Abstract
The “language network” is remarkably stable across language tasks but changes in response to injury to specific components or in response to “disconnection” of input to one component. We investigated network changes during language recovery, hypothesizing that language recovery takes place through distinct mechanisms: (a) reperfusion; (b) recovery from diaschisis; (c) recovery from structural disconnection; and (d) “reorganization” of language, whereby various components assume function of a damaged component. We also tested the hypothesis that “reorganization” depends on: the language task, level of performance, size and site of stroke, and time post onset. We tested these hypotheses in five participants who had structural, perfusion, and functional imaging utilizing spelling, reading, word generation, and picture naming tasks at acute and subsequent stages after ischaemic stroke. These cases illustrate different mechanisms of aphasia recovery or illustrate that reorganization of language acutely depends on individual variables in addition to size and site of stroke.
Despite the many variables that can influence the activation (the blood-oxygen-level-dependent, BOLD, response) correlated with tasks, there exists a relatively small set of areas of the brain that are reliably activated across a wide range of language tasks in healthy participants. These areas include the left posterior inferior frontal cortex (“Broca's area”, including pars triangularis and pars opercularis), precentral gyrus, premotor cortex, posterior superior temporal cortex (Wernicke's area), angular gyrus, and posterior middle and inferior temporal cortex (e.g., Parker Jones et al., Citation2012; Postman-Caucheteux et al., Citation2010; Prabhakaran et al., Citation2007; Segaert, Menenti, Weber, Petersson, & Hagoort, Citation2012). For example, shows activations across a variety of tasks that activate this “language network”. Reading and writing tasks typically also engage fusiform gyrus and supramarginal gyrus or posterior intraparietal sulcus (Beeson et al., Citation2003; Cohen, Dehaene, Vinckier, Jobert, & Montavont, Citation2008; Purcell, Napoliello, & Eden, Citation2011; Purcell, Turkeltaub, Eden, & Rapp, Citation2011; Turkeltaub, Eden, Jones, & Zeffiro, Citation2002). Depending on the design of the task and the control task, not all of these areas show significant activation, and not all areas reliably show activation in all participants (Poline, Vandenberghe, Holmes, Friston, & Frackowiak, Citation1996). Although in normal dextral participants activation during language tasks is reliably left-hemisphere dominant, there is usually some activation in areas of the right hemisphere that are homologous to the “language network”.
Figure 1. The “language network”: Areas of the brain frequently activated across very different language tasks. (A) Areas activated in association with silent word generation in normal controls (Prabhakaran et al., Citation2007); (B) areas of activation associated with word retrieval (naming and oral reading minus saying, “1, 2, 3”; Parker Jones et al., Citation2012); (C) areas of activation associated with syntax repetition (Segaert et al., Citation2012); and (D) areas of activation associated with reading (blue) and spelling (green; Rapp & Lipka, Citation2011). [To view this figure in colour, please see the online version of this journal.]
![Figure 1. The “language network”: Areas of the brain frequently activated across very different language tasks. (A) Areas activated in association with silent word generation in normal controls (Prabhakaran et al., Citation2007); (B) areas of activation associated with word retrieval (naming and oral reading minus saying, “1, 2, 3”; Parker Jones et al., Citation2012); (C) areas of activation associated with syntax repetition (Segaert et al., Citation2012); and (D) areas of activation associated with reading (blue) and spelling (green; Rapp & Lipka, Citation2011). [To view this figure in colour, please see the online version of this journal.]](/cms/asset/21a68f6f-a175-4bf0-a11a-76bb10b5df5f/pcgn_a_875467_f0001_c.jpg)
This robust “language network” must change in response to focal injury to one of the critical nodes of the network (e.g., Broca's area). The network also may change in response to functional or structural “disconnection” of input to one component. For example, the thalamus is often considered the “gateway to the cortex” and provides functional activation of cortical regions, sometimes through direct white matter tracts, or sometimes through indirect influences. An infarct in the thalamus can result in inactivation of a critical node of the network. Dysfunction of a cortical region due to a remote lesion is referred to as diaschisis and was initially described by Von Monakow (Citation1914). Diaschisis has been demonstrated using single photon emission computed tomography (SPECT) and positron emission tomography (PET) imaging (Olsen, Bruhn, & Oberg, Citation1986; Szelies et al., Citation1991; Vallar et al., Citation1988) and occasionally with dynamic contrast perfusion-weighted imaging (PWI) (Lin et al., Citation2009). Not surprisingly, focal infarct or inactivation of one or more nodes of the language network usually results in some degree of language impairment. Nevertheless, language usually recovers over time, despite persistent damage to that “critical node.” The purpose of our investigation was to determine how the language network changes in response to acute stroke and how it continues to change in order to account for language recovery after stroke.
