?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Dietary nitrate (NO3–) supplementation can reduce the oxygen cost of submaximal exercise, but this has not been reported consistently. We hypothesised that the number of step transitions to moderate-intensity exercise, and corresponding effects on the signal-to-noise ratio for pulmonary O2, may be important in this regard. Twelve recreationally active participants were assigned in a randomised, double-blind, crossover design to supplement for 4 days in three conditions: 1) control (CON; water); 2); PL (NO3–-depleted beetroot juice); and 3) BR (NO3–-rich beetroot juice). On days 3 and 4, participants completed two 6-min step transitions to moderate-intensity cycle exercise. Breath-by-breath
O2 data were collected and
O2 kinetic responses were determined for a single transition and when the responses to 2, 3 and 4 transitions were ensemble-averaged. Steady-state
O2 was not different between PL and BR when the
O2 response to one-, two- or three-step transition was compared but was significantly lower in BR compared to PL when four-step transitions was considered (PL: 1.33 ± 0.34 vs. BR: 1.31 ± 0.34 L·min−1, P < 0.05). There were no differences in pulmonary
O2 responses between CON and PL (P > 0.05). Multiple step transitions may be required to detect the influence of NO3− supplementation on steady-state
O2.
Introduction
Dietary inorganic nitrate (NO3–) supplementation, via pharmacological (i.e., NO3– salts) and vegetable juice (i.e., beetroot juice) sources, is the only putative nutritional ergogenic aid reported to lower steady-state pulmonary O2 uptake ( O2) during submaximal exercise (Bailey et al., Citation2009; Cermak et al., Citation2012; Larsen et al., Citation2007; Whitfield et al., Citation2016). These findings have recently been confirmed at the muscular level by Nyberg et al. (Citation2021) who reported that acute NO3− supplementation reduced leg muscle
O2 during knee extensor exercise. This improvement in exercise economy following NO3– supplementation may be related to the elevation of nitric oxide (NO), nitrite (NO2–), and/or S-nitrosothiol bioavailability and subsequent nitroso signalling (Lundberg et al., Citation2008; Piknova et al., Citation2021). The candidate mechanisms responsible include enhanced mitochondrial efficiency (i.e., lower O2 cost of ATP production; Larsen et al., Citation2011; cf., Whitfield et al., Citation2016) and/or muscle contractile efficiency (i.e., lower ATP cost of muscle force production; Bailey et al., Citation2010). It is notable, however, that not all studies have observed a lower pulmonary O2 uptake during submaximal exercise following NO3– supplementation (Betteridge et al., Citation2016; Boorsma et al., Citation2014; Oskarsson & McGawley, Citation2018; Sandbakk et al., Citation2015).
Important factors influencing the possible effect of dietary NO3– supplementation on exercise economy include the intensity and duration of the exercise task and the aerobic fitness of the study participants (Jones et al., Citation2018). However, an under-appreciated consideration that may contribute to the disparate results reported in previous studies is that pulmonary O2 inherently contains “noise” (i.e., breath-to-breath variability) which is superimposed on the “true” value (Koga, Citation2005; Lamarra et al., Citation1987). It is feasible that study designs employing a single exercise test, such as a one-step transition from a baseline to a moderate-intensity power output on the cycle ergometer, may be insufficient to reveal potentially small differences between experimental conditions (Linnarsson, Citation1974; Whipp et al., Citation1982). A higher signal-to-noise ratio can be achieved by repeating the exercise protocol and ensemble-averaging the responses of all transitions to enhance the underlying
O2 response (Koga, Citation2005; Lamarra et al., Citation1987). It is of interest in this regard that several earlier studies which reported a reduced submaximal
O2 following NO3– supplementation included multiple exercise tests, including an ensemble-average of four (Bailey et al., Citation2010, Citation2009; Lansley et al., Citation2011; Porcelli et al., Citation2015) or two (Porcelli et al., Citation2016; Rienks et al., Citation2015; Vanhatalo et al., Citation2010; Wylie et al., Citation2013, Citation2016) step transitions. In contrast, the majority of studies that reported no significant effect of NO3– on exercise economy employed only one transition (Bescós et al., Citation2011; Betteridge et al., Citation2016; Boorsma et al., Citation2014; Flueck et al., Citation2016; Kocoloski & Crecelius, Citation2018; Muggeridge et al., Citation2015; Nyakayiru et al., Citation2017; Wickham et al., Citation2019; Wilkerson et al., Citation2012). It is clearly important that pulmonary
O2 measurements have sufficient fidelity to reveal the hypothesised effects of an intervention. This is especially true when those effects might be relatively small, such as following dietary NO3– supplementation, which is expected to lower
O2 at a fixed submaximal work rate by ~3% compared to the control or placebo condition (Jones et al., Citation2021; Wylie et al., Citation2013). The role of the number of step transitions on possible differences in steady-state
O2 between dietary NO3– and placebo conditions is presently unknown and requires investigation.
To date, investigations that have administered beetroot juice to investigate the influence of NO3– supplementation on the physiological responses to exercise have typically provided NO3–-depleted beetroot juice as the placebo (Kelly et al., Citation2013; Lansley et al., Citation2011; Wylie et al., Citation2013, Citation2016). However, in addition to NO3–, beetroot (Beta vulgaris) contains an array of phytochemicals (Clifford et al., Citation2017; Wootton-Beard & Ryan, Citation2012) such as flavonoids and betalains (Shepherd et al., Citation2015). These bioactive polyphenolic compounds are found in both NO3–-rich and NO3–-depleted beetroot juice and could exert protective antioxidant properties (Davies et al., Citation1982; Mason et al., Citation2016) and facilitate the NO3–-NO2–-NO pathway (Gago et al., Citation2007; Lundberg et al., Citation2008; Peri et al., Citation2005). Indeed, indirect markers of NO bioavailability (e.g., arterial flow-mediated dilation) have been increased following polyphenol supplementation via, for example, dark chocolate (Hooper et al., Citation2012), grape (Li et al., Citation2013), and Montmorency cherry supplementation (Aboo Bakkar et al., Citation2019). These data suggest the potential for polyphenol compounds in beetroot juice to increase NO bioavailability and to induce NO-mediated physiological effects, independent of the effects of NO3−. Given that NO3–-rich beetroot juice has been reported to induce more pronounced favourable physiological effects compared to NO3– salts (Flueck et al., Citation2016; Thompson et al., Citation2018), further research is required to understand the potential influence of the polyphenol content of NO3–-depleted beetroot juice on the physiological responses to exercise.
