23, 24, 33 and 39). The deepest water (from well 39) has isotopically light δ13C ratios, which are possibly evidence of bacterially-mediated sulphate reduction (Edmunds et al. Citation1995).
Fig. 5 Relationship between mineral and water saturation indexes (SI) of calcite, dolomite, gypsum and halite.
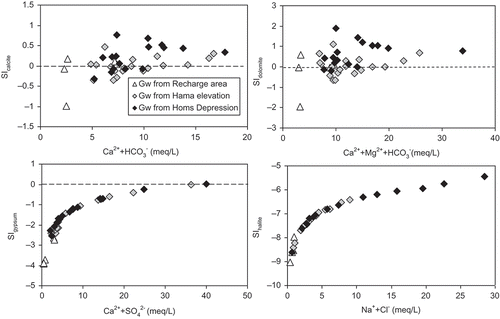
The mineralogical constraints of the water–rock interactions are confirmed by examination of the saturation indexes (). Saturation indexes and carbon partial pressures (P CO2) of all groundwater samples were calculated using the geochemical code NETPATH 2.0 (Plummer et al. Citation1994). Taking into account the accuracy of the field measurements of pH and alkalinity, and the uncertainties of the other analytical data, the waters are in equilibrium with respect to calcite if the saturation index ranges from –0.1 to +0.1, and from –0.2 to +0.2 for dolomite (Nordstrom and Ball Citation1989). Accordingly, our results show that the groundwater samples in the recharge area are in equilibrium with calcite and dolomite at low P CO2 conditions (10−4 to 10−3), and are strongly under-saturated with respect to gypsum and chloride. Most groundwater samples from the unconfined aquifer of the Hama Uplift and confined aquifer of the Homs Depression are in equilibrium or over-saturated with respect to calcite and dolomite, and some approach saturation with respect to gypsum. The P CO2 values of these samples are nearly an order of magnitude higher than those of the recharge area (10−2). All the groundwater samples are under-saturated with respect to halite, but for several groundwater samples from the Homs Depression, the saturation state for halite is about four orders of magnitude higher than in the groundwater from the Hama Uplift and recharge area.
Table 2 Isotopic composition of groundwater samples collected from the Upper Cretaceous aquifer system in the Orontes basin. See for location of sites
Table 3 Mean isotopic composition values of the three groundwater clusters of the Upper Cretaceous aquifer in the Orontes basin
4.2 Stable isotope composition in the groundwater
The stable isotope composition of oxygen and hydrogen is used to determine the origin of the groundwater with respect to the source of precipitation, the altitude of the recharge area and the approximate groundwater age.
The stable isotope composition of modern precipitation was monitored at Bloudan station (1550 m) during the period 2000–2008 and at Kadmous station (750 m) during the period 2005–2008. These two highland stations represent the regional groundwater recharge area of the Anti-Lebanon and Coastal Mountains, respectively (). The δ2H versus δ18O relationship for all monthly rainwater samples is defined by the following equation (): δ2H = 7.9δ18O + 20‰ (Al-Charideh Citation2011a). The stable isotope composition of precipitation in this region is similar to that which defines the Eastern Mediterranean meteoric water line (EMWL: δ2H = 8δ18O + 22‰, Gat and Carmi Citation1970), which differs from the global meteoric water line (GMWL: δ2H = 8δ18O + 10‰, Craig Citation1961) by a strong deuterium excess (+22‰) due to strong primary evaporation.
Fig. 6 δ18O–δ2H diagram of groundwater samples from the Upper Cretaceous aquifer in the Orontes basin. The mean and standard deviations of the δ values of the rainwater collected at the monitoring stations Bloudan and Kadmous are also shown.