A previous, longitudinal functional magnetic resonance imaging (fMRI) study of language recovery in 14 stoke patients who had repeated fMRI studies of auditory sentence comprehension in the acute, subacute, and chronic phases of stroke proposed a model of language reorganization based on the average performance of these stroke patients (Saur et al., Citation2006). They proposed that aphasia recovery as reflected in fMRI is characterized by: little activation (in either hemisphere) during the acute phase; a large increase in activation in the bilateral network, with peak activation in the right homologue of Broca's area in the subacute phase; and a shift of language back to the left-hemispheric “language cortex” in the chronic phase. This shift back to the normal left-hemisphere language network was seen particularly in patients who showed good recovery. The authors assume there exists a preexisting bilateral language network, which is normally left-hemisphere dominant, including Broca's area and Wernicke's area, along with right-hemisphere homologues. The right-hemisphere homologues are normally engaged in language tasks to some degree, but do not have a dominant role. It cannot be determined from fMRI alone whether right-hemisphere “activation” that correlates with language in normal controls represents activation of downstream neuronal function or active inhibition (active suppression of downstream neuronal function by inhibitory neurotransmitters) in those areas. Likewise, some of the activation of right-hemisphere homologues during stroke recovery could be maladaptive rather than facilitative. Suppression of right-hemisphere Broca's area in chronic stroke can improve speech production (Barwood et al., Citation2011; Hamilton et al., Citation2010; Martin et al., Citation2004, Citation2009a, 2009b; Naeser et al., Citation2005; Thiel et al., Citation2001; Weiduschat et al., Citation2011). Another recent fMRI study of auditory comprehension of 9 recovered aphasic individuals and 18 nonrecovered aphasic individuals with left middle cerebral artery (MCA) stroke showed that dominant right-hemisphere activation was associated with poor recovery (Szaflarski, Allendorfer, Banks, Vannest, & Holland, Citation2013). However, other recent studies indicate that facilitating activation of the right hemisphere can improve language after stroke (Mylius, Zouari, Ayache, Farhat, & Lefaucheur, Citation2012).