Therefore, the purpose of this study was twofold: 1) to investigate whether the number of transitions to a moderate work rate would influence the pulmonary O2 response outcome following NO3–-rich beetroot juice supplementation (BR) compared to NO3–-depleted beetroot juice supplementation (PL); and 2) to investigate whether PL supplementation would influence indices of NO bioavailability or the pulmonary
O2 response to exercise compared to a water control condition (CON). We hypothesised that the O2 cost of exercise would be significantly lower following BR compared to PL supplementation when multiple step transitions were ensemble-averaged but not when only one exercise test was performed in each condition. We also hypothesised that PL supplementation would not significantly increase resting plasma [NO3–] and [NO2–] or influence
O2 kinetics during moderate-intensity exercise compared to CON.
Methods
Participants
Eight recreationally active males (mean ± SD: age 30 ± 8 years; body mass 74 ± 7 kg; height 1.77 ± 0.05 m; O2peak 48 ± 8 mL·kg−1·min−1) and four recreationally active females (mean ± SD: age 27 ± 6 years; body mass 64 ± 14 kg; height 1.63 ± 0.06 m;
O2peak 38 ± 5 mL·kg−1·min−1) volunteered to participate for this study. The protocols, risks and benefits of participating were explained prior to them providing written informed consent. This study was approved by the Institutional Research Ethics Committee and conformed to the code of ethics of the Declaration of Helsinki.
Experimental overview
Participants reported to the laboratory on eight separate occasions over a ~4–5-week period (). Prior to experimental testing, participants completed a ramp incremental cycling exercise test for the determination of O2peak and gas exchange threshold (GET). Breath-by-breath pulmonary gas exchange was measured continuously during the incremental test and averaged over consecutive 10 s periods. The data collected during the incremental tests were used to calculate individualised work rates at 80% GET for subsequent moderate-intensity step tests. The participants returned to the laboratory 2–3 days later for familiarisation to the double step test protocol. Over the remaining six laboratory visits, participants completed moderate-intensity step exercise tests following three separate 4-day supplementation periods with water (CON), NO3–-depleted placebo (PL) or NO3–-rich beetroot juice (BR) for the determination of the O2 cost of moderate-intensity exercise and
O2 kinetics. The three supplementation periods were administered in a randomised, double-blinded, crossover experimental design. Participants completed two moderate-intensity step tests on days 3 and 4 of each dietary condition (CON, PL and BR). The step tests comprised 3 min of baseline cycling at 20 W followed by a step increment to a moderate-intensity (80% GET) constant work rate, which was maintained for 6 min. Following a 7 min period of passive rest, this was repeated. In total, the participants therefore completed four identical step tests.
Supplementation
Following the initial ramp test, participants underwent three 4-day supplementation periods in which they consumed 2 × 70 mL doses per day of CON (negligible NO3– content per 50 mL; Buxton Natural Still Water, Nestlé UK Ltd., UK), PL (~0.04 mmol of NO3– per 70 mL; Beet It Sport, James White Drinks Ltd., Ipswich, UK) or BR (~6.2 mmol of NO3– per 70 mL; Beet It Sport, James White Drinks Ltd., Ipswich, UK) separated by at least a 5-day wash-out period. Each 70 mL beetroot juice beverage contained 72 kcal energy and 15.4 g of carbohydrate. The CON was provided in 50 mL plastic tubes, which were placed in a small transparent plastic bag. On days 1 and 2 of each supplementation period, participants consumed one 70 mL beverage in the morning and one in the evening, whereas on the experimental days (days 3 and 4), they consumed 2 × 70 mL of their allocated beverage in the morning ~2.5 h prior to the exercise (Wylie et al., Citation2013). For the duration of the study, participants were asked to maintain their habitual physical activity and dietary intake but to avoid consuming foods high in dietary NO3– such as beetroot, kale, spinach and rocket, and to refrain from taking any other dietary supplements or using antibacterial mouthwash due to its detrimental effect on oral nitrate reduction (Govoni et al., Citation2008). Experimental visits were performed at the same time of day (± 2 h). Participants recorded their activity and diet during the 24 h prior to the first experimental visit and were asked to repeat these for subsequent visits. Participants were also instructed to arrive at the laboratory having avoided strenuous exercise and alcohol in the 24 h preceding, and caffeine in the 8 h preceding, each experimental visit.
Measurements
Venous blood samples were obtained at rest on days 3 and 4, and were drawn into a 6 mL lithium-heparin tube (Vacutainer, Becton-Dickinson, New Jersey, USA) and centrifuged at 4000 rpm and 4°C for 8 min, within 2 min of collection. Plasma was subsequently extracted and frozen at −80°C for later deproteinization and analysis of [NO3–] and [NO2–] as described previously (Tan et al., Citation2018).
During all exercise tests, breath-by-breath pulmonary gas exchange was measured with participants wearing a nose clip and breathing through a low-dead-space, low-resistance mouthpiece and impeller turbine assembly (Jaeger Triple V). The inspired and expired gas volume and gas concentration signals were continuously sampled at 100 Hz, the latter with paramagnetic O2 and infrared CO2 analysers (Jaeger Oxycon Pro, Hoechberg, Germany) via a capillary line connected to the mouthpiece. The gas analysers were calibrated before each test with gases of known concentration, and the turbine volume transducer was calibrated with a 3-L syringe (Hans Rudolph, Kansas City, MO). The volume and concentration signals were time-aligned by accounting for the delay in the capillary gas transit and the analyser rise time relative to the volume signal.
Data analysis procedures
The breath-by-breath O2 data from each test were initially examined to exclude errant breaths caused by coughing, swallowing, sighing, etc. and values lying more than 4 standard deviations from the local mean were deleted. The breath-by-breath data were subsequently linearly interpolated to give 1 s values. To assess the influence of the number of transitions, the second-by-second data from two-, three- and four-step transitions were time aligned to the start of exercise and ensemble averaged; that is, the mean
O2 for each 1 s period was calculated to create a “composite” response for 2, 3 and 4 transitions. The baseline
O2 was defined as the mean
O2 measured during “unloaded” cycling between 150 and 30 s before the start of exercise. The first 20 s after the onset of exercise (i.e., the phase I response) was not considered in the model fit. Subsequently, a single exponential model was used to analyse the
O2 responses for one, two, three and four transitions, as described in the following equation:
where O2 (t) represents the absolute
O2 at a given time t;
O2baseline represents the mean
O2 in the baseline period; Ap, TDp and
the amplitude, time delay and time constant, respectively, describing the phase II increase in
O2 above baseline. The mean response time (MRT) was calculated by fitting a single exponential curve to the data with no time delay from the onset to the end of exercise.
