ABSTRACT
The objective of this research was to evaluate the groundwater chemical and bacteriological quality in the Barranquita-Knutzen basin (Córdoba, Argentina). The main purpose was to trace contamination through the examination of bacteriological parameters and antibiotic resistance of Escherichia coli, relating them to surface water, hydrogeological features and land use. Thus, 40 water samples were collected. The major chemical components and bacterial indicators were determined and antibiotic resistance was analysed using standard methods. Multivariate factorial analysis showed that the first principal component (PC) reveals the process of water mineralization, while the second and the third PCs explain a low percentage of variance, but collect chemical constituents and total and faecal coliform bacteria, respectively, revealing specific contamination situations. The observed antibiotic resistance profiles of E. coli and their relation with the land uses revealed that the source of faecal contamination in water is mainly from animal residues.
Editor R. Woods; Associate editor S. Lyon
Introduction
Pollution affecting surface water and groundwater is one of the priority issues in many research, management and even policy groups. In recent decades, there has been increasing concern about the final destination of numerous chemical and biological pollutants, which have a strong impact on water bodies (Katz et al. Citation2009, Kuroda et al. Citation2012, Pawar Citation2013). Pathogenic bacteria, pesticides, fertilizers and industrial and pharmaceutical by-products are typical examples (Chin Citation2006). In agricultural ecosystems, agrochemicals, dairy farms and extensive and, especially, intensive breeding of cows, pigs and poultry are potentially contaminating sources (Cirelli and Mortier Citation2005, Pruden Citation2009, Hafner et al. Citation2016).
Water quality assessment includes the study of physical, chemical and/or biological indicators (Chin Citation2006). In order to make an accurate diagnosis when groundwater is studied, the mentioned indicators cannot be measured and evaluated ignoring the hydrogeological conditions, since the degree of groundwater contamination depends strongly on them. Among the most important features to take into account, the unsaturated zone (UZ) thickness, the permeability of the UZ sediments and the groundwater flow direction can be mentioned (Foster et al. Citation2002). Moreover, as has been demonstrated in many parts of the world, as well as in the region where this study was made (Becher Quinodoz et al. Citation2017), groundwater is hydraulically related to surface water in different ways. This aspect implies that it is better to evaluate groundwater–surface water interconnections and the quality of both ecosystems. Furthermore, it is important to take into consideration the contaminant sources features, such as temporal and spatial distributions. Several investigations have demonstrated the importance of measuring different contamination indicators to assess groundwater quality (Plummer and Long Citation2007, Sinclair et al. Citation2009, Chidya et al. Citation2016). However, few have made complete studies taking into account the relationship between bacterial indicators, hydrogeological aspects and land use (Kuroda et al. Citation2012, Chen et al. Citation2016).
The detection of faecal contamination is relevant when groundwater is used for consumption, since it indicates the possible presence of pathogenic micro-organisms and, as a consequence, the potential risk to human health (Ibekwe et al. Citation2011, Carlos et al. Citation2012). Although Escherichia coli is the best indicator of faecal contamination, its presence in the water does not provide definitive information about its possible origin (Unno et al. Citation2010, Ibekwe et al. Citation2011). This is the reason why phenotypic methods, such as antibiotic resistance profiles of E. coli, have been used as a tool to elucidate the origin of the contamination source in various aquatic environments (Meays et al. Citation2004, Gourmelon et al. Citation2007, Ksoll et al. Citation2007, Ibekwe et al. Citation2011). In rural areas, antibiotics are used in veterinary medicine in a prophylactic way (antiparasites and antibiotics) or as growth promoters (antibiotics used in sub-therapeutic doses), generating selective pressure on indicators of faecal contamination. This selective pressure may be a useful criterion for identifying the contamination sources of E. coli in water by assessing antimicrobial sensitivities (Ibekwe et al. Citation2011). Moreover, the use of these compounds in agro-ecosystems, coupled with the intensification of animal husbandry in increasingly smaller areas, increases surface water and groundwater contamination with the above-mentioned substances (Kummerer Citation2003, Cirelli and Mortier Citation2005, Pruden Citation2009).
In addition, it is important to highlight, as stated by the World Health Organization (WHO Citation2016), that antibiotic resistance is one of the biggest threats to global health, food security and current development. It can affect anyone, of any age, in any country. Even though antibiotic resistance occurs naturally, the misuse of antibiotics in humans and animals is accelerating the process. Thus, a growing number of infections – such as pneumonia, tuberculosis and gonorrhoea – are becoming harder to treat as the antibiotics used become less effective. Antibiotic resistance leads to longer hospital stays, higher medical costs and increased mortality (WHO Citation2016).
The identification of faecal contamination sources by microbial source-tracking methods, such as antimicrobial sensitivities, has been evaluated in various studies (Simpson et al. Citation2002, Vogel et al. Citation2007). However, few complete studies investigated the relationship between source-tracking methods, hydrogeological aspects and land use (Tran et al. Citation2015). When groundwater is studied, it is necessary to know the basic hydrogeological conditions of the sampling environment, such as the aquifer layer being sampled and the relationships with other environmental features. Therefore, an integrated study that includes analysis of the profiles of antibiotic resistance related to physico-chemical and hydrological characteristics and land use could provide a more complete result. The application of such contaminant source-tracking strategies may offer a more efficient means to identify pollution sources and effective means of remediation. As was stated by Tran et al. (Citation2015), the suitability of chemical and microbial markers for faecal pollution source tracking varies from region to region, depending on many factors such as land-use patterns, population of humans and grazing animals, hydroclimatic influences, geology and characteristics of pollution sources.
