ABSTRACT
We investigated whether a single heart rate clamped cycling session under systemic hypoxia affects the recovery of physical and psycho-physiological responses from residual fatigue compared to normoxia. On separate occasions, twelve trained males performed a 3-d acute training camp scenario. On days 1 and 3, participants cycled for 60 min at a constant heart rate (80% of ventilatory threshold). On day 2, fatigue was induced through a simulated team game circuit (STGC), followed by a 60-min intervention of either: (1) heart rate clamped cycling in normoxia; (2) heart rate clamped cycling in hypoxia (simulated altitude ~ 3500 m); or (3) no cycling. Countermovement jump height and leg stiffness were assessed before and after every session. Perceptual fatigue was evaluated daily. Compared to baseline, jump height decreased at all timepoints following the STGC (all p < 0.05). Leg stiffness and cycling power output only decreased immediately following the STGC, with a 48% further decrease in cycling power output in hypoxia compared to normoxia (p < 0.05). Perceived fatigue, decreased sleep quality, and increased muscle soreness responses occurred on day 3 (p < 0.05). A single heart rate-clamped cycling session in hypoxia reduced mechanical output without affecting recovery of physical performance and perceptual measures from residual fatigue induced through team sport activity.
Introduction
Team sport athletes commonly engage in intensified training periods, such as training camps lasting 10–15 days, to prepare for competition. One of the key aims of these camps is to enhance athletes’ fitness level, as highlighted by Racinais et al. (Citation2021), who showed an increase in maximal oxygen uptake in elite rugby union players following a 10-d camp. To elicit adaptations, athletes must undertake training at high intensity with substantial workloads during these camps. However, this elevated external work can also lead to increased mechanical stress, which may cause excessive tissue damage, thereby increasing the probability of injury (Galloway et al., Citation2013).
To prevent injury during training camps, it is necessary to allow adequate recovery time for structural and functional adaptations of the musculoskeletal systems following a high mechanical load training session (Vanrenterghem et al., Citation2017). However, the recovery of biomechanical constraints (e.g., synthesis of soft tissues in response to stress) typically takes longer than physiological supercompensation (e.g., increased cardiac output) (Vanrenterghem et al., Citation2017). Additionally, the presence of residual fatigue from previous training sessions can negatively impact performance and the outcomes of subsequent sessions. Studies have shown that performance measures, including 20-m sprint time, leg extension, leg flexion, and hip flexion strength, remain reduced for up to 48 h following the completion of a simulated team game circuit (STGC) (Ingram et al., Citation2009). Therefore, to maximise the physiological adaptations while minimising the risk of musculoskeletal injury, and mitigating the decline in physical performance resulting from residual fatigue during a training camp, it may be beneficial to reduce external work while maintaining an elevated internal demand over multiple training sessions. Consequently, a training method is needed that can provide physiological stimuli with lower external work, allowing for better maintenance of training quality in subsequent sessions.
One possible intervention for reducing external work during exercise without compromising internal demand is the use of systemic hypoxia. Hypoxia has the potential to affect exercise responses such that internal demand on physiological processes associated with oxygen delivery to exercising muscles are elevated, even at the same external load compared to normoxia conditions (Girard et al., Citation2021). Through this effect of hypoxia, recent research conducted in our laboratory demonstrated that metabolic, cardiorespiratory, and neuromuscular responses can be maintained between normoxia and simulated altitudes up to ~3500 m, despite a gradual reduction in cycling power output (PO) with increased hypoxia severity (~12.3% per 500 increment above 2500 m) (Li et al., Citation2023b). Here, exercise intensity was controlled across environmental conditions using an automatic heart rate (HR) clamp (Li et al., Citation2023a). These findings suggest that combining a HR clamp with hypoxia can effectively reduce the external load of exercise without lowering internal demands in an acute scenario. Specifically, these finding suggest that a simulated altitude of 3500 m can provide the greatest decrease in cycling power output without affecting other indices of internal load. However, one limitation of our previous study is that participants were well-rested during the exercise session, which may not accurately represent the typical fatigue levels experienced by athletes during a training camp scenario. Therefore, it is unknown whether the effects of residual fatigue on performance measures can be mitigated by the reduction in external work performed in hypoxia compared to normoxia.