The signal-to-noise ratio of the O2 response in the CON condition was calculated for one, two, three and four transitions by dividing the mean
O2 response during the final 60 s of exercise by the standard deviation of the
O2 response during the final 60 s (Beltrame et al., Citation2020; Koga, Citation2005).
Statistical analyses
A one-way analysis of variance (ANOVA) with repeated measures was used to analyse differences in the O2 uptake responses and plasma [NO3–] and [NO2–] between CON, PL and BR. Significant main and interaction effects were further explored using Fisher’s LSD post hoc comparisons. In addition, a paired t-test was used to analyse differences in the end-exercise steady-state O2 response between BR vs. PL to provide a comparable approach to previous studies, which typically include only these two conditions. A one-way ANOVA was used to analyse differences in the signal-to-noise ratio between one, two, three and four transitions in the CON condition. Statistical significance was accepted at P < 0.05. Results are presented as mean ± SD unless otherwise stated.
Results
All participants reported consuming all servings of each supplement at the correct time and confirmed that they had maintained their habitual exercise and dietary habits prior to each testing visit. There were no reports of adverse reactions, gastrointestinal distress or discomfort following the ingestion of supplements.
Plasma [NO3–] and [NO2–]
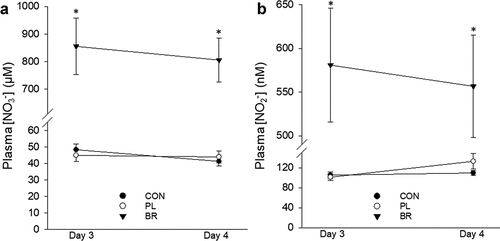
There was a main effect by condition (P < 0.01), but no effect by time (P > 0.05) on plasma [NO3–] and [NO2–] () and (b)). Plasma [NO3–] averaged over intervention days 3 and 4 was higher in BR (831 ± 225 µM) compared to PL (45 ± 53 µM, P < 0.01) and CON (45 ± 8 µM, P < 0.01) with no significant difference between PL and CON (P > 0.05). Plasma [NO2–] averaged over intervention days 3 and 4 was higher in BR (569 ± 225 nM) compared to PL (117 ± 53 nM, P < 0.01) and CON (107 ± 24 nM, P < 0.01) with no significant difference between PL and CON (P > 0.05).
Figure 2. Mean ± SE plasma nitrate (NO3–; panel A) and nitrite (NO2–; panel B) at rest following: 1) CON, water supplementation (solid circles); 2) PL, nitrate-depleted beetroot juice (open circle); and 3) BR, nitrate-rich beetroot juice (solid triangle). * = significantly different from CON and PL (P < 0.05).
Pulmonary gas exchange
ANOVA revealed no main effects by condition (CON, PL, BR) on pulmonary ventilation and gas exchange variables at baseline or during steady-state moderate-intensity cycling exercise when data were averaged over four-step transitions ().
Table 1. Mean ± SD ventilatory and gas exchange dynamics during moderate-intensity exercise following supplementation with water (CON), nitrate-depleted beetroot juice (PL) and nitrate-rich beetroot juice (BR) over four-step transitions
When data were analysed using paired-samples t-tests, end-exercise O2 was significantly lower in BR compared to PL and CON for four transitions (PL: 1.33 ± 0.34 vs. BR: 1.31 ± 0.34 L·min−1, P < 0.05), but not for one, two or three transitions (P > 0.05, ). There was no difference between PL and CON. The signal-to-noise ratio of the steady-state
O2 response progressively increased as the number of transitions increased from 1 to 4 ()). Averaged across all conditions, the signal-to-noise ratio for four transitions (28 ± 11) was 12% higher than for three transitions (25 ± 9), 37% higher than for two transitions (21 ± 8) and 90% higher than for one transition (15 ± 8) (all P < 0.05; )). The improvement in the signal-to-noise ratio when more transitions are ensemble-averaged is illustrated for an individual participant in .
Figure 3. Steady-state end-exercise O2 over one-, two-, three- and four-step transitions (1 T, 2 T, 3 T, 4 T) from unloaded to moderate-intensity exercise following control (CON, panel A), supplementation with nitrate-depleted beetroot juice (PL, panel B) and nitrate-rich beetroot juice (BR, panel C). Panel D shows the increase in signal-to-noise ratio of end-exercise
O2 (mean of CON, PL and BR) as the number of step transitions increases. #, different from 4 T in PL (paired t-test); *, different from signal-to-noise ratio in 4 T.
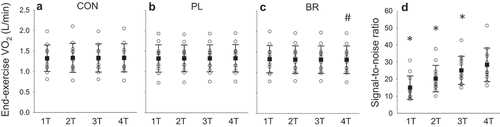
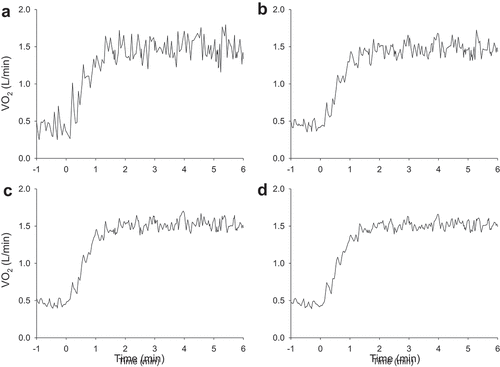
Figure 4. Illustration of improvement in the signal-to-noise ratio for pulmonary O2 when considering 1 step transition (panel A) and 2 (panel B), 3 (panel C) and 4 (panel D) transitions in one individual. The mean ± SD steady-state
O2 was 1.48 ± 0.14, 1.47 ± 0.09, 1.52 ± 0.06 and 1.51 ± 0.05 L·min−1 for one, two, three and four transitions, respectively, and the corresponding signal-to-noise ratios were 11, 17, 24 and 28. With each additional transition, the measured steady-state
O2 becomes closer to the “true” value.
Discussion
The principal novel findings of the present study were that 1) there was a significant reduction in the O2 cost of exercise following BR compared to PL supplementation when four (but not one, two or three) step exercise transitions were ensemble-averaged; and 2) PL supplementation did not elevate NO bioavailability or influence the pulmonary O2 responses compared to CON. These findings are consistent with our hypotheses and indicate that the number of exercise transitions may be important in detecting the influence of NO3– on the O2 cost of submaximal exercise. In addition, these results indicate that despite NO3–-depleted beetroot juice containing bioactive polyphenolic compounds (i.e., flavonoids, betalains, ascorbic acid; Shepherd et al., Citation2015), NO3–-depleted beetroot juice is an appropriate placebo control for scientific investigations examining the influence of dietary NO3– on the physiological responses to exercise.