The groundwater of the south of Córdoba Province (Argentina), which is almost entirely in sedimentary aquifers, is not unrelated to this problem. The interaction between water and human activities such as farming and livestock has generated important environmental problems, recognized by the regional community, lacking diagnosis in many cases and management measures in others (Blarasin et al. Citation2005). For several years, different physical and chemical indicators (water levels, electrical conductivity (EC), nitrates, chlorides, among others) have been measured in different ecosystems in this region, and some environmental changes have been identified in relation to water dynamics and quality (Blarasin et al. Citation2008). Moreover, some results about resistant bacteria have been found already (Gambero et al. Citation2016). However, the need to expand indicators is increasingly evident, since they are useful to assess the relationships among environmental variables, detecting anomalies and causes and consequences of environmental changes. In this way, it is possible to contribute to the improvement of the water resources and environmental management.
In this context, the main hypothesis of this study is that concentrated animal husbandry impacts unconfined aquifers (groundwater) and streams (surface water) and that this impact can be traced via Escherichia coli phenotypic characterization. Accordingly, the objective of this work is to trace aquifer contamination through analysis of bacteriological parameters and antibiotic resistance of E. coli, relating them to surface water and groundwater features and to land use. The study is made in the Barranquita-Knutzen basin, located in Córdoba province (Argentina).
Study area
Climate, land use and hydrogeology
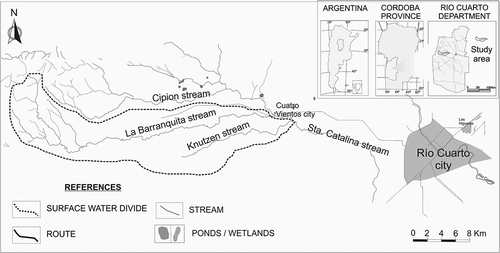
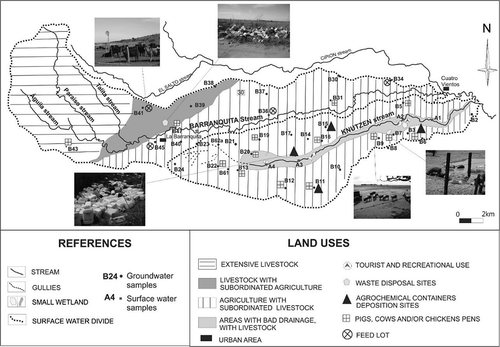
The Barranquita-Knutzen basin covers an area of 292 km2. The basin is located in the Chacopampeana Plain, in the south of Córdoba Province, Argentina (). The climate is dry sub-humid with little water excess. Hydroclimatic data were obtained from the La Aguada Series (1994–2011) (Giuliano Citation2013). The average annual precipitation (Pa) is 732 mm, being distributed into two periods, the wettest being November–March with 70% of the total precipitation. The wettest year of the series was 1998, with 1080 mm/year and the driest was 2011 with 430 mm/year. Water for human consumption and livestock activities is mostly supplied by the unconfined aquifer. The land use is mainly farming, which includes soybean, wheat and corn crops, grown by means of large amounts of pesticides and fertilizers (phosphates, sulphates and urea). Some farmers indicate that they use 100–120 kg urea/ha. Livestock activity includes breeding of cows, pigs, sheep, horses and poultry, and it is worth mentioning that cattle frequently go to the streams to drink water ().
The unconfined aquifer is made up of loess sediments (mainly very fine sands and silts) interlayered with palaeochannels that lie at different depths (sands and gravels with high hydraulic conductivity). The loess sediments present partial and local cementation with calcium carbonate. The general groundwater flow is in almost a west–east direction, from the piedmont to the lowland areas (), but different local flow directions can be observed. The depth of the water table is variable, ranging from 2.5 to 24.0 m b.g.l. This parameter and groundwater flow are mainly controlled by the relief topography (Giuliano Albo and Blarasin Citation2014). As can be seen in , groundwater feeds the water courses (gaining streams), except in the piedmont sector where groundwater flow lines diverge indicating the presence of a recharge area. The streams generally show low flows and low flow velocity (less than 0.5 m3/s and 0.3 m/s respectively), especially in the Knutzen basin (Giuliano Citation2013). The lithology of the UZ and water table depth are key factors that control the aquifer recharge and the aquifer vulnerability to contamination (Giuliano Albo and Blarasin Citation2014). The upper basin is characterized by a UZ with coarser sediments, but the water table level is deepest (up to 24 m b.g.l.), while mid and lower basins exhibit shallow water table depths (< 2.5 m b.g.l.) and finer sediments (sand and silts).
Figure 3. Study area showing sampling sites, groundwater flow direction and electrical conductivity (EC) in the Barranquita-Knutzen basin, Córdoba, Argentina.