The importance of the Saur study was in providing direct evidence that the language network is dynamic. The areas of brain that are responsible for a particular language task change over time. Therefore, a previous debate over whether perilesional areas or right-hemisphere homologues of language cortex are more critical for aphasia recovery is too simplistic. There is evidence that both perilesional areas (Heiss et al., Citation1997; Heiss, Kessler, Thiel, Ghaemi, & Karbe, Citation1999; Karbe, Kessler, Herholz, Fink, & Heiss, Citation1995; Thiel et al., Citation2001; Warburton, Price, Swinburn, & Wise, Citation1999) and right-hemisphere homologues (Cappa et al., Citation1997; Leff, Scott, Rothwell, & Wise, Citation2001; Musso et al., Citation1999; Ohyama et al., Citation1996; Thompson, den Ouden, Bonakdarpour, Garibaldi, & Parrish, Citation2010; Thompson, Riley, den Ouden, Meltzer-Asscher, & Lukic, Citation2013; Thulborn, Carpenter, & Just, Citation1999; Weiller et al., Citation1995) can assume the functions of damaged components of language cortex. Saur and colleagues provided evidence that time post stroke is one of the variables that influences whether right-hemisphere or left-hemisphere areas are more likely to be dominant in auditory comprehension. Size and site of stroke also influence the extent to which right-hemisphere homologues of language cortex are engaged in recovery. For example, Sebastian and Kiran (Citation2011) studied oral picture naming and semantic judgement in eight stroke participants and eight age- and gender-matched controls with fMRI. In the semantic judgement task, site of lesion influenced activation: Stroke participants without a lesion involving the left frontal region showed left-dominant activation of inferior frontal gyrus (IFG) similarly to normal controls. In contrast, those with left frontal lesions showed bilateral activation in IFG. On the oral picture naming task, size of lesion influenced activation: Although all participants showed bilateral IFG activation, there was a significant negative correlation between the laterality index and lesion volume. That is, larger lesions predicted more right lateralized activation. Likewise, Marcotte et al. (Citation2012) reported that although poor recovery was not associated with volume of lesion, poor recovery was associated with damage to pars triangularis of Broca's area (left Brodmann's area 45). Good recovery with treatment was associated with activation of left Brodmann's area 4 (precentral gyrus) and area 6 (premotor cortex).
Thus, the variables underlying aphasia recovery are likely to be complex. Many studies have evaluated how the language network changes in response to treatment (Berthier et al., Citation2011; Cappa, Citation2011; Fridriksson, Citation2010; Fridriksson et al., Citation2007; Meinzer et al., Citation2013; Rochon et al., Citation2010; Szaflarski et al., Citation2013) and how the relationships between components of the network change after stroke (Price & Crinion, Citation2005; Warren, Crinion, Lambon Ralph, & Wise, Citation2009) or how activation in particular areas predicts outcome (e.g., Crinion & Price, Citation2005). Furthermore, there are likely to be several distinct mechanisms underlying aphasia recovery (Kertesz & Munoz, Citation1997; Kiran, Citation2012). Despite the recognition of these facts, there have been few systematic, longitudinal, multimodality imaging studies of individuals from the acute stage of stroke through the chronic stage to attempt to uncover the various mechanisms that contribute to recovery and the variables that influence each mechanism. Here we touch on this issue, by testing two hypotheses: (a) There are distinct mechanisms of recovery of language functions that have different time courses beginning at the acute stage of stroke; and (b) reorganization of the language network—in which one area of the brain (perhaps part of a preexisting bilateral “language network”) assumes the function of a damaged area—is influenced by factors other than size and site of stroke and time post onset. We show that which parts of the brain are recruited during performance of language varies for distinct language tasks and across individuals with the same size and site of lesion at the same time post onset (i.e., is also influenced by individual factors that affect level of task performance).
METHOD
Participants
Five participants (3 men, 2 women) with acute ischaemic left-hemisphere stroke were recruited from the inpatient units or the Emergency Department at Johns Hopkins Hospital for this longitudinal study. These participants are part of an ongoing project, the Stroke Cognitive Outcome and REcovery (SCORE) study. Participants ranged in age from 39–62 years (mean age = 54). All participants obtained at least 10th grade education and gave informed consent (if they demonstrated intact comprehension) or their closest relative or legal representative consented (if they had impaired comprehension) to the study according to the Human Subjects Protocol for Johns Hopkins University. Additional exclusion criteria included: known uncorrected visual or hearing loss, ongoing sedation or reduced level of consciousness, contraindication for MRI (e.g., implanted ferrous metal), primary haemorrhage on imaging, and previous neurological disease (except stroke).