The influence of the number of transitions on pulmonary O2 uptake responses to moderate-intensity exercise
BR significantly lowered the O2 cost of exercise by ~2% compared to PL when ensemble-averaged over four-step exercise transitions but not when ensemble-averaged over one, two or three transitions. These results are consistent with earlier studies, although the absolute magnitude of difference was somewhat lower than the 3–5% reduction in O2 that has been reported in some studies (Bailey et al., Citation2009; Lansley et al., Citation2011; Larsen et al., Citation2007; Vanhatalo et al., Citation2010). The important original observation in the present study, consistent with our hypothesis, was that statistical significance was only attained following the averaged response of four-step transitions, a result which is almost certainly related to a reduced error (i.e., improved signal-to-noise ratio) achieved with each additional transition. Our findings indicate that inherent noise in the pulmonary gas exchange responses to exercise (Lamarra et al., Citation1987) has the potential to obscure identification of physiological effects of NO3– supplementation. Accordingly, it is possible that the inability of previous investigations to detect a significant effect of NO3− on steady-state
O2 employed too few exercise transitions (one: Bescós et al., Citation2011; Kocoloski & Crecelius, Citation2018; Wickham et al., Citation2019; two: Breese et al., Citation2013; Christensen et al., Citation2013; Kelly et al., Citation2014), to appropriately characterise the response.
Nyberg et al. (Citation2021) recently reported that acute dietary NO3− supplementation reduced skeletal muscle O2 during moderate-intensity knee extension exercise by ~10%, providing the first direct evidence that dietary NO3− ingestion enhances efficiency at the muscular level. While pulmonary
O2 measurements have been shown to provide a good approximation of contracting muscle
O2 (Grassi et al., Citation1996; Krustrup et al., Citation2009; Poole et al., Citation1991), it remains essential to consider the temporal and spatial dissociations between the two compartments, along with contributions from other organs and muscle groups to the pulmonary
O2 signal (Lamarra et al., Citation1987). In addition, inter-breath fluctuations related, in part, to changes in tidal volume, can also serve to obscure and confound assessment of muscle
O2 from measurement of pulmonary
O2 (Koga, Citation2005). As evidenced by the present study, averaging procedures both within (e.g., interpolation to second-by-second values from less frequent breath-by-breath measurements) and across (e.g., ensemble-averaging of multiple repeat transitions) exercise bouts are appropriate when pulmonary gas exchange measurements are used with the purpose of providing a non-invasive window on muscle metabolic processes (Keir et al., Citation2014).
The p of the
O2 response during the step transition from unloaded to moderate-intensity exercise was not influenced by BR compared to PL, a finding which is consistent with previous studies (Bailey et al., Citation2009; Bescós et al., Citation2011; Breese et al., Citation2013; Ghiarone et al., Citation2017; Kelly et al., Citation2014). Moreover, increasing the averaged number of transitions from 1 to 4 had no effect on the influence of NO3– supplementation on
O2
p. The literature suggests that dietary NO3– supplementation may reduce
O2
p in situations where O2 delivery may be impaired such as when breathing a hypoxic inspirate (Kelly et al., Citation2014) and during exercise protocols that require a relatively greater recruitment of type II muscle fibres such as during step transitions from moderate- to severe-intensity cycling (Breese et al., Citation2013) or when cycling at a high cadence (Bailey et al., Citation2015). Overall, these results are in agreement with the “tipping point” theory, which suggests that when O2 delivery is sufficient (e.g., healthy individuals performing moderate-intensity exercise), increases in O2 delivery relative to demand (e.g., following NO3– supplementation) would not be expected to influence
O2
p (Poole & Jones, Citation2012). In addition, given that NO3– supplementation may improve blood flow and contractile function specifically in type II muscle fibres (Ferguson et al., Citation2013; Hernández et al., Citation2012), and that predominantly type I muscle fibres would be recruited during moderate-intensity exercise, effects on
O2
p during a step transition from unloaded to moderate-intensity exercise might be considered unlikely.
The effect of NO3–-depleted beetroot juice supplementation on indices of NO bioavailability and pulmonary O2 responses during moderate-intensity exercise
Short-term NO3–-depleted beetroot juice supplementation (i.e., placebo) did not influence plasma [NO3–] and [NO2–] which is consistent with a previous report (Lansley et al., Citation2011). Importantly, there was no difference in plasma [NO3–] and [NO2–] between PL and CON, suggesting that concentrated NO3–-depleted beetroot juice is not effective at altering indices of NO bioavailability despite beetroot juice containing phytochemical compounds (Georgiev et al., Citation2010; Ninfali & Angelino, Citation2013; Shepherd et al., Citation2015; Wootton-Beard & Ryan, Citation2012). However, consistent with previous studies, NO3–-rich beetroot juice supplementation increased plasma [NO3–] and [NO2–] by ~1750% and ~420%, respectively, compared to CON and PL (Wylie et al., Citation2013).
Recent studies suggest that more pronounced effects on plasma [NO3–] and [NO2–] may occur following NO3– supplementation administered in vegetable juice form (i.e., beetroot juice) compared to a pharmaceutical form (i.e., NO3– salts) which could indicate an independent or synergistic effect of polyphenols within the former (Flueck et al., Citation2016; Jonvik et al., Citation2016; McIlvenna et al., Citation2017; Thompson et al., Citation2018). For example, Thompson et al. (Citation2018) reported that 4 weeks of sprint training combined with NO3–-rich beetroot juice supplementation lowered systolic blood pressure by ~5% and increased exercise tolerance by ~71%; in contrast, 4 weeks of sprint training combined with NO3– salt supplementation lowered systolic blood pressure by ~2% and increased exercise tolerance by ~42%. Although it is possible that physiological effects following NO3–-rich beetroot juice supplementation may be partly related to phytochemicals interacting synergistically with NO3–, the data from the present study provide evidence that NO3–-depleted beetroot juice, per se, does not contribute to the elevation of plasma [NO3–] and [NO2–] and thus is unlikely to induce NO-mediated effects.
A possible explanation for the lack of effect of NO3–-depleted beetroot juice supplementation on NO bioavailability could be low phytochemical bioavailability (Clifford et al., Citation2015). To our knowledge, only two studies that have investigated polyphenol supplementation have measured plasma [NO3–] and/or [NO2–] and these found no influence of Montmorency tart cherry powder (~256 mg·d−1 anthocyanins; Aboo Bakkar et al., Citation2019) or concentrate (74 mg cyanidin-3-glucoside and 179 mg gallic acid; Keane et al., Citation2018, Citation2016) on plasma [NO3–] and/or [NO2–]. However, the relative concentration and type of each phytochemical within beetroot juice could be important for any potential physiological effects to occur. In support of this interpretation, a meta-analysis concluded that ~500 mg of flavonoids was required to influence vascular function (Kay et al., Citation2012), which is an indirect measure of NO bioavailability. However, beetroot juice contains drastically less, containing only ~4 mg of flavonoids per 100 ml (Shepherd et al., Citation2015). Therefore, although it is acknowledged that phytochemicals may impact physiological responses to exercise through NO-mediated mechanisms (Bowtell & Kelly, Citation2019), the constituents of NO3–-depleted beetroot juice (i.e., placebo) do not independently contribute to NO bioavailability following NO3–-rich beetroot juice supplementation.