Accordingly, the aim of this study was to determine the effects of a single session of reduced mechanical load under systemic hypoxia (~3500 m) compared to normoxia or no training (control) on the recovery of physical and psycho-physiological responses from residual fatigue in a 3-day training camp scenario. We hypothesised that performing an endurance cycling training session in hypoxia, with reduced mechanical work compared to normoxia, would alleviate the effects of residual fatigue caused by a training session conducted prior to cycling.
Methods
Participants
Twelve males (mean±SD: age 26 ± 3.3 years; stature 1.76 ± 0.07 m; body mass 76.1 ± 6.7 kg; peak oxygen uptake 47.6 ± 6.7 ml.min−1.kg−1) were recruited to participate in this study. Participants who trained for ~6 hours per week were classified as “Trained/Development” (Tier 2) using established criteria (McKay et al., Citation2021). A power calculation for repeated measures ANOVA (α = 0.05, 1-β = 0.95) was conducted using G*POWER (version 3.1.9.3) to determine the sample size, which suggested a sample size of 10 for our primary variable: cycling power output. The effect size (Cohen’s f) was 1.73, and the critical F-value of 4.74 was determined from previous works (Amann & Dempsey, Citation2008). All participants had no medical conditions, were injury-free, and had resided in Perth, Western Australia (near sea-level) for at least six months before the investigation. Ethical approval was obtained from the Human Research Ethics Committee at The University of Western Australia (ROAP2022/ET000298) in accordance with the Declaration of Helsinki, and participants provided signed informed consent before inclusion in the study.
Experimental design
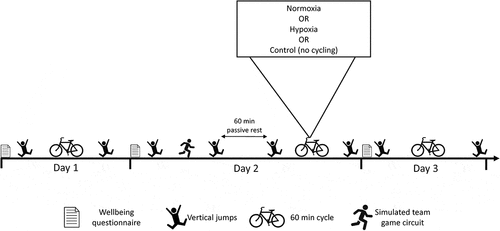
This study used a randomised cross-over design. Participants first completed a familiarisation session to determine their HR corresponding to 80% of ventilatory threshold (VT) through an incremental cycling test. The incremental cycling test involved a ramped increase of external workload at 25 W.min−1 until exhaustion. Expired gases were collected continuously using an indirect calorimetry system (TrueOne 2400, Parvo Medics, Inc., Utah, USA) providing rolling 15-s averages of oxygen uptake (VO2), carbon dioxide production (VCO2), and minute ventilation (VE). The criteria of an increase in VE/VO2 with no increase in VE/VCO2 and departure from the linearity of VE was used to determine VT (Racinais et al., Citation2014). At least 48 h after the familiarisation session, three experimental trials were conducted, each separated by at least five days, with each trial consisting of three consecutive days (). Participants completed each trial in a counter-balanced order and were blinded to the conditions. Each visit began with a well-being questionnaire (McLean et al., Citation2010) and a standardised warm-up consisting of 10 min of self-paced continuous cycling at sense of effort corresponding to five out of ten on the Borg CR-10 scale (Christian et al., Citation2014). On day 1, participants then performed pre-cycling vertical jump testing, followed by a 60-min HR-clamped cycling protocol in normoxia, and then a post-cycling vertical jump test. On day 2, participants completed the STGC in normoxia, followed by 60 min of passive rest (with recorded and replicated food intake for each trial during the test). After the rest period participants underwent one of three experimental conditions: (1) a 60-min HR-clamped cycling protocol completed in normoxia, (2) a 60-min HR-clamped cycling protocol completed in hypoxia, or (3) no cycling (i.e., 60 min of passive rest in normoxia). Vertical jump testing was conducted immediately before and after both the STGC and the 60-min experimental conditions. Day 3 was performed using exactly the same procedures as day 1 and was also completed in normoxia. This experimental design allowed us to compare the acute impacts of cycling in normoxia versus in hypoxia (i.e., reduced mechanical load) or no exercise (control) on the recovery of physical and psycho-physiological responses from residual fatigue in a training camp scenario.