All participants had clinical MRI that included diffusion-weighted imaging (DWI) and PWI at Day 1. Participants were also given a battery of language and cognitive tests, including the Western Aphasia Battery (Kertesz, Citation1982), Apraxia Battery for Adults–2 (Dabul, Citation2000), and Johns Hopkins Lexical Battery (unpublished). These tests were administered in the acute (1–5 days) and follow-up time points. (Please see and for details.) Multimodality MR research imaging (fMRI; diffusion tensor imaging, DTI) sessions were scheduled at 2–5 days and 2–6 months (and 1-year follow-up is planned). All individuals participated in one or more research imaging sessions, but Participant 1 did not engage in fMRI.
Table 1. Participant 1 performance on the Johns Hopkins lexical battery
Table 2. Western Aphasia Battery and additional tasks
MRI scans
Clinical scans
Perfusion and diffusion MR images were acquired as a part of the clinical standard of care on a Siemens 3.0 T Magnetom Trio scanner, equipped with a 8-channel head coil. High-resolution diffusion-weighted images were acquired using a single-shot echo-planar imaging (EPI) pulse sequence with the following parameters: generalized autocalibrating partially parallel acquisitions (GRAPPA) parallel imaging (acceleration factor of 2), field of view (FOV) 220 mm × 220 mm, matrix 160 × 160, time to repetition (TR) = 5700 ms, echo time (TE) = 87 ms, EPI factor 160, phase partial Fourier factor 6/8, 40 slices, slice thickness 4 mm, b = 0 s mm–2, and b = 1000 s mm–2. PWI were acquired using echo planer imaging with the following parameters: GRAPPA parallel imaging (acceleration factor of 2), FOV 230 mm × 230 mm, TR = 1640 ms, TE = 35 ms, matrix 128 × 128. 20 cc of gadolinium was administered, using a power injector, with a 10-s delay, at a rate of 5 cc s–1. We analysed the time-to-peak (TTP) maps and considered an area of hypoperfusion any voxels where the TTP in the affected (left) hemisphere was >4 s delayed compared to the voxels in the right hemisphere.
Research scans
Research MR images were acquired on a 3-T Philips Achieva MRI scanner, equipped with a 32-channel head coil. The MRI acquisition protocol was 50 minutes long and included the following: magnetization prepared rapid gradient echo (MPRAGE), fluid-attenuated inversion recovery (FLAIR), and BOLD acquisitions. The language paradigm timing was synchronized to the BOLD acquisition using the radio frequency (RF) signal from the RF trigger box. BOLD images were acquired using: EPI in the axial plane, TR = 2-s 160 volumes with an additional 8 s of dummy scans, 32-channel head coil, a 240 mm × 240 mm in field of view 139.5 mm, scan resolution 80 × 80, ascending slice scan order, 90° flip angle, and TE = 30 ms. MPRAGE images were acquired in the sagittal plan, using a multishot, turbo field echo pulse sequence with an in-plane resolution 256 mm × 240 mm, TR = 6.8 ms, TE = 3.1 ms, minimum TI = 850 ms, and 170 slices.
fMRI paradigms
fMRI data were acquired using four block design paradigms: orthographic (“spelling”) task, picture naming, reading, and word fluency. Each paradigm consisted of eight blocks, beginning with a control block, followed by alternating experimental and control blocks. The duration of each block was 40 seconds.
The orthographic task (requiring retrieval of the spelling of the word) consisted of 80 words written in upper-case letters, each containing a single missing letter. Participants were instructed to name the missing letter silently. The control blocks incorporated 80 words, matched in length and frequency to the words in the active block, written with upper-case letters, with the exception of a single lower-case letter. Participants were instructed to name the single lower-case letter silently. In both the task blocks and the control blocks, the participant silently named a letter; however, for the task block the participant had to retrieve the spelling of the word to correctly name the missing letter. Each word was projected for 2 s. The picture naming task consisted of 80 coloured objects in the active block. Participants were instructed to name each picture silently. The control blocks contained alternating chequerboard images, for which the participant was instructed to name “chequerboard” silently. For both the task block and the control block, the participant silently produced a word; however, for the task block the participant had to retrieve the picture name.