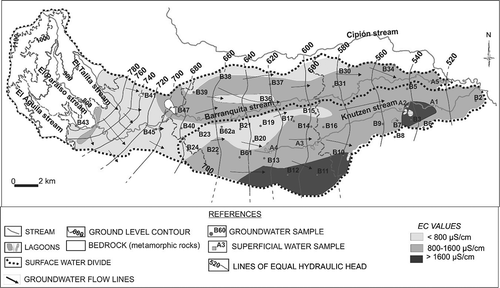
The EC values of surface water and groundwater are in the ranges 508–2050 µS/cm and 654–1078 µS/cm, respectively (). The water geochemical types are variable, from calcium-sodium bicarbonate to sodium sulphate, and depend on the geochemical processes, described for this aquifer in Giuliano Albo and Blarasin (Citation2014), which are predominantly salt dissolution and cation exchange and, to a lesser extent, mineral hydrolysis.
Methods
Water sampling
Environmental water samples (n = 40) were collected in the wet season (spring) during October 2008, a sampling year during which the precipitation was equal to the average annual precipitation (732 mm/year). Thirty-six samples were collected from groundwater and four from surface water, the latter limited by accessibility. The water samples (500 mL) were taken for bacteriological indicator analysis according to the Argentine Food Code (AFC Citation2012). All samples were collected aseptically in sterile bottles, stored at 4°C and analysed within 24 h of collection in the laboratory of the National University of Rio Cuarto (NURC, Argentina). The chemical characteristics of surface water and groundwater were measured in a duplicated sample in the Geochemistry Laboratory of the Department of Geology (NURC) following Standard Methods for Examination of Water and Wastewater (APHA Citation2005) procedures (Giuliano Albo and Blarasin Citation2014). Typical guidelines in hydrogeological surveys were followed. Thus, to ensure the representativeness of groundwater samples, each well was emptied three times. The bottles were treated and cleaned in the laboratory, and the water samples were filtered in the field. Moreover, typical in situ measurements were taken with a multiparametric probe (Hanna HI 9828): temperature (T), dissolved oxygen (DO), pH and electrical conductivity (EC). The chemical variables analysed were Na+, K+, Mg2+, Ca2+, HCO3−, Cl−, SO42− and NO3− using Merck chemical products (Argentina) through titration (Mg2+, Ca2+, HCO3−, Cl−), flame photometry (Na+ and K+), turbidimetry (SO42−) and ion selective electrode methods (NO3−).
Bacteriological analysis
The determination of heterotrophic plate counts (HPC) was carried out in plate count agar, incubated at 35°C for 24 h. The total (TC) and thermotolerant or faecal coliforms (FC) were determined through the multiple-tube fermentation (MTF) technique. Probability tables (McCrady tables) were used to determine the most probable number (MPN) and to estimate the number of coliform organisms per 100 mL of water. The TC were incubated in MacConkey broth (Britania, Argentina) at 35°C for a period of 24–48 h and FC in BRILA broth (brilliant green bile lactose 2% broth) (Britania, Argentina) at 44.5°C for 24 h. The presence of E. coli was determined in 100 mL of the sample in MacConkey broth incubated at 35°C for 24–48 h. Then, an aliquot was spread onto EMB agar (eosin methylene blue) (Britania, Argentina) plates and incubated at 35°C for 24 h. Isolates were confirmed as E. coli by using a series of biochemical tests, including indole, Voges-Proskauer, methyl red tests, and the inability to grow on citrate agar (IMViC test) (Britania, Argentina). The presence of Pseudomonas aeruginosa was determined on a volume of 100 mL of sample in asparagine broth incubated at 35°C for a period of 24–48 h. The isolation was carried out in cetrimide agar (Britania, Argentina) plates and colonies were confirmed by the following biochemical tests: oxidase, growth at 42°C, and pigment production in pseudomonas agar P and F (Britania, Argentina). The methodology was carried out according to APHA (Citation2005) and AFC (Citation2012).
Antibiotic resistance analysis
The isolated and identified strains of E. coli were evaluated for resistance to antibiotics by the plaque diffusion method using six antibiotic discs (Bauer et al. Citation1996, Gambero et al. Citation2016). They correspond to the drugs most commonly used in the treatment of infections caused by gram-negative bacilli in both humans and animals and on their use as a food additive and as growth promoters in animals according to Laplumé et al. (Citation2011) (). An E. coli inoculum was prepared in tripticasa soya broth (Britania, Argentina) of approximately 2 × 108 cfu/mL, whose turbidity corresponded to tube number 0.5 on the McFarland scale. Therefore, 200 μL of the cultivation was placed in 5 mL of sterile physiological solution and the optical density (600 nm) of each mixture was adjusted to about 0.08. The bacterial suspension was inoculated onto plates with 150 mm of Mueller Hinton agar (Britania, Argentina) and then the commercial antibiotic (Britania, Argentina) discs were put in place. The plates were incubated at 35°C for 18–20 h. Diameters (in mm) of the clear areas of growth inhibition around each antibiotic disk were measured with a precision caliper. The criterion of sensitivity or resistance to each antimicrobial was determined as established by CLSI (Clinical and Laboratory Standard Institute Citation2016). E. coli strain ATCC 25922 was used as a control.
Table 1. Tested antibiotics.
Statistical analysis
For statistical analysis of bacterial contents and physico-chemical variables, the SSPS v.11.5 package was used. Associations between bacterial counts and NO3− were analysed by Pearson (R) correlation. The multivariate analysis was performed using the factorial method by principal components (PC) to determine possible relationships between bacterial contents (TC and FC) and physico-chemical variables (EC, T, DO, pH, HCO3−, SO42−, Cl−, Na+, K+, Ca2+, Mg2+ and NO3−). The variable TDS (total dissolved salts) was not considered in the multivariate analysis, because it is represented by the EC.