The cycling bouts were completed in a normobaric hypoxia chamber (40 m3) using nitrogen injection (flow rate 270 L∙min−1, b-Cat BV S879, VPSA S325 V16, Van Amerongen, Biezenwei 6, The Netherlands) to achieve hypoxia (FiO2 = 13.6%). The chamber was maintained at temperate ambient conditions (air temperature: 21.9 ± 1.0°C; relative humidity: 46.1 ± 8.3%). The STGC took place in an indoor gymnasium with parquetry flooring and varnish under ambient conditions (air temperature: 18.4 ± 2.2°C; relative humidity: 54.1 ± 8.7%). All experimental trials were performed at a similar time of day (±2 h). Participants were instructed to avoid vigorous exercise, caffeine and alcohol for 24 h prior to and during the entire duration of the experimental trials. Water consumption was permitted ad libitum during all sessions.
Heart rate clamped cycling protocol
Participants cycled for 60 min at a HR corresponding to 80% VT1. The resistance on the electronically-braked cycle ergometer (Wahoo kickR, power trainer v5, Wahoo fitness Inc., Atlanta, GA, USA) was automatically adjusted by a custom-made application (AutoHR) to maintain HR at the desired level (Li et al., Citation2023a). The Wahoo kickR has previously been demonstrated as a reliable tool to measure cycling PO. For instance, in three separate cycling time trials performed on the Wahoo kickR, the coefficient of variation for recorded power output was 3.4% (Zadow et al., Citation2016). Additionally, typical errors between 50–400 W across two separate occasions were reported to be 1.3% (Hoon et al., Citation2016). The AutoHR app can wirelessly connect to HR monitors and electronically-braked ergometers via Bluetooth Low Energy. It adjusts the resistance using a proportional-integral controller based on previous works (Kawada et al., Citation1999). The app monitored both the target and actual HR, as well as the instantaneous cycling PO at sampling frequency of 1 Hz. Cycling PO was adjusted based on the differences between the target and actual HR. The participant’s HR was continuously measured throughout the HR clamped cycling protocol using a chest strap monitor (Polar H10, Kempele, Finland) paired with the AutoHR app. Additionally, oxygen saturation (SpO2) was estimated every 15 min via fingertip pulse oximetry (Palmsat 2500, Nonin Medical Incs, USA). Ratings of sense of effort, which were used for the perceptually regulated warm-up, and perceptual responses (i.e., ratings of perceived exertion [RPE], perceived leg discomfort, and perceived breathing difficulty) were recorded at similar times to SpO2 (ever 15 min) using modified BorgCR10 scales (Christian et al., Citation2014). The “sense of effort scale” was assessed by the question: “How hard are you trying?” with the anchor points provided ranging from 0 (“no effort”) to 10 (“maximum effort”). Perceived lower-limb discomfort, RPE, and perceived breathing difficulty were assessed from the questions: “How heavy do your legs feel?”, “What is your overall perceived exertion?”, and “How difficult does it feel to breath?”, respectively. All questions were printed together with the modified BorgCR10 scales with anchor points provided ranging from 0 (“nothing at all”) to 10 (“maximal”).
Simulated team game circuit
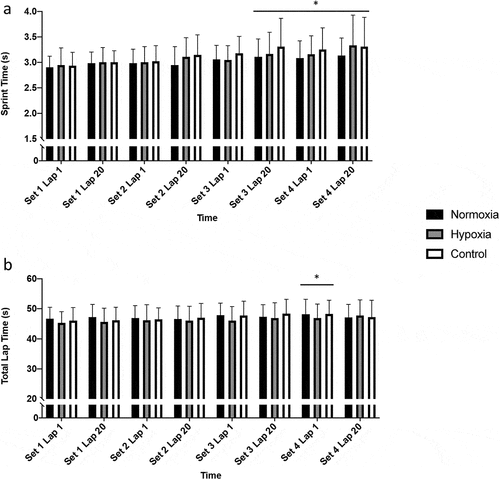
Participants completed a modified version of the STGC, originally designed by Bishop et al. (Citation2001), to induce fatigue. The protocol involved four sets of 20 × 1-min laps, with a 5-min rest period between each set. Each lap included team sport movements such as walking, jogging, striding, sprinting, agility and vertical jumps, covering a total distance of 92 m per lap. Sprint times for the initial 15 m and the total lap times for the first and last lap of each set during the STGC were recorded using electronic timing gates (SmartSpeed, Fusion Sport, QLD, Australia).