The reading task consisted of 40 words, and participants were instructed to silently read the word. The control blocks contained alternating symbol strings, and participants were instructed to passively view the letter strings. Each active and control stimulus was projected for four seconds.
The design of the word fluency task was based on the silent word generation task that was published in a list of paradigms presented at the American Society of Functional Neuroradiology (ASFNR) 2007 annual meeting (Yetkin et al., Citation1998). Our word fluency task utilized a 30-second control block, followed by alternating, 30-second, active and control blocks for four minutes. During the active blocks, images of a single letter were presented for 10 seconds at a time. Participants were instructed to silently name as many words as possible that began with the letter presented on the screen without repeating words. During the control blocks, participant were asked to passively view a series of nonsense symbols, each presented for two seconds. Each participant participated in one of the task based on language deficit noted at initial language testing.
The word fluency paradigm was implemented using an INVIVO Esys fMRI projection system. The orthographic task, picture naming, and reading paradigms were implemented using an Optimo PK320 pico-projector system, operated in conjunction with Psychtoolbox.
fMRI data analysis
fMRI processing
fMRI images were converted from the Philips enhanced DICOM format to the NifTi format, using DCM2nii package, and analysed in AFNI ® Version 2.56b, using a generalized linear model (GLM) analysis (Cox, Citation1996). The GLM model design included six motion parameters in addition to the time course fMRI signal. fMRI volumes with more than 2 mm of motion were censored from the GLM analysis. fMRI images were analysed in their native space, with no lesion masking, and the resulting statistical maps were false discovery rate (FDR) corrected. The preprocessing to the GLM analysis included (in order): slice timing correction, transformation to the cardinal axis, temporal filter, three-dimenional volume registration, 6.0-Hz spatial filter, and application of a mask, rescaled voxel to have a mean of 100.
Activation tables were generated, using the GUI interface in AFNI ® Version 2.56b, from individual, FDR corrected p < .05, stroke subject statistical maps. The minimum cluster size was set to 20 voxels, and clusters were created using the third-nearest neighbour clustering method.
fMRI difference images
High-resolution MPRAGE and BOLD EPI images were coregistered into Montreal Neurological Institute (MNI) template space using the MNI_avg152T1_tlrc and TT_EPI_tlrc templates, respectively. Statistical parametric maps were calculated in this standard space using the workflow described above, and difference-statistical maps (DSMs) were calculated to infer the change between two statistical maps acquired from the same study participants at different time points, or the change between two study participants’ statistical maps acquired using the same BOLD acquisition and paradigm.
fMRI lateralization index
The lateralization index (LI) was calculated to determine hemispheric lateralization in specific regions of interest (ROIs) in the “language network” at each time point, using the following formula.
Where VR and VL denote the number of active voxels in an ROI located in the right and left hemispheres, respectively. The lateralization index calculations were performed on FDR corrected statistical maps calculated with an alpha level of p < .05.
The lateralization index was computed in the following ROI (components of the “language network”): superior, middle, and inferior frontal gyri; superior, middle, and inferior temporal gyri; fusiform gyrus, and angular and supramarginal gyri. MNI templates for each ROI were used to mask the FDR corrected statistical map in MNI space, and the active voxels in each ROI (in left and right hemispheres) at p < .05 were counted.
Participant training
Participants were trained outside the MRI scanner, before fMRI imaging was done. During training, each participant was asked to overtly perform the tasks in order to determine performance accuracy. However, during the actual fMRI scans, participants were asked to silently perform the task in order to minimize motion-related artefacts associated with overt language tasks. A technician from the research team reviewed the paradigm with each participant before image acquisition. Research participants were selected based on compliance criteria established during this training session. Participants who showed less than 75% accuracy on a specific task, during training, were excluded from data acquisition using that task.
Lesion volume analysis
Volumes of infarct and hypoperfusion were measured by a technician blinded to the behavioural data and hypotheses of the study, using Olea Sphere ® 2.2 software (Olea Medical, 2012).