Results
Groundwater and surface water bacteriological quality
The results of bacteriological analysis of groundwater samples and human consumption aptitude are shown in . Thus, 19% (seven) wells were positive for faecal coliforms (4–430 MPN/100 mL) and 22% (eight) wells were positive for E. coli. Moreover, the presence of Ps. aeruginosa was observed in 22% of the samples. In general, groundwater bacteriological contamination was high in 30% of the samples because of high levels of bacteriological indicators. The most affected samples were those extracted from wells B6, B13, B16, B17, B24, B31, B38, B39, B43, B61 and B62a.
Table 2. Bacteriological groundwater quality. HPC: heterotrophic plate count, TC: total coliforms, AFC: Argentine Food Code.
The results of bacteriological analysis of surface water samples showed higher values of HPC and TC than for groundwater samples. Only one sample showed Ps. aeruginosa (). In addition, it was detected that two samples were positive for FC (75 and 900 MPN/100 mL) and all the samples were positive for E. coli.
Table 3. Bacteriological surface water quality. HPC: heterotrophic plate count, TC: total coliforms, FC: faecal coliforms.
Association between chemical indicators and bacterial counts
The results of the chemical analysis used as a basis for this study are shown in . The analysis of association between geochemical indicators and bacterial counts was performed for the total groundwater and surface water samples. The correlation analysis was statistically insignificant (r = −0.133 and r = −0.114) between TC, FC and nitrate, respectively, and it was significant for TC compared to FC.
Table 4. Values of chemical parameters in surface water and groundwater.
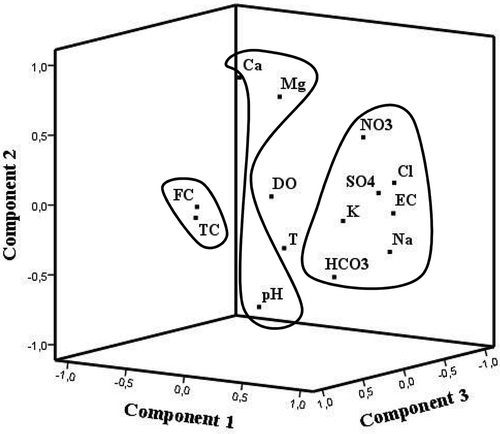
The factor analysis by PC ( and ) revealed three principal components, which explained 71% of the total variance. The first component, which explained 35% of the total variance, gathers the following parameters: EC, HCO3−, SO42−, Cl−, Na+, K+ and NO3−, which show, in general, high correlation. The second component (20% of the total variance), collects Ca2+ and Mg2+ moderately associated with NO3−, and all of those opposed to pH. Finally, the third component (16% of the total variance) contains the total and faecal coliform variables, showing high correlation. Variables T and DO are poorly correlated with the other variables.
Land use and antibiotic resistance of E. coli strains
The land-use types where the E. coli strains were isolated are shown in . From a hydraulic point of view and the behaviour of wells in the basin, the land use surrounding the sites was especially taken into consideration (300 m). Of the total groundwater samples (n = 7), in six samples, agriculture with subordinate livestock activity is highlighted, while in one only sample (B62a), livestock activity only is observed. In addition, in all the samples the livestock activity is intensive (concentrated animal), mainly identified by pig, sheep and cow pens. On the other hand, at the sampling sites for surface water it was observed that cattle drink water in the streams sporadically and surrounding farming activities were observed. From total water samples, 17 E. coli isolates were recovered (n = 9 from the streams and n = 8 from the unconfined aquifer) and tested for their resistance to antibiotics. All isolates were resistant to one or more antibiotics. A different resistance pattern was observed between the strains from the surface water and those recovered from groundwater. The first and second most prevalent antibiotic resistance was demonstrated in relation to ampicillin and tetracycline in both ecosystems. The highest resistance to ampicillin and tetracycline was found in groundwater samples ().
Table 5. Rotated component matrix (varimax method).
Table 6. Land-use characteristics and strains of E. coli isolated at each site in the Barranquita-Knutzen basin.
Table 7. Antibiotic resistance pattern of E. coli isolates from groundwater and surface water. AMP: ampicillin, TET: tetracycline, CEF: cephalotine, CIP: ciprofloxacin, AMC: amoxicillin + clavulanic acid and CHL: chloramphenicol.
Discussion
Bacteriological groundwater quality measurements were compared to the Argentine Food Code (AFC, Citation2012) water quality standards. The results indicate that 53% of the samples do not fit to those standards. Most of the groundwater samples were affected by faecal contamination. This result can be linked to the land use detected around the wells, such as pens of sheep, pigs, goats, cows and horses. All surface water samples were affected by contamination showing the highest bacterial counts, as well as E. coli. The elevated bacterial counts and the presence of E. coli along the sampling stream sites could be linked to cattle that drink the water, as was detected in the field. The high bacterial contents are favoured by low stream flow and low flow velocity, especially in the Knutzen basin, a situation that decreases the chances of hydrodynamic dispersion. Merlo et al. (Citation2017) state that the bacterial community composition in a river of Córdoba province was very variable among sites and water flow periods. For the same river, Pasquini et al. (Citation2012) described water chemical contamination in relation to surrounding soils.