Well-being questionnaire
The well-being questionnaire (McLean et al., Citation2010) consisted of five items, including fatigue, sleep quality, general muscle soreness, stress levels, and mood, which are rated on a scale of 1 to 5. For example, fatigue is rated from 1 (“very fresh”) to 5 (“always tired”).
Vertical jump testing
The vertical jump test battery comprised three maximal countermovement jumps, with a 1-min rest between each trial, followed by a series of ten maximal hops. All jumps were performed on a force platform (AccuGait-Optimised, Advanced Mechanical Technology Incs., Watertown, MA, USA), which recorded vertical ground reaction forces at a rate of 200 Hz. Participants were instructed to keep their hands on their hips to eliminate the influence of arm swing. For the countermovement jumps, participants began in a standing position, then squatted down and extended their knees in one continuous motion. Participants were instructed to perform take-offs and landings in a consistent upright position, maintaining extension of their knees and ankles during the flight phase. Jump height was estimated from the flight time of the highest jump (Glatthorn et al., Citation2011). During the hops, participants were instructed to jump ten times to the highest possible point with stiff knees (“ankle jumps”) and with as brief a ground contact time as possible. Leg stiffness was calculated from contact and flight times using a previously developed method (Dalleau et al., Citation2004).
Data processing
For each HR clamped cycling session, the mean values of PO and HR were calculated by averaging the values obtained for 60 min. Mean values for jump height and leg stiffness were calculated for each instance of jump testing (Day 1 pre-cycling, Day 1 post-cycling, Day 2 pre-STGC, Day 2 post-STGC, Day 2 pre-intervention, Day 2 post-intervention, Day 3 pre-cycling, Day 3 post-cycling). Jump height and leg stiffness values were expressed relative to Day 1 pre-cycling, making Day 1 our maximal baseline marker (i.e., 100%).
Statistical analysis
A General Linear Mixed Model using the R (Team RC. R Core Team, 2020) package lme4 was used to analyse the data for the effects of condition (normoxia, hypoxia, control), time (day) and their interaction. A random intercept was also included in the model to account for baseline levels and inter-individual homogeneity. All models were estimated using Restricted Maximum Likelihood. Visual inspections of residual plots did not reveal any obvious deviations from homoscedasticity or normality. The p values were obtained using Type II Wald F tests with Kenward-Roger degrees of freedom as implemented in the R package car. Post-hoc pairwise-comparisons using the Tukey HSD tests were performed using the R package emmeans if a significant main effect (condition, time, condition × time) was observed. Partial eta squared (ηp2, with ηp2 ≥0.06 representing a moderate and ηp2 ≥0.14 a large effect) values were calculated for effect sizes (Cohen, Citation2013). All data are expressed as mean ± SD.
Results
Simulated team game circuit
There were no significant interaction or condition effects observed for 15-m sprint times and total lap times (all p > 0.09). A main effect of time was noted for both sprint times and total lap times (p < 0.01, ηp2 = 0.08 and 0.02, respectively). Compared to Set 1 Lap 1, 15-m sprint times increased between Set 3 Lap 20 and Set 4 Lap 20 (p < 0.05). Total lap time was longer only at Set 4 Lap 1 compared to Set 1 Lap 1 (p < 0.05, ).