Fibre tract analysis
Lesion disruption calculation
The intensity-thresholding and segmentation features in ROIEditor Version 1.6 (Mori et al., Citation2008) were used to reproducibly define the enhancing portion of each subject's FLAIR image as the stroke lesion. A whole brain probabilistic fibre tract atlas was mapped to the JHUMNISS atlas and its corresponding brain parcellation map (JHUMINSSBPM) using the previously published methods by Zhang et al. (Citation2010). The probability atlas was then used to determine the voxelwise probabilistic density of each fibre tract (Zhang et al., Citation2010).
A fibre tract disruption ratio for each fibre tract projection from a given anatomical area was calculated by averaging the voxelwise probabilistic density for each fibre tract over the total number of voxels in the lesion. Vl is defined as the total number of voxels in the lesion, and Pxy is defined a voxelwise probability density for a given fibre tract in the probabilistic fibre tract atlas. The lesion disruption ratio, Rl , for each fibre tract, is defined as:
Where Pxy is summed over all the voxels in the lesion.
Cortical volume disruption calculation
A single cortical volume disruption ratio, Rc , was calculated for each cortical volume, using the ratio of the total number of fibre tracts in each cortical volume to the number of totally or partially, lesion-disrupted fibre tracts associated with that same cortical volume. The cortical volumes were defined by JHU-MNI-SS (Mori et al., Citation2008). Fv is defined as the total number of fibre tracts associated with each cortical volume. Lc is defined as the number of partially or totally, lesion-disrupted fibre tracts. The cortical volume disruption ratio was calculated as:
RESULTS
In this paper we report five case studies from our longitudinal study, the Stroke Cognitive Outcome and REcovery (SCORE) study, which test the following hypotheses: (a) There are distinct mechanisms of recovery from aphasia after stroke with different time courses; and (b) reorganization (which area “takes over” the function of a damaged area “language cortex”) depends not only on the time post onset, but also on the language task being used (and the level of performance of that language task), as well as the lesion location and size and individual factors (perhaps age, education, previous language competence, bilingualism, etc.).
Participant 1: Reperfusion of critical parts of “language cortex” in acute stroke
Participant 1 is a 62-year-old right-handed professor of political science, who was fluent in several languages. He had authored textbooks in three languages. He experienced sudden onset word-finding difficulty and right-arm weakness while on a beach in another country. He was evaluated at the local hospital, where he was found to have an old subcortical right-hemisphere stroke (which had been asymptomatic) and new (acute) left subcortical and cortical infarct. He was also found to have complete occlusion of both internal carotid arteries. He was not considered to be a candidate for any therapy to improve blood flow by opening the carotid artery (because such therapies are unsuccessful when the carotid is completely occluded). His wife requested a transfer to Johns Hopkins when he became acutely unable to speak.
On admission to our hospital (approximately 24 hours after acute worsening of his speech), Participant 1 had well-articulated but anomic speech, with frequent word-finding pauses and circumlocutions, and both semantic and phonemic paraphasias in conversation and naming tasks. Semantic paraphasias in naming included: accordion → “harmonica”, ostrich → “swan”. Phonemic paraphasias in naming included: heart → “cart”; anchor → “ankler”. He was aware of his naming errors and attempted self-correction. He was anomic in Spanish, Arabic, and English. He reported that he was usually unable to recall words in the other languages he spoke, although occasionally recalled a name in Arabic or Spanish, when he could not recall the name in English (he was not tested in other languages). His word comprehension was relatively spared. He understood conversation well, but had trouble with comprehension of syntactically complex sentences and questions. Written naming and spelling to dictation were not tested because he had trouble using his right hand, due to ideomotor apraxia. In spelling aloud, he made phonologically plausible errors (e.g., door → d-o-r-r), and many omissions due to difficulty naming letters. Oral reading was intact, other than self-corrected articulatory errors. He was able to read pseudowords. His language scores (in English) are given in . Based on language testing, Participant 1 was assumed to have a relatively selective impairment in accessing lexical representations (or linking semantic representations to lexical representations via modality-independent lemma-level representations), along with mild bucco-facial apraxia and impairment in comprehension of syntactically complex sentences without contextual cues.