As was stated, the presence of faecal coliform bacteria and E. coli in water represents a risk to consumer’s health and to the environment, since they indicate the possible existence of pathogenic micro-organisms. The transmission of E. coli pathogenic strains through contaminated recreational, consumption, irrigation and sewage waters is well documented (Hunter et al. Citation2000, Price et al. Citation2006, Hamner et al. Citation2007, Mull and Hill Citation2009).
The results of factorial analysis by PC analysis () are strongly conditioned to the major number of groundwater samples. They show that the first component, which usually explains general aspects or processes, is formed by EC, HCO3−, SO42−, Cl−, Na+, K+ and describes the water salinization factor, moderately associated with NO3− ions, whose high value in pollution situations can be significant as a contributor to water salinity. The second component, formed by Ca2+ and Mg2+, slightly associated with NO3−, represents nutrients that can pass from organic to inorganic forms by degradation of organic matter (OM) and become available to dissolve in water. Similar observations have been made for a landfill leachate-affected aquifer (Roling et al. Citation2001). The ions Ca2+ and Mg2+ are inversely correlated with pH and moderately with HCO3− which would allow us to interpret carbonate dissolution linked to the acidity generated by the OM arrival and its decomposition in this aerobic environment (C6H12O6 + 6O2 → 6CO2 + 6H2O). The anomalous Ca2+ values observed in samples B43 and B45 (72 and 66.4 mg/L, respectively) and Mg2+ in samples B43, B62a, B47 and B41 (20.97, 31.7, 52.19 and 40 mg/L, respectively) correspond to wells that are surrounded by cattle. Also in these samples, high levels of bacteriological indicators and high concentrations of nitrate (range: 13–60 mg/L) were observed. Analysing all these variables together, these samples can be interpreted as local contamination situations. Finally, the third component, made up of total and faecal coliform bacteria, highly correlated, indicates local pollution situations derived from faecal sources. For example, sample B43, strongly impacted by bacterial contamination, shows high FC counts (430 MPN/100 mL for both cases) and E. coli presence. These results may be explained by the surrounding livestock (goat, pig and poultry pens). Variables DO and T did not have a significant weight in any of the three components, indicating that they are not controlling variables in this situation.
The NO3− is an excellent indicator of contamination in the agro-ecosystem. As a result, higher values than natural background (estimated as 10 mg/L NO3− by Giuliano Albo and Blarasin Citation2014), indicate contamination mainly from livestock wastes and organic and inorganic fertilizers. According to the aerobic environment that predominates in the soil and the entire unsaturated zone and the important quantities of dissolved oxygen in the aquifer, the main species of N is always nitrate because of the NH4+ oxidation (NH4+ + 2O2 (Nitrosomones) NO2− + O2 (Nitrobacter) NO3−). Very low NH4+ values (0.06–0.56 mg/L) have been determined in a few groundwater samples (Giuliano Albo et al. Citation2015). A wide range of NO3− values was found in water samples (3.0–192.5 mg/L (). However, it may be highlighted that a correlation between bacterial counts and NO3− was not found, which may indicate that NO3− originated from inorganic sources, e.g. fertilizer. Similar results were obtained by Perdomo et al. (Citation2001), Picone et al. (Citation2003) and Rodríguez et al. (Citation2012). Giuliano Albo et al. (Citation2015) used NO3− isotopes to trace the origin of groundwater contamination in this regional agro-ecosystem. They concluded that, although there are numerous wells contaminated with nitrates derived from fertilizers, the highest values come from point contamination generated by livestock.
The hydrogeological setting plays an important role in groundwater contamination, especially the depth of the water table and unsaturated zone lithology, parameters that control the aquifer vulnerability to contamination. Thus, the UZ exerts protection on the aquifer through physical (advection, dispersion and dilution), chemical (adsorption, oxidation, hydrolysis, redox reaction, dissolution) and biological (degradation or reduction) processes, which produce the degradation or attenuation of contaminants, influencing their arrival at the aquifer (Díaz Delgado et al. Citation2005). However, as was observed in this study, many contaminants persist through infiltration and reach the aquifer, in spite of the fact that the UZ lithology found was represented by fine sands and silts, often cemented with carbonate salts. The most contaminated wells (B6, B13, B17, B31, B43 and B61) showed shallow depths of the water table (2.58–13.2 m b.g.l.). This aspect is also greatly influenced by the contaminant load (duration, concentration, etc.). In this case, the bacteriological analysis carried out on samples taken near livestock breeding sites showed high concentrations of total and faecal coliform and E. coli.
In the studied area, analysis of samples along both ecosystems (streams and aquifer) showed that the antibiotic resistance of E. coli populations was mainly resistant to a single antibiotic (ampicillin, AMP), with some isolates having resistance to two antibiotics. These results coincide with those of other studies (Webster et al. Citation2004, Laroche et al. Citation2010), in which bacterial low multi-antibiotic resistance rates have been discovered in rural environments. The highest percentages of resistance were observed for AMP followed by tetracycline (TET). In the surface environment the observed resistance profile was AMP–TET, whereas in groundwater three patterns are highlighted: AMP–TET, AMP–CEF (cephalotine) and AMP–AMC (amoxicillin + clavulanic acid), with AMP resistance being the most prevalent. Strains isolated from surface water samples showed lower percentages of antibiotic resistance than those isolated from groundwater. In addition, resistant E. coli isolates were found at sites A1, A3 and A4; a result that would indicate diffuse sources of faecal contamination arriving at the stream.