From a practical perspective, the results of this study indicate that the innate breath-to-breath noise in pulmonary gas exchange measurements has the potential to obscure the effect of dietary nitrate on the O2 cost of submaximal exercise. Researchers should be cognisant of this point when designing studies to investigate the efficacy of dietary nitrate supplementation. Specifically, single step exercise transitions are unlikely to provide the fidelity required to ascertain differences in steady-state O2 between experimental and placebo conditions. The results of this study also affirm that a nitrate-depleted beetroot juice serves as an appropriate placebo control in such studies since the potential (non-nitrate) bioactive compounds do not independently influence steady-state
O2. A potential limitation of the present study is that we did not assess exercise performance and thus it is not clear whether the small reduction in the O2 cost of submaximal exercise with BR compared to PL would have translated into a meaningful difference in performance.
Conclusion
In conclusion, the O2 cost of moderate-intensity cycling exercise was found to be significantly reduced by NO3–-rich beetroot juice supplementation when responses were ensemble-averaged over four-step transitions but not one-, two- or three-step transitions. In addition, NO3–-depleted beetroot juice supplementation did not significantly influence plasma [NO3–] and [NO2–] or the pulmonary O2 responses measured during moderate-intensity exercise when compared to a water control condition. These data have important methodological and practical implications, including that numerous step transitions (i.e., 4) may be required to detect significant changes to the O2 cost of exercise following NO3– supplementation, and that NO3–-depleted beetroot juice is an effective placebo for investigating the potential physiological effects of NO3– supplementation.
Acknowledgments
We thank Dr Chris Thompson for reading an earlier draft of this manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Aboo Bakkar, Z., Fulford, J., Gates, P. E., Jackman, S. R., Jones, A. M., Bond, B., & Bowtell, J. L. (2019). Montmorency cherry supplementation attenuates vascular dysfunction induced by prolonged forearm occlusion in overweight middle-aged men. Journal of Applied Physiology, 126(1), 246–254. https://doi.org/10.1152/japplphysiol.00804.2018
- Bailey, S. J., Fulford, J., Vanhatalo, A., Winyard, P. G., Blackwell, J. R., DiMenna, F. J., Wilkerson, D. P., Benjamin, N., & Jones, A. M. (2010). Dietary nitrate supplementation enhances muscle contractile efficiency during knee-extensor exercise in humans. Journal of Applied Physiology, 109(1), 135–148. https://doi.org/10.1152/japplphysiol.00046.2010
- Bailey, S. J., Varnham, R. L., DiMenna, F. J., Breese, B. C., Wylie, L. J., & Jones, A. M. (2015). Inorganic nitrate supplementation improves muscle oxygenation, O2 uptake kinetics, and exercise tolerance at high but not low pedal rates. Journal of Applied Physiology, 118(11), 1396–1405. https://doi.org/10.1152/japplphysiol.01141.2014
- Bailey, S. J., Winyard, P., Vanhatalo, A., Blackwell, J. R., Dimenna, F. J., Wilkerson, D. P., Tarr, J., Benjamin, N., & Jones, A. M. (2009). Dietary nitrate supplementation reduces the O2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. Journal of Applied Physiology, 107(4), 1144–1155. https://doi.org/10.1152/japplphysiol.00722.2009
- Beltrame, T., Gois, M. O., Hoffmann, U., Koschate, J., Hughson, R. L., Moraes Frade, M. C., Linares, S. N., da Silva Torres, R., & Catai, A. M. (2020). Relationship between maximal aerobic power with aerobic fitness as a function of signal-to-noise ratio. Journal of Applied Physiology, 129(3), 522–532. https://doi.org/10.1152/japplphysiol.00310.2020
- Bescós, R., Rodriguez, F. A., Iglesias, X., Ferrer, M. D., Iborra, E., & Pons, A. (2011). Acute administration of inorganic nitrate reduces VO2peak in endurance athletes. Medicine & Science in Sports & Exercise, 43(10), 1979–1986. https://doi.org/10.1249/MSS.0b013e318217d439
- Betteridge, S., Bescós, R., Martorell, M., Pons, A., Garnham, A. P., Stathis, C. C., & McConell, G. K. (2016). No effect of acute beetroot juice ingestion on oxygen consumption, glucose kinetics, or skeletal muscle metabolism during submaximal exercise in males. Journal of Applied Physiology, 120(4), 391–398. https://doi.org/10.1152/japplphysiol.00658.2015
- Boorsma, R. K., Whitfield, J., & Spriet, L. L. (2014). Beetroot juice supplementation does not improve performance of elite 1500-m runners. Medicine & Science in Sports & Exercise, 46(12), 2326–2334. https://doi.org/10.1249/MSS.0000000000000364
- Bowtell, J., & Kelly, V. (2019). Fruit-derived polyphenol supplementation for athlete recovery and performance. Sports Medicine, 49(Suppl 1), 3–23. https://doi.org/10.1007/s40279-018-0998-x
- Breese, B. C., McNarry, M. A., Marwood, S., Blackwell, J. R., Bailey, S. J., & Jones, A. M. (2013). Beetroot juice supplementation speeds O2 uptake kinetics and improves exercise tolerance during severe-intensity exercise initiated from an elevated metabolic rate. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 305(12), R1441–R1450. https://doi.org/10.1152/ajpregu.00295.2013
- Cermak, N. M., Gibala, M. J., & van Loon, L. J. (2012). Nitrate supplementation’s improvement of 10-km time-trial performance in trained cyclists. International Journal of Sport Nutrition and Exercise Metabolism, 22(1), 64–71. https://doi.org/10.1123/ijsnem.22.1.64
- Christensen, P. M., Nyberg, M., & Bangsbo, J. (2013). Influence of nitrate supplementation on VO2 kinetics and endurance of elite cyclists. Scandinavian Journal of Medicine & Science in Sports, 23(1), e21–e31. https://doi.org/10.1111/sms.12005
- Clifford, T., Contantinou, C. M., Keane, K. M., West, D. J., Howatson, G., & Stevenson, E. J. (2017). The plasma bioavailability of nitrate and betanin from beta vulgaris rubra in humans. European Journal of Nutrition, 56(3), 1245–1254. https://doi.org/10.1007/s00394-016-1173-5
- Clifford, T., Howatson, G., West, D. J., & Stevenson, E. J. (2015). The potential benefits of red beetroot supplementation in health and disease. Nutrients, 7(4), 2801–2822. doi:10.3390/nu7042801