Vertical jumps
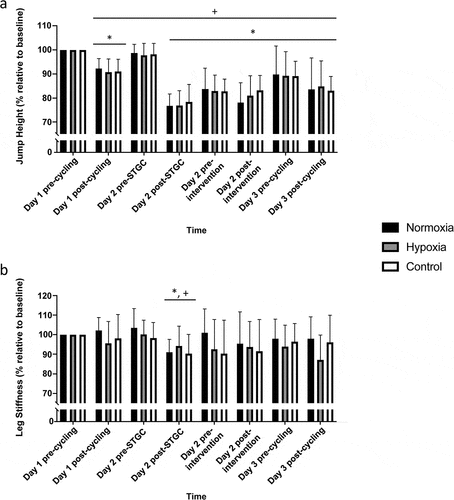
No significant interaction or condition effects were noted for jump height and leg stiffness (all p > 0.69, ). There was a significant main effect of time on jump height and leg stiffness (both p < 0.01, ηp2 = 0.08 and 0.01, respectively). Post-hoc analysis revealed that other than Day 2 pre-intervention, jump height was significantly lower at all other timepoints when compared to Day 1 pre-cycling (0.37 ± 0.06 m; p < 0.05). Compared to Day 1 pre-cycling (26.8 ± 6.4 kN.m−1) and all other timepoints, leg stiffness was only lower at Day 2 post-STGC (24.7 ± 6.4 kN.m−1; p < 0.05).
Figure 3. Jump height (a) and leg stiffness (b) measured pre and post each exercise bout.
Heart rate clamped cycling
For all cycling sessions, mean HR was (126.1 ± 7.3 bpm). Across the three days, there was an interaction effect between condition and time on cycling PO (p < 0.01, ηp2 = 0.10, ). Post-hoc analysis revealed no difference between conditions on day 1 (95.8 ± 25.1 W) and day 3 (95.9 ± 23.7 W), whereas on day 2 cycling PO (39.4 ± 15.4 W) was significantly reduced by 46.8 ± 18.0% (range: 12.8–75.9%) in hypoxia compared to normoxia (p < 0.01, ).
Figure 4. Mean power output (a), rating of perceived exertion (b), sense of effort (c), leg discomfort (d), and breathing difficulty (e) during each 60-min heart rate clamped cycling session. Perceived fatigue (f), sleep quality (g), muscle soreness (h), mood (i), and stress (j) reported from the well-being questionnaire on arrival to the laboratory each day.
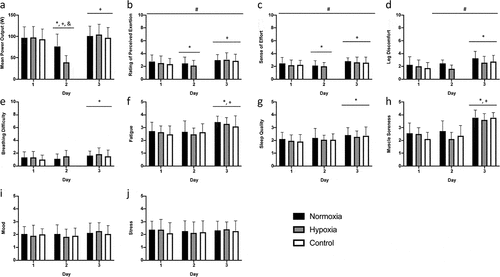
During the cycling sessions, a significant interaction between condition and time was present only for leg discomfort (p = 0.04, ηp2 = 0.02, ). Post-hoc analysis showed that leg discomfort was greater in normoxia on day 2 and day 3 (p < 0.05, ). Significant condition and time effects were present in RPE (ηp2 = 0.02 and 0.08, respectively), sense of effort (ηp2 = 0.02 and 0.10, respectively), and leg discomfort (ηp2 = 0.08 and 0.17, respectively) (all p < 0.05, ). Sense of effort and RPE were significantly higher in normoxia compared to all other conditions, and were different on day 2 compared to other days (p < 0.01, ). Breathing difficulty only exhibited a time effect (p < 0.01, ηp2 = 0.05, ). Across all conditions, breathing difficulty increased on day 3 compared to day 1 (p < 0.01, ).
Well-being questionnaire
No interaction or condition effects were present in the responses provided in the well-being questionnaire (all p > 0.19, ). There was a time effect in perceived fatigue, sleep quality, and muscle soreness (all p < 0.01, ηp2 = 0.20, 0.08 and 0.52, respectively). Post-hoc analysis revealed that perceived fatigue and muscle soreness were elevated and sleep quality worsened on day 3 compared to day 1 (all p < 0.05).
Discussion
The main findings from the current study indicate that, compared to normoxia, performing a single HR clamp cycling session in hypoxia with reduced mechanical output did not affect the recovery of physical performance and perceptual measures from residual fatigue induced by team sport activity. Consequently, these findings contradict our initial hypothesis, suggesting that, despite reduced mechanical work compared to normoxia, hypoxia does not alleviate the effects of residual fatigue caused by a training session conducted prior to cycling.