In contrast, in the groundwater environment, high percentages of resistance and the observed resistance profiles demonstrate the arrival of faecal contamination from more concentrated point sources. The resistance to ampicillin and tetracycline may suggest that these two antimicrobials are present throughout the basin since they had the most common antimicrobial resistance detected in the water samples. The frequency of resistance to both antibiotics is interesting, since ampicillin and tetracycline are used frequently in food-producing animals as medicine and food additives (Ibekwe et al. Citation2011). These results coincide with those obtained by other authors who have demonstrated a predominant spread of this resistance in the faecal bacterial population isolated from aquatic environments (Mackie et al. Citation2006, Fluckey et al. Citation2007, Sapkota et al. Citation2007, Alexander et al. Citation2008).
In the studied area, it was found that E. coli isolated from groundwater with resistance to AMP and TET was related to sites surrounded by cattle, which could be the main sources of faecal contamination in the unconfined aquifer. In other samples (B43 and B62a), E. coli showed resistance to amoxicillin + clavulanic acid and cephalotine. Near these sites, cattle activity (pigs, goats and a bovine feed lot) and the on-site sanitation systems belonging to the farmer’s house were surveyed. Resistance to these antibiotics is not surprising since the use of these antimicrobials is related to different therapeutic uses for human diseases (Ibekwe et al. Citation2011). Therefore, at these sites, inefficient or badly designed on-site sanitation systems could be the main source of faecal contamination.
Conclusions
This study showed that both groundwater and surface water from the Barranquita-Knutzen basin were impacted by pollution. Although the unsaturated zone provides important protection for the unconfined aquifer by attenuating the arrival of bacteria, the bacteriological quality analysis showed that 53% of the groundwater samples were unsuitable for drinking water. The surface environment was more affected, with all samples showing high concentrations of the different bacterial indicators.
However, the association between the geochemical indicators and the bacterial counts, using multivariate factorial analysis by principal components, allowed a better explanation of the geochemical scenario. The first PC reveals the process of water mineralization by grouping those chemical components that contribute most to water salinity. The second and third PCs explain a low percentage of variance, but collect chemical components such as Ca2+ and Mg2+, associated with NO3− and total and faecal coliform bacteria, respectively, which reveal carbonate dissolution and specific situations of contamination.
Finally, the observed profiles of antibiotic resistance of E. coli and their relation with the type of land use revealed that the source of faecal contamination in groundwater and surface water is mainly animal residues. In both ecosystems, we observed a high percentage of E. coli resistant to veterinary antimicrobials (AMP, TET) and sensitivity or a very low percentage of resistance to antibiotics frequently used in human medicine (CIP, AMC and CMP). However, the difference in the antibiotic resistance profiles observed between the two ecosystems shows that groundwater is more affected by the contamination sources than the surface water, a feature that is considered to be linked to the lower hydrodynamic dispersion that occurs in this sedimentary aquifer.
Although it would be of great interest to continue with water monitoring in this basin, the results obtained in this study demonstrate the significant role of surface water and groundwater in the spreading of antibiotic resistant bacteria.
Acknowledgements
This work was supported by PID 35/08, PICT 474/15 and Secyt UNRC.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- AFC (Argentine Food Code), 2012. Law 18284/69. Chapter XII. Water drinks, water and sparkling water. Art 982 [online] (Buenos Aires, Argentine). Available from: http://anmat.gov.ar
- Alexander, T., et al., 2008. Effect of subtherapeutic administration of antibiotics on the prevalence of antibiotic-resistant Escherichia coli bacteria in feedlot cattle. Applied and Environmental Microbiology, 74, 4405–4416. doi:10.1128/AEM.00489-08
- APHA, AWWA y WPCF, 2005. American Public Health Association & Eaton, Andrew D & Water Environment Federation & American Water Works Association. In: Standard methods for the examination of water and wastewater, 21st. Washington, DC.
- Bauer, A., et al., 1996. Antibiotic susceptibility testing by a standardized single disk method. American Journal of Clinical Pathology, 45, 493–496. doi:10.1093/ajcp/45.4_ts.493
- Becher Quinodoz, F., et al., 2017. Assessing interactions between groundwater and surface water in a Pampean stream. Hydrology Research, 48 (4), 1045–1057. ISSN Online 2224-7955. doi:10.2166/nh.2016.100
- Blarasin, M., et al., 2005. Aguas superficiales y subterráneas en el Sur de Córdoba: una perspectiva geoambiental. UniRio, 1, 240.
- Blarasin, M., et al., 2008. Indicadores para evaluar cambios ambientales en acuíferos. Consideraciones sobre el fondo natural de la calidad de agua. In: M. Cantú, A. Becker, and J. Bedano, eds. Evaluación de la sustentabilidad ambiental en sistemas agropecuarios. Río Cuarto, Argentina: Editorial UNRC, 69–80.
- Carlos, C., et al., 2012. Use of Escherichia coli BOX-PCR fingerprints to identify sources of fecal contamination of water bodies in the State of São Paulo, Brazil. Journal of Environmental Management, 93, 38–43. doi:10.1016/j.jenvman.2011.08.012
- Chen, C.S., et al., 2016. Simulation of groundwater contaminant transport at decommissioned landfill site: A case study, Tainan city, Taiwan. International Journal Environmental Research and Public Health, 13, 467–488. doi:10.3390/ijerph13050467
- Chidya, R.S., et al., 2016. Evaluation of groundwater quality in rural areas of northern Malawi: case of zombwe extension planning area in Mzimba. Physics and Chemistry of the Earth, 93, 55–62. doi:10.1016/j.pce.2016.03.013
- Chin, D., 2006. Water-quality engineering in natural systems. Hoboken, NJ: John Wiley & Sons, Inc.