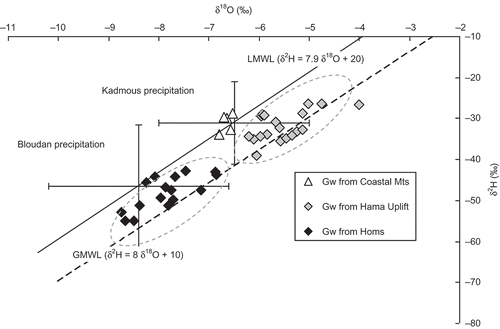
The results of the isotopic composition determinations of the groundwater samples collected from the study area, together with the deuterium excess (dex) and tritium values, are reported in and . The plot of the δ2H values versus the δ18O values of the groundwater samples shows three well distinguished clusters (Fig. 6 and ). The delta values of the groundwater samples from the Coastal Mountains form a cluster on the Local Meteoric Water Line (LMWL), indicating that only westerly storms approaching via the Mediterranean Sea have recharged this groundwater. However, the delta values of groundwater samples from the unconfined aquifer of the Hama Uplift and the deep confined aquifer of the Homs Depression areas form separate clusters between the LMWL and the GMWL (). This means that both groundwaters are recharged by westerly Mediterranean and by northern continental storms, which fit to the GMWL. There is no evidence for isotopic evaporation effects as both clusters fit to regression lines with a slope near 8. There is one exception (well no. 7), which is situated in the unconfined part and contains some tritium. Irrigation return flow might have been admixed because intensive agriculture is practised in this area as shown by the high NO3 (40 mg/L) concentration.
The groundwater of the confined aquifer section is more depleted in heavy isotopes than that of the unconfined aquifer (). Two factors may be responsible: (i) the altitude effect: the δ18O value of rainwater decreases with increasing altitude, and (ii) the palaeoclimatic effect: the δ18O values of Pleistocene groundwater are by 1.5 to 1.8‰ more negative than those of the Holocene groundwater (Bath et al. Citation1979). As the difference of 2.5‰ between the means of the two clusters (–5.5‰, Hama; and –8.01‰, Homs) is much larger than 1.8‰, the palaeoclimatic effect alone cannot explain these results. The plot of δ18O and dex versus groundwater 14C age () reveals the required information. The calibrated groundwater 14C ages were calculated with a constant initial 14C value of 60 pmC (Geyh Citation1992).
Fig. 7 The δ18O (blue) and dex (green) values plotted vs groundwater 14C age (A0 = 60 pmC) form six distinct clusters which are discussed in the text.
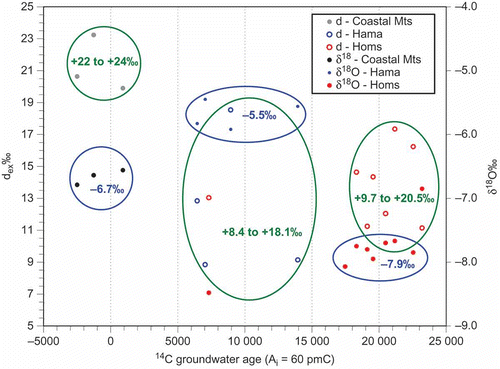
Groundwater of the Coastal Mountains is continuously recharged and very young. Its dex values of +20 to +24‰ indicate that Mediterranean storms are the source of this groundwater, similar to results for spring waters from both the Anti-Lebanon and Coastal Mountains (Al-Charideh Citation2011a).
Well no 12 is an exception; the groundwater in the Cretaceous Hama Uplift aquifer is aged between 5000 and 9000 years Before Present (BP). Therefore, the palaeoclimatic effect is excluded. The mean of the δ18O values of the groundwaters from the Coastal Mountains and the Hama Uplift differ by 1.2‰ (). This can be attributed to the altitude effect as the Hama Uplift recharge area is about 400 m lower than the Coastal Mountains (Fig. 1), assuming an altitude effect of –0.23‰/100 m as for the Anti-Lebanon Mountains (Al-Charideh and Abou Zakhem Citation2010). The wide range of the dex values (8–18‰) provides evidence that both Mediterranean and continental storms feed the groundwater recharge. It is noteworthy that groundwater well nos. 6, 20 and 21, with dex values >18‰ are located in the most western part of the study area. There is a topographic window between the Coastal Mountains and the northern part of the Anti-Lebanon Mountains allowing Mediterranean storms to penetrate directly. Northern continental precipitation has been responsible for the recharge of the groundwater pumped from well nos. 8, 9, 12, 16 and 17 in the Hama Uplift aquifer.