Physical performance
A key finding was that there were no differences in the recovery of physical performance markers (i.e., maximal countermovement jump height, leg stiffness, cycling PO) between the hypoxia and normoxia conditions after STGC-induced fatigue. However, residual fatigue was evident through reduced countermovement jump height on day 3, while cycling PO and leg stiffness had recovered at this time point. This suggests that the residual fatigue observed on day 3 May be attributed to a loss of muscular efficiency in the stretch-shortening cycle. In support, fatigue induced by a game of soccer can impair countermovement jump height for up to 72 h due to disruptions in the transition from the eccentric to the concentric phase of the jump (Silva et al., Citation2018). Comparatively, the quick recovery of leg stiffness in our study suggests that the STGC did not induce significant muscle damage, as prolonged decreases in leg stiffness are indicative of muscle damage (Byrne et al., Citation2004). It appears that the STGC did not sufficiently disrupt neuromuscular integrity to warrant a reduction in mechanical load on the musculoskeletal system during cycling. Therefore, hypoxia exposure during a 60-min clamped HR cycling session did not affect the recovery profiles of physical performance markers. This finding may explain why there was no difference observed in the recovery of physical performance markers between the hypoxia and normoxia conditions in the current study. Overall, our results suggest that a single endurance cycling session in hypoxia does not alter the recovery of physical performance markers from team sport-induced fatigue compared to cycling in normoxia.
Effect of hypoxia on cycling power output
Our study was the first to investigate the effects of hypoxia on cycling PO and HR-clamped cycling when participants were in a pre-fatigued state. We found that acute hypoxia exposure resulted in a significant reduction in cycling PO of ~ 47% compared to normoxia, despite participants cycling at similar HR levels. These findings suggest that fatigue amplifies the effect of hypoxia on the interplay between internal and external loads during exercise. In a previous study (Li et al., Citation2023b), it was found that simulating an altitude of 3500 m at the same clamped HR (80% VT) led to only a ~ 34% reduction in cycling PO. It is noteworthy, that participants across the two studies exhibited varying fitness levels, as indicated by differences in maximal oxygen uptake (57.0 vs 47.6 ml.min−1.kg−1 in the current study). However, one possible reason for this disparity in the reduction of mechanical load may be attributed to a decrease in central motor drive when participants are fatigued, which is further exacerbated by hypoxia and results in additional reductions in exercise capacity at a given HR. Notably, peripheral fatigue was found to be lower at exhaustion after high-intensity cycling in hypoxia, indicating that fatigue originating from the central nervous system was increased in hypoxia, causing exercise cessation (Amann & Dempsey, Citation2008). Consequently, the effect of hypoxia on central motor drive may have been heightened when participants were pre-fatigued, leading to a greater reduction in cycling PO compared to when participants were in a rested state.
Perceptual responses
Perceived fatigue was evident during the experimental protocol, as indicated by the responses on the well-being questionnaire. Specifically, participants reported increased fatigue, decreased sleep quality, and elevated muscle soreness on day 3. This is consistent with previous findings indicating that perceived muscle soreness remains elevated for up to 48 h following a soccer match before returning to baseline values (Rampinini et al., Citation2011). Importantly, in our study, the responses on the well-being questionnaire did not differ significantly between conditions. This suggests that despite a lower mechanical load during cycling after team sport activity, perceptual fatigue remains unaltered.
Although there were statistically significant differences in perceptual responses during HR clamped cycling between conditions, these differences may not have practical relevance. For example, on day 2, participants reported leg discomfort scores that were ~ 0.8 lower in hypoxia than in normoxia. However, since the BorgCR10 scale uses increments of 1, such a small difference may not have meaningful implications. Accordingly, it appears that performing a single endurance cycling session in hypoxia had no substantial impact on the perceptual responses during cycling compared to normoxia.
Limitations
It has previously been discussed that the recovery of physical performance from residual fatigue is dependent on a multitude of factors such as age, training history, and playing position (Paul et al., Citation2015). Accordingly, a limitation of our study is that our findings in “trained/development” males may not be generalisable across the entire spectrum of athletes. As such, further investigations are needed in other populations. Nonetheless, our results may provide an initial indication that a single HR clamped cycling session does not significantly affect the recovery from residual fatigue.