- Davies, K. J., Quintanilha, A. T., Brooks, G. A., & Packer, L. (1982). Free radicals and tissue damage produced by exercise. Biochemical and Biophysical Research Communications, 107(4), 1998. https://doi.org/10.1016/S0006-291X(82)80124-1
- Ferguson, S. K., Hirai, D. M., Copp, S. W., Holdsworth, C. T., Allen, J. D., Jones, A. M., Musch, T. I., & Poole, D. C. (2013). Impact of dietary nitrate supplementation via beetroot juice on exercising muscle vascular control in rats. The Journal of Physiology, 591(2), 547–557. https://doi.org/10.1113/jphysiol.2012.243121
- Flueck, J. L., Bogdanova, A., Mettler, S., & Perret, C. (2016). Is beetroot juice more effect than sodium nitrate? The effects of equimolar nitrate dosages of nitrate-rich beetroot juice and sodium nitrate on oxygen consumption during exercise. Applied Physiology, Nutrition, and Metabolism, 41(4), 421–429. https://doi.org/10.1139/apnm-2015-0458
- Gago, B., Lundberg, J. O., Barbosa, R. M., & Laranjinha, J. (2007). Red wine-dependent reduction of nitrite to nitric oxide in the stomach. Free Radical Biology and Medicine, 43(9), 1233–1242. https://doi.org/10.1016/j.freeradbiomed.2007.06.007
- Georgiev, V. G., Weber, J., Kneschke, E. M., Denev, P. N., Bley, T., & Pavlov, A. I. (2010). Antioxidant activity and phenolic content of betalain extracts from intact plants and hair root cultures of the red beetroot beta vulgaris cv. Detroit dark red. Plant Foods for Human Nutrition, 65(2), 105–111. https://doi.org/10.1007/s11130-010-0156-6
- Ghiarone, T., Ataide-Silva, T., Bertuzzi, R., McConnell, G. K., & Lima-Silva, A. E. (2017). Effect of acute nitrate ingestion on VO2 response at different exercise intensity domains. Applied Physiology, Nutrition, and Metabolism, 42(11), 1127–1134. https://doi.org/10.1139/apnm-2017-0198
- Govoni, M., Jansson, E. A., Weitzberg, E., & Lundberg, J. O. (2008). The increase in plasma nitrite after a dietary nitrate load is markedly attenuated by an antibacterial mouthwash. Nitric Oxide: Biology and Chemistry / Official Journal of the Nitric Oxide Society, 19(4), 333–337. https://doi.org/10.1016/j.niox.2008.08.003
- Grassi, B., Poole, D. C., Richardson, R. S., Knight, D. R., Erickson, B. K., & Wagner, P. D. (1996). Muscle O2 uptake kinetics in humans: Implications for metabolic control. Journal of Applied Physiology, 80(3), 988–998. https://doi.org/10.1152/jappl.1996.80.3.988
- Hernández, A., Schiffer, T. A., Ivarsson, N., Cheng, A. J., Bruton, J. D., Lundberg, J. O., Weitzberg, E., & Westerblad, H. (2012). Dietary nitrate increases tetanic [Ca2+] i and contractile force in mouse fast-twitch muscle. The Journal of Physiology, 590(15), 3575–3583. https://doi.org/10.1113/jphysiol.2012.232777
- Hooper, L., Kay, C., Abdelhamid, A., Kroon, P. A., Cohn, J. S., Rimm, E. B., & Cassidy, A. (2012). Effects of chocolate, cocoa, and flavan-3-ols on cardiovascular health: A systematic review and meta-analysis of randomized trials. The American Journal of Clinical Nutrition, 95(3), 740–751. https://doi.org/10.3945/ajcn.111.023457
- Jones, A. M., Thompson, C., Wylie, L. J., & Vanhatalo, A. (2018, August). Dietary nitrate and physical performance. Annual Review of Nutrition, 38(1), 303–328. https://doi.org/10.1146/annurev-nutr-082117-051622
- Jones, A. M., Vanhatalo, A., Seals, D. R., Rossman, M. J., Piknova, B., & Jonvik, K. L. (2021). Dietary nitrate and nitric oxide metabolism: Mouth, circulation, skeletal muscle, and exercise performance. Medicine & Science in Sports & Exercise, 53(2), 280–294. https://doi.org/10.1249/MSS.0000000000002470
- Jonvik, K. L., Nyakayiru, J., Pinckaers, P. J., Senden, J. M., van Loon, L. J., & Verdijk, L. B. (2016). Nitrate-rich vegetables increase plasma nitrate and nitrite concentrations and lower blood pressure in healthy adults. The Journal of Nutrition, 146(5), 986–993. https://doi.org/10.3945/jn.116.229807
- Kay, C. D., Hooper, L., Kroon, P. A., Rimm, E. B., & Cassidy, A. (2012). Relative impact of flavonoid composition, dose and structure on vascular function: A systematic review of randomised controlled trials of flavonoid-rich food products. Molecular Nutrition & Food Research, 56(11), 1605–1616. https://doi.org/10.1002/mnfr.201200363
- Keane, K. M., Bailey, S. J., Vanhatalo, A., Jones, A. M., & Howatson, G. (2018). Effects of Montmorency tart cherry (L. Prunus Cerasus) consumption on nitric oxide biomarkers and exercise performance. Scandinavian Journal of Medicine & Science in Sports, 28(7), 1746–1756. https://doi.org/10.1111/sms.13088
- Keane, K. M., George, T. W., Constantinou, C. L., Brown, M. A., Clifford, T., & Howatson, G. (2016). Effect of Montmorency tart cherry (Prunus Cerasus L.) consumption on vascular function in men with early hypertension. The American Journal of Clinical Nutrition, 103(6), 1531–1539. https://doi.org/10.3945/ajcn.115.123869
- Keir, D. A., Murias, J. M., Paterson, D. H., & Kowalchuk, J. M. (2014). Breath-by-breath pulmonary O2 uptake kinetics: Effect of data processing on confidence in estimating model parameters. Experimental Physiology, 99(11), 1511–1522. https://doi.org/10.1113/expphysiol.2014.080812
- Kelly, J., Vanhatalo, A., Bailey, S. J., Wylie, L. J., Tucker, C., List, S., Winyard, P. G., & Jones, A. M. (2014). Dietary nitrate supplementation: Effects on plasma nitrite and pulmonary O2 uptake dynamics during exercise in hypoxia and normoxia. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 307(7), R920–R930. https://doi.org/10.1152/ajpregu.00068.2014
- Kelly, J., Vanhatalo, A., Wilkerson, D. P., Wylie, L. J., & Jones, A. M. (2013). Effects of nitrate on the power-duration relationship for severe-intensity exercise. Medicine & Science in Sports & Exercise, 45(9), 1798–1806. https://doi.org/10.1249/MSS.0b013e31828e885c
- Kocoloski, G. M., & Crecelius, A. R. (2018). Effects of single-dose dietary nitrate on oxygen consumption during and after maximal and submaximal exercise in healthy humans: A pilot study. International Journal of Exercise Science, 11(3), 214–225. https://pubmed.ncbi.nlm.nih.gov/29795728
- Koga, S. (2005). Measuring VO2 kinetics: The practicalities. In A. M. Jones & D. C. Poole (Eds.), Oxygen uptake kinetics in sport, exercise and medicine (pp. 39–61). Routledge.