The groundwater in the confined aquifer of the Homs Depression could be hydraulically connected with the open aquifer of the Hama Uplift area although the visible faults may act as barriers (Fig. 2(a)). The groundwater is aged between 17 000 and 24 000 years BP, with exception of that from well no. 39 located close to the Anti-Lebanon Mountains ((a)). The groundwater 14C ages might be too young if the well catchment area is covered by sediments and an initial 14C value of around 84 pmC, rather than 60 pmC, is valid. In any case, the palaeoclimatic effect has to be taken into account for the Pleistocene groundwater. However, the difference of 2.5‰ between the means of the corresponding δ18O values to that of the groundwater from the Hama Uplift aquifer is too large and a complementary isotopic altitude effect must have modified the isotopic composition.
The spatial distributions of the dex values suggest that the catchment area of this groundwater might be situated in the northern piedmont of the Anti-Lebanon Mountains. The confined Homs Depression aquifer is close to this piedmont. Both Mediterranean (detected as predominant in samples nos. 26 and 27) and continental storms (predominant in sample nos. 31, 38 and 40) participated in the groundwater recharge. Well no. 39, from which the outlier sample was collected, is situated within the piedmont of the Anti-Lebanon Mountains. The δ18O value is more negative and the 14C age is lower than those values of all other samples possibly due to a somewhat higher and locally restricted catchment area and a shorter flow distance.
Table 4 Application of 14C age correction models for groundwater in the Orontes basin
4.3 Tritium and 14C residence time
Tritium values help to identify the presence of modern groundwater in samples which might have been recharged or admixed after about 1960. Admixture may be due to leakage through the confining bed, hydraulic shortcuts in the aquitards or defects in the well casings.
Tritium values of between 2 to 4 TU for the groundwater samples from the Coastal Mountains correspond to mean residence times of several decades. This is in agreement with those of the spring water discharging from the high Anti-Lebanon and Coastal Mountains (Al-Charideh Citation2011a). The majority of groundwater samples from the unconfined aquifer of the Hama Uplift contain tritium at between 2 TU and the detection limit of <0.4 TU, evidence that some groundwater recharge is occurring, and have higher mean residence times of 100 years, or even more. Most groundwater samples from the confined aquifer of Homs Depression had tritium values below the detection limit, as expected for Pleistocene groundwater in a confined aquifer. However, well nos. 29, 32, 35 and 37 (all 0.6 TU) might contain some modern groundwater.
Radiocarbon activities and δ13C values are commonly used to identify carbon origin in groundwater. In fact, atmospheric waters are “marked” by dissolution/precipitation processes taking place during water–rock interactions (Clark and Fritz Citation1997).
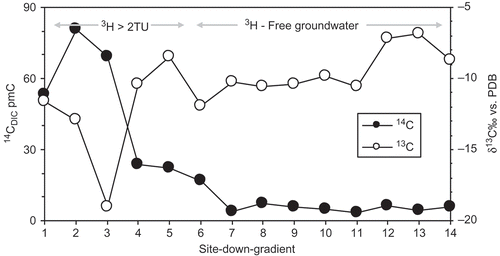
The δ13C contents of the TDIC ranges from –19.9 to –11.6‰ versus PDB for the recharge area, and from –12 to –6.9‰ for the unconfined and confined aquifer system of Hama Uplift and Homs Depression (). The 14C activities vary widely from 80.6 to 4% pmC. The highest values were measured in the recharge area which reveals the existence of modern recharge. The 14C activities decrease rapidly moving from the unconfined to the confined aquifer, ranging between 23 to 9 pmC in samples from the unconfined aquifer of Hama Uplift, to very low, between 7 and 4% pmC, in the confined aquifer which implies long water residence time (). Data indicate that groundwater is evolving from recent water with high 14C activities and low δ13C values towards older groundwater relatively enriched in 13C. This argument relates to different water–rock interaction conditions enhanced by different groundwater residence times (). Longer water residence times enhance equilibration with aquifer carbonate minerals resulting in lower 14C activity and more enriched δ13C compositions (Bath et al. Citation1979, Clark and Fritz Citation1997, Mook and De Vries Citation2000). As mentioned above, the several depleted values of δ13C observed in the confined aquifer could be explained by a larger contribution of organic matter resulting in a δ13C depleted source of carbon (Edmunds et al. Citation1995), or by different recharge regimes such as palaeo-recharge (Zourai et al. 2011).