Cranial nerve examination was intact. He had no dysarthria. Motor examination revealed normal strength but right ideomotor apraxia. He was unable to imitate meaningless gestures and had difficulty producing some meaningful gestures with the right hand. Stance and gait were normal. He had no sensory deficits.
Participant 1 underwent MRI with DWI and PWI. His MRI showed subcortical infarcts and hypoperfusion in the watershed cortical areas in the left inferior temporal cortex between the middle cerebral artery (MCA) territory and the posterior cerebral artery (PCA) territory (including part of Brodmann area, BA, 37), and in the frontal cortex, between the MCA territory and the anterior cerebral artery (ACA) territory (, Panel A). In an effort to restore blood flow to these areas, he received an investigational therapy, temporary blood pressure elevation. This intervention is based on evidence from animal studies that in ischaemic (hypoperfused) tissue, there is a loss of autoregulation, so that increasing systemic mean arterial blood pressure results in a linear increase in blood flow in that area (Astrup, Branston, & Lassen, Citation1977; Hillis, Kane, et al., 2001; Hillis et al., Citation2003; Hillis, Wityk, et al., 2001). The intervention was associated with improved blood flow in the previously hypoperfused tissue (, Panel B), and Participant 1 showed a concomitant increase in naming of both pictures and objects presented for tactile exploration (, Panel C). Picture naming improved from 0/17 to 15/17 correct between Day 1 and Day 5 (Fisher's exact, FE: p = .00000015); tactile naming improved from 1/17 to 17/17 (FE: p = .00000002).
Figure 2. (A) Participant 1's DWI (left) and PWI (right) before intervention to restore blood flow shows hypoperfusion of left frontal and inferior temporal cortex, and acute infarcts in subcortical watershed areas. TTP = time-to-peak. (B) DWI and PWI post intervention shows reperfusion of left frontal and inferior temporal cortex (and unchanged acute subcortical infarcts). (C) Language testing shows improvement in oral picture naming and oral naming of objects from tactile input after reperfusion of left frontal and inferior temporal cortex. [To view this figure in colour, please see the online version of this journal.]
![Figure 2. (A) Participant 1's DWI (left) and PWI (right) before intervention to restore blood flow shows hypoperfusion of left frontal and inferior temporal cortex, and acute infarcts in subcortical watershed areas. TTP = time-to-peak. (B) DWI and PWI post intervention shows reperfusion of left frontal and inferior temporal cortex (and unchanged acute subcortical infarcts). (C) Language testing shows improvement in oral picture naming and oral naming of objects from tactile input after reperfusion of left frontal and inferior temporal cortex. [To view this figure in colour, please see the online version of this journal.]](/cms/asset/ef8bfb3b-2065-45f6-ab80-68e24d0d7e6f/pcgn_a_875467_f0002_c.jpg)
Further evidence that the intervention (and restored blood flow) was responsible for his improvement in naming was indicated by the fact that there was a strong correlation between naming and mean arterial pressure (r = .82, p = .04; see ).
Figure 3. Temporal correlation between mean arterial pressure and oral picture naming accuracy for Participant 1. The two solid lines at the top of represent times when Participant 1 was receiving intervention to increase blood pressure.
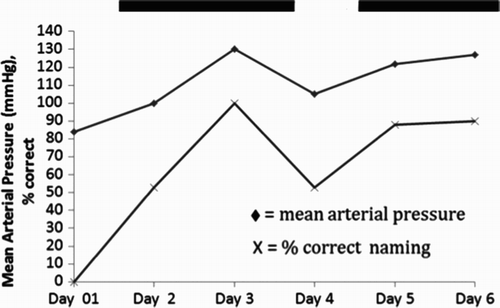
Repeat language testing immediately post intervention and at long-term follow-up confirmed that Participant 1 showed lasting improvement in language ().