- Krustrup, P., Jones, A. M., Wilkerson, D. P., Calbet, J. A., & Bangsbo, J. (2009). Muscular and pulmonary O2 uptake kinetics during moderate- and high-intensity sub-maximal knee-extensor exercise in humans. The Journal of Physiology, 587(Pt 8), 1843–1856. https://doi.org/10.1113/jphysiol.2008.166397
- Lamarra, N., Whipp, B. J., Ward, S. A., & Wasserman, K. (1987). Effect of interbreath fluctuations on characterising exercise gas exchange kinetics. Journal of Applied Physiology, 62(5), 2003–2012. https://doi.org/10.1152/jappl.1987.62.5.2003
- Lansley, K. E., Winyard, P. G., Fulford, J., Vanhatalo, A., Bailey, S. J., Blackwell, J. R., DiMenna, F. J., Gilchrist, M., Benjamin, N., & Jones, A. M. (2011). Dietary nitrate supplementation reduces the O2 cost of walking and running: A placebo-controlled study. Journal of Applied Physiology, 110(3), 591–600. https://doi.org/10.1152/japplphysiol.01070.2010
- Larsen, F. J., Schiffer, T. A., Borniquel, S., Sahlin, K., Ekblom, B., Lundberg, J. O., & Weitzberg, E. (2011). Dietary nitrate improves mitochondrial efficiency in humans. Cell Metabolism, 13(2), 149–159. https://doi.org/10.1016/j.cmet.2011.01.004
- Larsen, F. J., Weitzberg, E., Lundberg, J. O., & Ekblom, B. (2007). Effects of dietary nitrate on oxygen cost during exercise. Acta Physiologica, 191(1), 59–66. https://doi.org/10.1111/j.1748-1716.2007.01713.x
- Li, S. H., Tian, H. B., Zhao, H. J., Chen, L. H., & Cui, L. Q. (2013). The acute effects of grape polyphenols supplementation on endothelial function in adults: Meta-analyses of controlled trials. PLoS One, 8(7), e69818. https://doi.org/10.1371/journal.pone.0069818
- Linnarsson, D. (1974). Dynamics of pulmonary gas exchange and heart rate changes at start and end of exercise. Acta Physiologica Scandinavica, 415, 1–68. pubmed.ncbi.nlm.nih.gov/4621315
- Lundberg, J. O., Weitzberg, E., & Gladwin, M. T. (2008). The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nature Reviews Drug Discovery, 7(2), 156–167. https://doi.org/10.1038/nrd2466
- Mason, S. A., Morrison, D., McConell, G. K., & Wadley, G. D. (2016). Muscle redox signalling pathways in exercise. Role of antioxidants. Free Radical Biology & Medicine, 98, 29–45. https://doi.org/10.1016/j.freeradbiomed.2016.02.022
- McIlvenna, L. C., Monaghan, C., Liddle, L., Fernandez, B. O., Feelisch, M., Muggeridge, D. J., & Easton, C. (2017). Beetroot juice versus chard gel: A pharmacokinetic and pharmacodynamics comparison of nitrate bioavailability. Nitric Oxide: Biology and Chemistry / Official Journal of the Nitric Oxide Society, 64, 61–67. https://doi.org/10.1016/j.niox.2016.12.006
- Muggeridge, D. J., Sculthorpe, N., Grace, F. M., Willis, G., Thornhill, L., Weller, R. B., James, P. E., & Easton, C. (2015). Acute whole body UVA irradiation combined with nitrate ingestion enhances time trial performance in trained cyclists. Nitric Oxide: Biology and Chemistry / Official Journal of the Nitric Oxide Society, 48, 3–9. https://doi.org/10.1016/j.niox.2014.09.158
- Ninfali, P., & Angelino, D. (2013). Nutritional and functional potential of beta vulgaris cicla and ruba. Fiterapia, 89, 188–199. https://doi.org/10.1016/j.fitote.2013.06.004
- Nyakayiru, J. M., Jonvik, K. L., Pinckaers, P. J., Senden, J., van Loon, L. J., & Verdijk, L. B. (2017). No effect of acute and 6-day nitrate supplementation on VO2 and time-trial performance in highly trained cyclists. International Journal of Sport Nutrition and Exercise Metabolism, 27(1), 11–17. https://doi.org/10.1123/ijsnem.2016-0034
- Nyberg, M., Christensen, P. M., Blackwell, J. R., Hostrup, M., Jones, A. M., & Bangsbo, J. (2021, September 29). Nitrate-rich beetroot juice ingestion reduces skeletal muscle O2 uptake and blood flow during exercise in sedentary men. The Journal of Physiology, 599(23), 5203–5214. https://doi.org/10.1113/JP281995
- Oskarsson, J., & McGawley, K. (2018). No individual or combined effects of caffeine and beetroot-juice supplementation during submaximal or maximal running. Applied Physiology, Nutrition, and Metabolism, 43(7), 697–703. https://doi.org/10.1139/apnm-2017-0547
- Peri, L., Pietraforte, D., Scorza, G., Napolitano, A., Fogliano, V., & Minetti, M. (2005). Apples increase nitric oxide production by human saliva at the acidic pH of the stomach: A new biological function for polyphenols with a catechol group? Free Radical Biology and Medicine, 39(5), 668–681. https://doi.org/10.1016/j.freeradbiomed.2005.04.021
- Piknova, B., Schechter, A. N., Park, J. W., Vanhatalo, A., & Jones, A. M. (2021, October 16). Skeletal muscle nitrate as a regulator of systemic nitric oxide homeostasis. Exercise and Sport Sciences Reviews, 50(1) . https://doi.org/10.1249/JES.0000000000000272
- Poole, D. C., & Jones, A. M. (2012). Oxygen uptake kinetics. Comprehensive Physiology, 2(2), 933–996. https://doi.org/10.1002/cphy.c100072
- Poole, D. C., Schaffartzik, W., Knight, D. R., Derion, T., Kennedy, B., Guy, H. J., Prediletto, R., & Wagner, P. D. (1991). Contribution of exercising legs to the slow component of oxygen uptake kinetics in humans. Journal of Applied Physiology, 71(4), 1245–1260. https://doi.org/10.1152/jappl.1991.71.4.1245