Fig. 8 Evolution of 14C and δ13C values in the groundwater samples from the Upper Cretaceous aquifer in the study area.
In order to assess the groundwater residence time, different classic correction models of the initial activity within a carbonate aquifer have been used. These models are based on different mixing processes: models of pure chemical and isotopic mixing (Ingerson and Pearson Citation1964, Tamers Citation1975), and models of chemical mixing and isotopic exchange (Evans et al. Citation1979, Fontes and Garnier Citation1979, Salem et al. Citation1980, Eichinger Citation1983). The signatures for different carbon sources introduced in these calculations for our study have the following isotopic characteristics:
| a. | for biogenic carbon δ13Csoil = –21‰ vs V-PDB and 14C = 100% pmC | ||||
| b. | for carbonate matrix δ13Ccarb = 0‰ vs V-PDB and 14C = 0% pmC | ||||
Excluding the Tamers model, the 14C modelling indicates that the results of the five C14 correction models can be divided into two groups according to groundwater age range (). The first group includes the Pearson (Ingerson and Pearson Citation1964), Evans (Evans et al. Citation1979) and Eichinger (Citation1983) models which are based on isotope and chemical exchanges. The second group, from the IAEA (Salem et al. Citation1980) and Fontes and Garnier (Citation1979) models based on isotopic exchanges, lead to groundwater ages much greater than the first group; a maximum difference of 4000 years is observed. Consequently, adoption of the maximum range of the actual uncertainty of the different models into the error propagation leads to standard deviations of the calibrated groundwater 14C ages of ±5000. Therefore the spread of the calibrated ages is still within the actual confidence interval (). The Tamers model gives results similar to the first group for ages less than 15 000 years BP with a maximum difference of 3000 years less than the second group. However, this chemical model cannot be considered in this case study since the δ13C contents vary with the 14C activities (), which suggests an exchange between TDIC and the matrix of the carbonate aquifer through time.
Fig. 9 Relationship between δ18O and corrected age values for groundwater in the study area. Standard deviation shown by ± “whiskers” (5000 years for 14C age and 0.1‰ for δ18O).
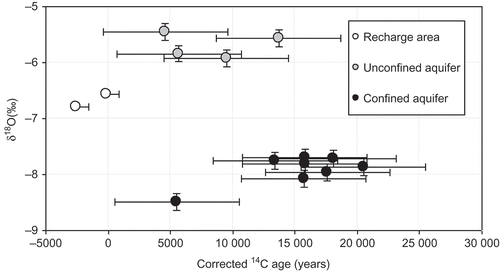
The IAEA and Fontes and Garnier models suggest exaggerated calibrated 14C ages for groundwater in the recharge area and the unconfined aquifer of Hama Uplift relative to those indicated by their stable isotope composition and tritium contents. Thus, in this case study, the first group of models (Pearson, Evans and Eichinger) seems to be more appropriate to adopt. Pearson ages are present day for the group in the recharge area, between 5000 and 13 750 years BP for the unconfined Cretaceous aquifer of Hama Uplift, and reach 21 500 years BP for the confined part of this aquifer in the Homs Depression.