- Cirelli, A.F. and Mortier, C., 2005. Evaluación de la condición del agua para consumo humano en Latinoamérica. Tecnologías solares para la desinfección y descontaminación del agua. Solar Safe Water, 1, 11–26.
- Clinical and Laboratory Standard Institute (CLSI), 2016. M100-S27. Performance standards for antimicrobial susceptibility testing. Wayne, PA: CLSI.
- Díaz Delgado, C., Alberich, M., and Lopez Vera, F., 2005. Hidrologia subterránea. In: C. Díaz Delgado and F. Lopez Vera, eds. Recursos Hídricos. Conceptos básicos y estudios de casos en Iberoamérica. Montevideo, Uruguay: Piriguazú. Ediciones/CIRA-UAEM, III-3 to III-101.
- Fluckey, W.M., et al., 2007. Antimicrobial drug resistance of Salmonella and Escherichia coli isolates from cattle feces, hides, and carcasses. Journal Food Protection, 70, 551–556. doi:10.4315/0362-028X-70.3.551
- Foster, S., et al., 2002. Groundwater Quality Protection. A guide for water utilities, municipal authorities and environment agencies. Groundwater management advisory team. Co-sponsored by WHO – PAHO – CEPIS & UNESCO – ROSTLAC – PHI. Washington, DC: The World Bank.
- Gambero, M.L., Blarasin, M., and Bettera, S., 2016. Tracking contamination: antibiotic resistance and molecular characteristics of Escherichia coli isolated from groundwater in an agroecosystem. Argentina Rend. Online Soc.Geol. It., 39 (1), 829. Società Geologica Italiana, Roma. ISSN2035-8008. doi:10.3301/ROL.2016.63
- Giuliano Albo, J., Blarasin, M., and Panarello, H., 2015. Evaluación de la geoquímica e isótopos del nitrato en el acuífero libre de una llanura con actividad agropecuaria, Córdoba, Argentina. Revista Académica de la FI-UADY, 19, 24–38.
- Giuliano Albo, M.J. and Blarasin, M., 2014. Hidrogeoquímica y estimación del fondo natural de nitratos del agua subterránea en un agroecosistema del pedemonte de la sierra de Comechingones. Revista de la Asociación Geológica Argentina, 71 (3), 378–392. ISSN en línea 1851-8249. http://ppct.caicyt.gov.ar/index.php/raga/article/view/3096/4349.
- Giuliano, M.J., 2013. Evaluación por la contaminación por nitratos en aguas subterráneas de agroecosistemas mediante el uso de isótopos estables 15N – 15N, 18O – 16O, y otros solutos relacionados. Thesis (PhD). National University of Río Cuarto.
- Gourmelon, M., et al., 2007. Evaluation of two library independent microbial source tracking methods to identify sources of fecal contamination in French estuaries. Applied and Environmental Microbiology, 73, 4857–4866. doi:10.1128/AEM.03003-06
- Hafner, S.C., Harter, T., and Parikh, S.J., 2016. Evaluation of monensin transport to shallow groundwater after irrigation with dairy lagoon water. Journal of Environmental Quality, 45, 480–487. doi:10.2134/jeq2015.05.0251
- Hamner, S., et al., 2007. Ford isolation of potentially pathogenic Escherichia coli O157: H7from the Ganges River. Applied and Environmental Microbiology, 73, 2369–2372. doi:10.1128/AEM.00141-07
- Hunter, C., et al., 2000. Fecal bacteria in the waters of and upland area in Derbishire, England: the influence of agricultural land use. Journal Environment Qualitative, 29, 1253–1261. doi:10.2134/jeq2000.00472425002900040032x
- Ibekwe, A., Murinda, S., and Graves, A., 2011. Genetic diversity and antimicrobial resistance of Escherichia coli from human and animal sources uncovers multiple resistances from human sources. PLoS ONE, 6, 20819. doi:10.1371/journal.pone.0020819
- Katz, B., Griffin, D., and Davis, J., 2009. Groundwater quality impacts from the land application of treated municipal wastewater in a large karstic spring basin: chemical and microbiological indicators. Science of the Total Environment, 407, 2872–2886. doi:10.1016/j.scitotenv.2009.01.022
- Ksoll, W., et al., 2007. Presence and sources of fecal coliform bacteria in epilithic periphyton communities of lake superior. Applied and Environmental Microbiology, 73, 3771–3778. doi:10.1128/AEM.02654-06
- Kummerer, K., 2003. Significance of antibiotics in the environment. Journal of Antimicrobial Chemotherapy, 52, 5–7. doi:10.1093/jac/dkg293
- Kuroda, K., et al., 2012. Assessment of groundwater pollution in Tokyo using PPCPs as sewage markers. Enviromental Science Technology, 46, 1455–1464. doi:10.1021/es202059g
- Laplumé, H., et al., 19-20 May 2011. Multiresistance: a problem to be addressed in an interdisciplinary and interinstitutional way. INE-SADI “X Argentine Congress of the Argentine Society of Infectology – SADI. Mar del Plata: Argentina.