Practical applications
Hypoxia can be used during a single endurance cycling session to lessen the mechanical load of exercise while maintaining the target HR, particularly when individuals are fatigued from participating in team sport activity, as compared to normoxia. These sessions, which involve reduced mechanical stress, could be advantageous during team sport training camps since they do not appear to hinder the training stimulus or affect the recovery of physical performance and perceptual responses from residual fatigue. However, the repeated use of hypoxia in training sessions may begin to inhibit the effects of hypoxia on the reduction of mechanical load as athletes become more acclimatised to the environmental conditions (Burtscher et al., Citation2022). Therefore, future investigations are required to determine whether implementing hypoxia in multiple sessions throughout a training camp would impact physical performance and perceptual responses as residual fatigue accumulates from consecutive training sessions.
Conclusion
Performing a single HR-clamped cycling session in hypoxia while being pre-fatigued can alleviate the mechanical constraints of exercise without affecting the recovery of physical performance and perceptual measures from residual fatigue induced by team sport activity. Thus, hypoxia can be used to reduce the overall external load in an acute training camp scenario without negatively impacting other physiological and psychological responses.
Acknowledgments
The authors thank the participants for their dedication, commitment, and cooperation with this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Amann, M., & Dempsey, J. A. (2008). Locomotor muscle fatigue modifies central motor drive in healthy humans and imposes a limitation to exercise performance. Journal of Physiology, 586(1), 161–173. https://doi.org/10.1113/jphysiol.2007.141838
- Bishop, D., Spencer, M., Duffield, R., & Lawrence, S. (2001). The validity of a repeated sprint ability test. Journal of Science and Medicine in Sport, 4(1), 19–29. https://doi.org/10.1016/S1440-2440(01)80004-9
- Burtscher, M., Millet, G. P., & Burtscher, J. (2022). Hypoxia conditioning for high-altitude pre-acclimatization. Journal of Science in Sport and Exercise, 1, 1–15. https://doi.org/10.1007/s42978-021-00150-0
- Byrne, C., Twist, C., & Eston, R. (2004). Neuromuscular function after exercise-induced muscle damage: Theoretical and applied implications. Sports Medicine, 34(1), 49–69. https://doi.org/10.2165/00007256-200434010-00005
- Christian, R. J., Bishop, D. J., Billaut, F., & Girard, O. (2014). The role of sense of effort on self-selected cycling power output. Frontiers in Physiology, 5, 115. https://doi.org/10.3389/fphys.2014.00115
- Cohen, J. (2013). Statistical power analysis for the behavioral sciences. Academic Press.
- Dalleau, G., Belli, A., Viale, F., Lacour, J. R., & Bourdin, M. (2004). A simple method for field measurements of leg stiffness in hopping. International Journal of Sports Medicine, 25, 170–176. https://doi.org/10.1055/s-2003-45252
- Galloway, M. T., Lalley, A. L., & Shearn, J. T. (2013). The role of mechanical loading in tendon development, maintenance, injury, and repair. Journal of Bone and Joint Surgery, 95(17), 1620–1628. https://doi.org/10.2106/JBJS.L.01004
- Girard, O., Girard, I. M., & Peeling, P. (2021). Hypoxia conditioning: A novel therapeutic solution for load-compromised individuals to achieve similar exercise benefits by doing less mechanical work! British Journal of Sports Medicine, 55(17), 944–945. https://doi.org/10.1136/bjsports-2020-103186
- Glatthorn, J. F., Gouge, S., Nussbaumer, S., Stauffacher, S., Impellizzeri, F. M., & Maffiuletti, N. A. (2011). Validity and reliability of optojump photoelectric cells for estimating vertical jump height. Journal of Strength and Conditioning, 25(2), 556–560. https://doi.org/10.1519/JSC.0b013e3181ccb18d