Based on this correction, the calculated age indicates three groundwater recharge periods ():
| a. | Present-day recharge with δ18O similar to the modern rainwater, between –6.5 and –6.8‰, and 14C activities of 50–80 pmC. This water represents the recharge area of Coastal Mountains. Based on the stable and radioactive isotopic data, it is suggested that the present recharge continues to function, notably in the west, where the aquifer outcrops. | ||||
| b. | Holocene groundwater recharge, where the corrected age range is 5000 to 13 000 years BP. The groundwater has enriched δ18O values (–5.46 to –5.93‰) relative to modern groundwater. The shift in δ18O to higher values signifies warmer climatic conditions at the beginning of the Holocene (Bath et al. Citation1979). The groundwater of this group is found in the unconfined part of the Upper Cretaceous aquifer of the Hama Uplift. | ||||
| c. | Late Pleistocene groundwater recharge, where the corrected age ranges between 10 000 and 22 000 years BP. The groundwater has depleted δ18O values (–7.96 to –8.67‰) and low radiocarbon activities (3.9–7.2 pmC), which represent the groundwater of the confined aquifer of Homs Depression. It is suggest that the residence time of the water in the confined Cretaceous aquifer is considerably longer than that in the unconfined area. | ||||
The spatial distribution of the calibrated groundwater 14C ages calculated with any of the models used (Table 4) does not allow determination of the groundwater flow direction or estimation of the groundwater velocity. This may be due to the locally variable mixture of different old groundwaters flowing in various non-homogeneously distributed wide flow paths in the hard-rock aquifers. Another reason may be that groundwater is usually pumped only from a restricted section of the total water column of the aquifer, which differs from well to well.
In fact, these results of low stable isotope composition and 14C activities in the deep groundwater of the Upper Cretaceous aquifer in the Homs Depression are similar to those for the Upper Cretaceous aquifer in the Syrian Desert (Ferronsky et al. Citation1991), the Upper Jezireh in northeast Syria (Al-Charideh and Abou Zakhem Citation2009) and the Aleppo basin in northern Syria (Al-Charideh Citation2011b). According to the palaeoclimatic model of the Syrian Desert (Ferronsky et al. Citation1991), the period from 20 000 to 10 000 years BP can be characterized as being the climatic temperature minimum, when annual average temperatures were lower than modern ones by about 6°C. The main recharge events could have occurred and formed the major reservoir of groundwater in the Upper Cretaceous aquifer in Syria, in this period. Based on the similarity of the δ18O values and 14C activities in the deep groundwater from the Homs Depression, Aleppo basin, Syrian Desert and Upper Jezireh, one might suggest simultaneous recharge events. However, given the uncertainties regarding the contribution of 14C-free carbon by different factors, this inference should be considered with caution.
5 CONCLUSION
The chemical and isotopic characteristics of the groundwater in the Upper Cretaceous aquifers of the Orontes basin have revealed many important aspects of groundwater evolution in aquifers that have been created from carbonate rocks.
The hydrochemistry of groundwater from the Upper Cretaceous aquifer system varies in relation to different water–rock interaction processes. The dominant anions are HCO3 − for fresh water and SO4 2- and Cl− for the more saline waters. The geochemical processes generating the chemical composition of groundwater in the confined aquifer of the Homs Depression indicate a long-lasting water–rock interaction probably dominated by dissolution of halite and gypsum, and cation exchange of varying intensity could explain most of the observed spatial variability of the chemical groundwater composition.