- Laroche, E., et al., 2010. Transport of antibiotic-resistant Escherichia coli in a public rural karst water supply. Journal of Hydrology, 392, 12–21. doi:10.1016/j.jhydrol.2010.07.022
- Mackie, R., et al., 2006. Tetracycline residues and tetracycline resistance genes in groundwater impacted by swine production facilities. Animal Biotechnology, 17, 157–176. doi:10.1080/10495390600956953
- Meays, C., et al., 2004. Source tracking fecal bacteria in water: a critical review of current methods. Journal of Environmental Management, 73, 71–79. doi:10.1016/j.jenvman.2004.06.001
- Merlo, C., et al., 2017. Changes in the bacterial community composition of different habitats along a polluted river (Suquía River, Cordoba, Argentina). Ecología Austral, 27, 72–84.
- Mull, B. and Hill, V., 2009. Recovery and detection of Escherichia coli O157: h7in surface water, using ultrafiltration and Real-Time PCR. Applied and Environmental Microbiology, 75, 3593–3597. doi:10.1128/AEM.02750-08
- Pasquini, A., Formica, S., and Sacchi, G., 2012. Hydrochemistry and nutrients dynamic in the Suquía River urban catchment’s, Córdoba, Argentina. Environmental Earth Sciences, 65, 453–467. Special Issue: Geochemistry of the Earth’s Surface I Reunión Argentina de Geoquímica de la Superficie (IRAGSU 2009). doi:10.1007/s12665-011-0978-z
- Pawar, P., 2013. Monitoring of impact of anthropogenic inputs on water quality of mangrove ecosystem of Uran, Navi Mumbai, west coast of India. Marine Pollution Bulletin, 75, 291–300. doi:10.1016/j.marpolbul.2013.06.045
- Perdomo, C., Casanova, O., and Ciganda, V., 2001. Contaminación de aguas subterráneas con nitratos y coliformes en el litoral sudeste del Uruguay. Agrociencia, 5, 10–22.
- Picone, L.I., et al., 2003. Evaluación de nitratos y bacterias coliformes en pozos de la cuenca alta del arroyo pantanoso (Bs. As.). Revista Investigación Agropecuaria, 32, 99–110.
- Plummer, J.D. and Long, S.C., 2007. Monitoring source water for microbial contamination: evaluation of water quality measures. Water Research, 41, 3716–3728. doi:10.1016/j.watres.2007.05.004
- Price, B., et al., 2006. Classification tree method for bacterial source tracking with antibiotic resistance analysis data. Applied and Environmental Microbiology, 72, 3468–3475. doi:10.1128/AEM.72.5.3468-3475.2006
- Pruden, A., 2009. Occurrence and transport of antibiotics from CAFOs. In: L. Shore and A. Pruden, eds. Hormones and pharmaceuticals generated by concentrated animal feeding operations transport in water and soil. Emerging topics in ecotoxicology. New York: Springer Dordrecht Heidelberg London, 63–70.
- Rodríguez, S., et al., 2012. Relación del nitrato sobre la contaminación bacteriana del agua. Terra Latinoamericana, 30, 111–119.
- Roling, W., et al., 2001. Relationships between microbial community structure and hydrochemistry in a landfill leachate-polluted aquifer. Applied and Environmental Microbiology, 67, 4619–4629. doi:10.1128/AEM.67.10.4619-4629.2001
- Sapkota, A., et al., 2007. Antibiotic-resistant Enterococci and fecal indicators in surface water and groundwater impacted by concentrated swine feeding operation. Environ Health Perspectives, 115, 1040–1045. doi:10.1289/ehp.9770
- Simpson, J.M., Santo Domingo, J.W., and Reasoner, D.J., 2002. Microbial source tracking: state of the science. Environmental Science and Technology, 36, 5279–5288. doi:10.1021/es026000b
- Sinclair, A., et al., 2009. Growing season surface water loading of fecal indicator organisms within a rural watershed. Water Research, 43, 1199–1206. doi:10.1016/j.watres.2008.12.006
- Tran, N.H., Yew-Hoong Gin, K., and Ngo, H., 2015. Fecal pollution source tracking toolbox for identification, evaluation and characterization of fecal contamination in receiving urban surface waters and groundwater. Science of the Total Environment, 538, 38–57. doi:10.1016/j.scitotenv.2015.07.155
- Unno, T., et al., 2010. High diversity and abundance of antibiotic-resistant Escherichia coli isolated from humans and farm animal hosts in Jeonnam Province, South Korea. Science of the Total Environment, 408, 3499–3506. doi:10.1016/j.scitotenv.2010.04.046
- Vogel, J., et al., 2007. Identifying fecal sources in a selected catchment reach using multiple source-tracking tools. Journal of Environmental Quality, 36, 718–729. doi:10.2134/jeq2006.0246
- Webster, L.F., et al., 2004. Identification of sources of Escherichia coli in South Carolina estuaries using antibiotic resistance analysis. Journal of Experimental Marine Biology and Ecology, 298, 179–195. doi:10.1016/S0022-0981(03)00358-7
- WHO (World Health Organization), 2016. October Fact sheet [online]. Available from: http://www.who.int/mediacentre/factsheets/antibiotic-resistance/en/ [Accessed 22 Dec 2017].