- Hoon, M. W., Michael, S. W., Patton, R. L., Chapman, P. G., & Areta, J. L. (2016). A comparison of the accuracy and reliability of the Wahoo KICKR and SRM power meter. Journal of Science and Cycling, 5(3), 11–15. https://doi.org/10.28985/jsc.v5i3.240
- Ingram, J., Dawson, B., Goodman, C., Wallman, K., & Beilby, J. (2009). Effect of water immersion methods on post-exercise recovery from simulated team sport exercise. Journal of Science and Medicine in Sport, 12(3), 417–421. https://doi.org/10.1016/j.jsams.2007.12.011
- Kawada, T., Ikeda, Y., Takaki, H., Sugimachi, M., Kawaguchi, O., Shishido, T., Sato, T., Matsuura, W., Miyano, H., & Sunagawa, K. (1999). Development of a servo-controller of heart rate using a cycle ergometer. Heart and Vessels, 14(4), 177–184. https://doi.org/10.1007/BF02482304
- Li, S. N., Peeling, P., Scott, B. R., Peiffer, J. J., Shaykevich, A., & Girard, O. (2023a). Automatic heart rate clamp: A practical tool to control internal and external training loads during aerobic exercise. Frontiers in Physiology, 14(14), 590. https://doi.org/10.3389/fphys.2023.1170105
- Li, S. N., Peeling, P., Scott, B. R., Peiffer, J. J., Shaykevich, A., & Girard, O. (2023b). Maintenance of internal load despite a stepwise reduction in external load during moderate intensity heart rate clamped cycling with acute graded normobaric hypoxia in males. Journal of Science and Medicine in Sport, 26(11), 628–635. https://doi.org/10.1016/j.jsams.2023.09.006
- McKay, A. K., Stellingwerff, T., Smith, E. S., Martin, D. T., Mujika, I., Goosey-Tolfrey, V. L., Sheppard, J., & Burke, L. M. (2021). Defining training and performance caliber: A participant classification framework. International Journal of Sports Physiology and Performance, 17(2), 317–331. https://doi.org/10.1123/ijspp.2021-0451
- McLean, B. D., Coutts, A. J., Kelly, V., McGuigan, M. R., & Cormack, S. J. (2010). Neuromuscular, endocrine and perceptual fatigue responses during different length between-match microcycles in professional rugby league players. International Journal of Sports Physiology and Performance, 5(3), 367–383. https://doi.org/10.1123/ijspp.5.3.367
- Paul, D. J., Bradley, P. S., & Nassis, G. P. (2015). Factors affecting match running performance of elite soccer players, shedding some light on the complexity. International Journal of Sports Physiology and Performance, 10(4), 516–519. https://doi.org/10.1123/ijspp.2015-0029
- Racinais, S., Buccheit, M., & Girard, O. (2014). Breakpoints in ventilation, cerebral and muscle oxygenation, and muscle activity during an incremental cycling exercise. Frontiers in Physiology, 5, 142. https://doi.org/10.3389/fphys.2014.00142
- Racinais, S., Périard, J. D., Psicione, J., Bourdon, P. C., Cocking, S., Ihsan, M., Lacome, M., Nichols, D., Townsend, N., Travers, G., Wilson, M. G., & Girard, O. (2021). Intensified training supersedes the impact of heat and/or altitude for increasing performance in elite rugby union players. International Journal of Sports Physiology and Performance, 16(10), 1416–1423. https://doi.org/10.1123/ijspp.2020-0630
- Rampinini, E., Bosio, A., Ferraresi, I., Petruolo, A., Morelli, A., & Sassi, A. (2011). Match-related fatigue in soccer players. Medicine & Science in Sports & Exercise, 43(11), 2161–2170. https://doi.org/10.1249/MSS.0b013e31821e9c5c
- Silva, J. R., Rumpf, M. C., Hertzog, M., Castagna, C., Farooq, A., Girard, O., & Hader, K. (2018). Acute and residual soccer match-related fatigue: A systematic review and meta-analysis. Sports Medicine, 48(3), 539–583. https://doi.org/10.1007/s40279-017-0798-8
- Vanrenterghem, J., Nedergaard, N. J., Robinson, M. A., & Drust, B. (2017). Training load monitoring in team sports: A novel framework separating physiological and biomechanical load-adaptation pathways. Sports Medicine, 47(11), 2135–2142. https://doi.org/10.1007/s40279-017-0714-2
- Zadow, E. K., Fell, J. W., & Kitic, C. M. (2016). The reliability of a laboratory-based 4 km cycle time trial on a Wahoo KICKR power trainer. Journal of Science and Cycling, 5(3), 23–27.