The δ2H and δ18O results confirm this hydrogeological concept in general and improve the details. The groundwater in the Coastal Mountains is solely recharged by Mediterranean precipitation. The groundwater in the unconfined Hama Uplift aquifer and the confined Homs Depression aquifer is fed by the same precipitation but also recharged by continental rainfall further to the east. The groundwater of the unconfined Hama Uplift aquifer is recharged locally. Most groundwater in the confined Homs Depression aquifer might be recharged in the northern piedmont of the Anti-Lebanon Mountains at an altitude around 1900 m, though an inflow from the Hama Uplift aquifer cannot be excluded in the northeast. The lower recharge rate of the groundwater in the Hama Uplift aquifer, and even slower recharge of the Homs Depression aquifer is reflected by groundwater 14C ages of more than 5000 and over 22 000 years, respectively. The dispersed distribution of flow paths in the hard rock aquifers prevents determination of the flow velocity and estimation of the groundwater model ages. Another reason may be pumping from sections that deviate from the total aquifer. The calibrated groundwater 14C ages obtained using one of the models applied might be underestimated due to an incorrect choice of any of the parameters used, especially the initial δ13C and 14C values of the soil CO2 and soil carbonate.
Finally, in semi-arid regions such as the present area, where there are prolonged dry periods associated with little recharge and a high demand for groundwater for irrigation, environmental impact arises due to groundwater use. In this case, the extraction of groundwater appears to be exploitation of a non-renewable resource. Even if some recharge is taking place, it is not sufficient in volumetric terms to resolve the environmental problems. It is strongly recommended to improve and develop sustainable water resources management by using modern irrigation methods.
Acknowledgements
The author would like to thank Prof. I. Othman, Director General of AECS for use of their facilities during this study. We are also grateful to M. Geyh, W.R. Agha and S. Rammah for their helpful comments and useful discussion. The author thanks Z. Kattan, Head of the Geology Department at the AECS, and the staff of the laboratories for their cooperation in performing the isotopic and chemical analyses.
REFERENCES
- Abd El Samie , S.G. and Sadek , M.A. 2001 . Groundwater recharge and flow in the lower Cretaceous Nubian sandstone aquifer in the Sinai Peninsula, using isotopic techniques and hydrochemistry . Hydrogeology Journal , 9 : 378 – 389 .
- Al-Charideh , A. 2011a . Environmental isotope study of groundwater discharge from the large karst springs in west Syria: a case study of Figeh and Al-sin springs . Environmental Earth Science , 63 : 1 – 10 .
- Al-Charideh , A. 2011b . Geochemical and isotopic characterization of groundwater from shallow and deep limestone aquifer system of Aleppo basin (north Syria) . Environmental Earth Science , 65 ( 4 ) : 1157 – 1168 .
- Al-Charideh , A. and Abou Zakhem , B. 2009 . Geochemical and isotopic characterization of groundwater from the Paleogene aquifer of the Upper Jezireh, Syria . Environmental Earth Science , 59 : 1065 – 1078 .
- Al-Charideh , A. and Abou Zakhem , B. 2010 . Distribution of tritium and stable isotopes in precipitation in Syria . Hydrological Sciences Journal , 55 ( 5 ) : 832 – 843 .
- Bajjali , W. , Clark , I.D. and Fritz , P. 1997 . The artesian thermal groundwater of the northern Jordan: insights into their recharge history and age . Journal of Hydrology , 92 : 355 – 382 .
- Bath , A.H. , Edmunds , W.M. and Andrews , J.N. 1979 . “ Palaeoclimatic trends deduced for the hydrogeochemistry of a Triassic sandstone aquifer, UK ” . In Isotope hydrology , 545 – 567 . Vienna : International Atomic Energy Agency . In:
- Brew , G. 2001 . Tectonic and geologic evolution of Syria . GeoArabic , 6 ( 4 ) : 573 – 616 .
- Chapelle , F.H. and Knobel , L.L. 1983 . Aqueous geochemistry and the exchangeable cation composition of glauconite in the Aquia aquifer, Maryland . Ground Water , 21 ( 3 ) : 343 – 352 .
- Clark , I.D. and Fritz , P. 1997 . Environmental isotopes in hydrogeology , Boca Raton , FL : Lewis .
- Cook , P. and Herczeg , A.L. 2000 . Environmental tracers in subsurface hydrology , New York : Kluwer .