- Porcelli, S., Pugliese, L., Rejc, E., Pavei, G., Bonato, M., Montorsi, M., La Torre, A., Rasica, L., & Marzorati, M. (2016). Effects of a short-term high-nitrate diet on exercise performance. Nutrients, 8(9), E534. https://doi.org/10.3390/nu8090534
- Porcelli, S., Ramaglia, M., Bellistri, G., Pavei, G., Pugliese, L., Montorsi, M., Rasica, L., & Mazorati, M. (2015). Aerobic fitness affects the exercise performance responses to nitrate supplementation. Medicine & Science in Sports & Exercise, 47(8), 1643–1651. https://doi.org/10.1249/MSS.0000000000000577
- Rienks, J. N., Vanderwoude, A. A., Maas, E., Blea, Z. M., & Subudhi, A. W. (2015). Effect of beetroot juice on moderate-intensity exercise at a constant rating of perceived exertion. International Journal of Exercise Science, 8(3), 277–286. pubmed.ncbi.nlm.nih.gov/27182417
- Sandbakk, S. B., Sandbakk, Ø., Peacock, O., James, P., Welde, B., Stokes, K., Böhlke, N., & Tjønna, A. E. (2015). Effects of acute supplementation of L-arginine and nitrate on endurance and sprint performance in elite athletes. Nitric Oxide: Biology and Chemistry / Official Journal of the Nitric Oxide Society, 48, 10–15. https://doi.org/10.1016/j.niox.2014.10.006
- Shepherd, A. I., Gilchrist, M., Winyard, P. G., Jones, A. M., Hallmann, E., Kazimierczak, R., Rembialkowska, E., Benjamin, N., Shore, A. C., & Wilkerson, D. P. (2015). Effects of dietary nitrate supplementation on the oxygen cost of exercise and walking performance in individuals with type 2 diabetes: A randomized, double-blind, placebo-controlled crossover trial. Free Radical Biology and Medicine, 86, 200–208. https://doi.org/10.1016/j.freeradbiomed.2015.05.014
- Tan, R., Wylie, L. J., Thompson, C., Blackwell, J. R., Bailey, S. J., Vanhatalo, A., & Jones, A. M. (2018). Beetroot juice ingestion during prolonged moderate-intensity exercise attenuates progressive rise in O2 uptake. Journal of Applied Physiology, 124(5), 1254–1263. https://doi.org/10.1152/japplphysiol.01006.2017
- Thompson, C., Vanhatalo, A., Kadach, S., Wylie, L. J., Fulford, J., Ferguson, S. K., Blackwell, J. R., Bailey, S. J., & Jones, A. M. (2018). Discrete physiological effects of beetroot juice and potassium nitrate supplementation following 4-wk sprint interval training. Journal of Applied Physiology, 124(6), 1519–1528. https://doi.org/10.1152/japplphysiol.00047.2018
- Vanhatalo, A., Bailey, S. J., Blackwell, J. R., DiMenna, F. J., Pavey, T. G., Wilkerson, D. P., Benjamin, N., Winyard, P. G., & Jones, A. M. (2010). Acute and chronic effects of dietary nitrate supplementation on blood pressure and the physiological responses to moderate-intensity and incremental exercise. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 299(4), R1121–1131. https://doi.org/10.1152/ajpregu.00206.2010
- Whipp, B. J., Ward, S. A., Lamarra, N., Davis, J. A., & Wasserman, K. (1982). Parameters of ventilatory and gas exchange dynamics during exercise. Journal of Applied Physiology, 52(6), 1506–1513. https://doi.org/10.1152/jappl.1982.52.6.1506
- Whitfield, J., Ludzki, A., Heigenhauser, G. J., Senden, J. M., Verdijk, L. B., van Loon, L. J., Spriet, L. L., & Holloway, G. P. (2016). Beetroot juice supplementation reduces whole body oxygen consumption but does not improve indices of mitochondrial efficiency in human skeletal muscle. The Journal of Physiology, 594(2), 421–435. https://doi.org/10.1113/JP270844
- Wickham, K. A., McCarthy, D. G., Pereira, J. M., Cervone, D. T., Verdijk, L. B., van Loon, L. J. C., Power, G. A., & Spriet, L. L. (2019). No effect of beetroot juice supplementation on exercise economy and performance in recreationally active females despite increased torque production. Physiological Reports, 7(2), e13982. https://doi.org/10.14814/phy2.13982
- Wilkerson, D. P., Hayward, G. M., Bailey, S. J., Vanhatalo, A., Blackwell, J. R., & Jones, A. M. (2012). Influence of acute dietary nitrate supplementation on 50 mile time trial performance in well-trained cyclists. European Journal of Applied Physiology, 112(12), 4127–4134. https://doi.org/10.1007/s00421-012-2397-6
- Wootton-Beard, P. C., & Ryan, L. (2012). Combined use of multiple methodologies for the measurement of total antioxidant capacity in UK commercially available vegetable juices. Plant Foods for Human Nutrition, 67(2), 142–147. https://doi.org/10.1007/s11130-012-0287-z
- Wylie, L. J., Kelly, J., Bailey, S. J., Blackwell, J. R., Skiba, P. F., Winyard, P. G., Jeukendrup, A. E., Vanhatalo, A., & Jones, A. M. (2013). Beetroot juice and exercise: Pharmacodynamics and dose-response relationships. Journal of Applied Physiology, 15(3), 325–336. https://doi.org/10.1152/japplphysiol.00372.2013
- Wylie, L. J., Ortiz de Zevallos, J., Isidore, T., Nyman, L., Vanhatalo, A., Bailey, S. J., & Jones, A. M. (2016). Dose-dependent effects of dietary nitrate on the oxygen cost of moderate-intensity exercise: Acute vs. chronic supplementation. Nitric Oxide: Biology and Chemistry / Official Journal of the Nitric Oxide Society, 57, 30–39. https://doi.org/10.1016/j.niox.2016.04.004