- Coplen , T.B. 1996 . New guidelines for reporting stable hydrogen, carbon, and oxygen isotope-ratio data . Geochimica et Cosmochimica Acta , 60 : 3359 – 3360 .
- Coplen , T.B. , Wildman , J. and Chen , J. 1991 . Improvement in the gaseous hydrogen–water equilibration technique for hydrogen isotopes ration analysis . Analytical Chemistry , 63 : 910 – 912 .
- Craig , H. 1961 . Isotopic variations in meteoric waters . Science , 133 : 1702 – 1703 .
- Edmunds , W.M. , Smedley , P.L. and Spiro , B. 1995 . “ Controls on the geochemistry of sulphur in East Midlands Triassic aquifer, UK ” . In Isotopes in water resources management , 107 – 122 . Vienna : International Atomic Energy Agency . In
- Eichinger , E. 1983 . A contribution to the interpretation of the 14C groundwater ages considering the example of a partially confined sandstone aquifer . Radiocarbon , 25 : 347 – 356 .
- El Naka , A. and Al Kuisi , M. 2004 . Hydrogechemical modeling of the water seepages through Tannur Dam, southern Jordan . Environmental Geology , 45 : 1087 – 1100 .
- Epstein , S. and Mayeda , T.K. 1953 . Variations of 18O content of waters from natural sources . Geochimica et Cosmochimica Acta , 4 : 213 – 224 .
- Evans , G.V. 1979 . “ Some problems in the interpretation of isotope measurements in United-Kingdom aquifers ” . In Isotope hydrology , 639 – 708 . Vienna : International Atomic Energy Agency . In
- Ferronsky , V.I. , Polyakov , V.A. and Ferronsky , S.V. 1991 . “ Isotope variations in water in the hydrological cycle as a tool in a climate change mechanism study ” . In International symposium on isotope techniques in water resources development , 567 – 586 . Vienna : IAEA . In:
- Fontes , J.-Ch. and Garnier , J.M. 1979 . Determination of the initial 14C activity of the total dissolved carbon: a review of the existing models and new approach . Water Resources Research , 15 : 399 – 413 .
- Gat , J.R. and Carmi , I. 1970 . Evolution of the isotopic composition of atmospheric waters in the Mediterranean Sea Area . Journal of Geophysical Research , 75 : 3039 – 3048 .
- Gat , J.R. and Issar , A. 1974 . Desert isotope hydrology: water sources of Sinai . Geochimica et Cosmochimica Acta , 38 : 117 – 1131 .
- GCHS (General Company of Hydraulic Studies) . 2003 . Collection geological data from the deep wells and relevant report in the entire Orontes basin , Damascus , Syria : GCHS, Unpublished report .
- Geyh , M.A. 1992 . “ The 14C time-scale of groundwater. Correction and linearity ” . In Isotope techniques in water resources development , 167 – 177 . Vienna : International Atomic Energy Agency . In:
- Hatem , M. 1995 . New conception of underground water movement in the Cretaceous region . Geological Science Review (in Arabic) , 5 : 41 – 50 .
- IAEA (International Atomic Energy Agency) . 1980 . “ Arid zone hydrology: investigations with isotope techniques ” . In Proceedings of an advisory group meeting , Vienna : IAEA . In:
- IAEA (International Atomic Energy Agency) . 1983 . “ Palaeoclimatic and paleowaters: a collection of environmental isotope studies ” . In Proceedings of an advisory group meeting , Vienna : IAEA . In:
- Ingerson , E. and Pearson , F.J. 1964 . “ Estimation of age and rate of motion of groundwater by the 14C method ” . In Recent researches in the fields of atmosphere: hydrosphere, atmosphere and nuclear geochemistry , 263 – 283 . Tokyo : Maruzen, Sugawara Festival Volume . In:
- Jankowski , J. and Acworth , R.I. 1997 . Impact of depris-flow deposits on hydrogeochemical processes and the development of dryland salinity in the Yass River catchment, New South Wales. Australia . Hydrogeology Journal , 5 ( 4 ) : 71 – 88 .
- Kattan , Z. 2002 . Effects of sulphate reduction and geogenic CO2 incorporation on the determination of 14C groundwater ages—a case study of the Paleogene groundwater system in north-eastern Syria . Hydrogeology Journal , 10 : 495 – 508 .
- Love , A.J. 1994 . Groundwater residence time and palaeohydrology in the Otway Basin, South Australia . Journal of Hydrology , 153 : 157 – 187 .
- Magaritz , M. 1981 . The use of Na/Cl ratio to trace solute sources in semiarid zone . Water Resources Research , 17 : 602 – 608 .
- Mook , W.G. and De Vries , J.J. 2000 . Environmental isotopes in the hydrological cycle: principles and applications , 39 Paris : UNESCO, International Hydrological Programme .
- Nordstrom , D.K. and Ball , J.W. 1989 . Mineral saturation states in natural waters and their sensitivity to thermodynamic and analytic errors . Science Geological Bulletin , 42 ( 4 ) : 269 – 280 .
- Plummer , L.N. 1990 . Geochemical modeling of the Madison aquifer in part of Montana, Wyoming and South Dakota . Water Resources Research , 29 : 1981 – 2014 .
- Plummer , L.N. , Perstemon , E.C. and Parkhurst , D.L. 1994 . “ An interactive code (NETPATH) for modeling NET geochemical reaction along a flow PATH-version 2.0 ” . In US Geological Survey Water Resources Investigations Report 94-4169
- Ponikarov , V.O. 1967 . “ The geology of Syria, explanatory notes on the map of Syria, Scale 1/500 000, Part II ” . In In: Mineral deposits and underground water resource , Moscow : Technoexport .
- Rasoul Agha , W. 1999 . “ Deep non-renewable groundwater in Syria and future strategic options for the management of water resources ” . In International conference on regional aquifer systems in arid zones—managing non-renewable resources. In(Tripoli, Libya) (in Arabic)
- Salem , O. 1980 . “ Groundwater flow patterns in the western Libyan Arab Jamahiriya ” . In Arid zone hydrogeology: investigations with isotope techniques , 165 – 179 . Vienna : International Atomic Energy Agency . In:
- Sami , K. 1992 . Recharge mechanisms and geochemical processes in semi-arid sedimentary basin, Eastern Cape, South Africa . Journal of Hydrology , 139 : 27 – 48 .
- SDWC (Syrian–DutchWater Cooperation) . 2008 . Development of a numerical groundwater flow model for the larger Orontes Basin. Damascus , Syria : SDWC . 2008-U-R0408/B, Unpublished report
- Selkhozpromexport . 1979 . “ Hydrogeological and hydrological surveys and investigation in four areas of Syrian Arab Republic, Orontes area. Vol II ” . In Hydrogeology , 234 Tbilisi , USSR : Georgian State Institute for Design of Water Resources Development Project .
- Tamers , M.A. 1975 . Validity of radiocarbon dates on groundwater . Geophysical Surveys , 2 : 217 – 239 .
- Tamers , M.A. and Scharpensel , H.W. 1970 . “ Sequential sampling of radiocarbon in groundwater ” . In Isotope hydrology , 241 – 256 . Vienna : International Atomic Energy Agency . In:
- Vengosh , A. 2007 . New isotopic evidence for the origin of groundwater from the Nubian Sandstone Aquifer in the Negev, Israel . Applied Geochemistry , 22 : 1052 – 1073 .
- Wagner , W. and Geyh , M.A. 1999 . Application of environmental isotope method for groundwater studies in the ESCWA region , 129 New York : United Nations, Economic and Social Commission for Western Asia .
- Zouari , K. , Trabelsi , R. and Chkir , N. 2011 . Using geochemical indicators to investigate groundwater mixing and residence time in the aquifer system of Djeffara of Medenine (southeastern Tunisia) . Hydrogeology Journal , 19 : 209 – 219 .