Discussion of Case 1
Participant 1 showed recovery from aphasia, most likely as a result of restored blood flow to BA 45 in inferior frontal cortex, and BA 37 in left inferior temporal cortex. Although he showed improvement on a number of language functions, his most impressive recovery was in oral naming. BA 45 (pars triangularis of “Broca's area”) has been associated with impairments in naming (DeLeon et al., Citation2007; Newhart, Ken, Kleinman, Heidler-Gary, & Hillis, Citation2007). The lateral inferior temporal cortex portion of BA 37 is also an area that has been found to be critical for naming in studies by our lab (DeLeon et al., Citation2007; Hillis, Citation2002; Hillis et al., Citation2006; Raymer et al., Citation1997; Reineck, Agarwal, & Hillis, Citation2005), and by others (Raymer et al., Citation1997). Although Participant 1 also had subcortical infarcts that theoretically could have contributed to his language deficits, these lesions were still present when he recovered completely after reperfusion of the cortex. If his language deficits were due to the subcortical lesions (e.g., structural disconnection), he would not have recovered immediately after reperfusion of the cortex. Therefore, it is plausible that restoring blood flow to these two areas resulted in recovery of naming in the first week of stroke in Participant 1.
Participant 2: Functional right-hemisphere homologous “substitution” acutely due to diaschisis, followed by recovery from diaschisis
Participant 2 is a 53-year-old right-handed male painter, studying to be a nurse, who reported experiencing heaviness and numbness on the right side of his body when he woke up. He stated that he could not move his right leg and had to use his arm to lift his right leg off the bed. He was admitted to the hospital through the emergency department, where an initial clinical examination determined that he was alert and oriented in all spheres. His speech was predominantly fluent and grammatical with no paraphasias, but occasional pauses for word retrieval. Performance on language testing was normal except on word fluency (see ). Cranial nerves II–XII were determined to be intact. A motor exam revealed normal bulk, tone, and strength, but he had a right pronator drift. He had no sensory deficits at that time. Stance and gait were normal. The clinical MR imaging, including DWI and PWI, acquired the day of admission showed a subcentimetre area of diffusion restriction in the left thalamus, consistent with ischaemia. (, Panels A and B) However, there was minimal structural damage to white matter tracts from the thalamus to cortex, as we discuss later, in comparison with another patient. There was no significant hypoperfusion of the cortex indicated by dynamic contrast PWI. Participant 2 was enrolled into our longitudinal fMRI study and received periodic fMRI examinations during the recovery period.
Figure 4. DWI (Panel A) and apparent diffusion coefficient (ADC; Panel B) sequences show a subcentimetre area of diffusion restriction in the left thalamus for Participant 2. Functional magnetic resonance imaging (fMRI) data registered in Montreal Neurological Institute (MNI) space shows areas of activation associated with silent word generation at Day 3 (Panel C) and 8 weeks (Panel D). fMRI activation maps are false discovery rate (FDR) corrected for multiple comparisons and are displayed with a threshold of p < .05. A difference statistical map (Panel E) shows activation that is greater at 8 weeks than 3 weeks in yellow, and activation that is greater at 3 weeks than 8 weeks in blue. This last map shows change in statistical maps as a function of time. [To view this figure in colour, please see the online version of this journal.]
![Figure 4. DWI (Panel A) and apparent diffusion coefficient (ADC; Panel B) sequences show a subcentimetre area of diffusion restriction in the left thalamus for Participant 2. Functional magnetic resonance imaging (fMRI) data registered in Montreal Neurological Institute (MNI) space shows areas of activation associated with silent word generation at Day 3 (Panel C) and 8 weeks (Panel D). fMRI activation maps are false discovery rate (FDR) corrected for multiple comparisons and are displayed with a threshold of p < .05. A difference statistical map (Panel E) shows activation that is greater at 8 weeks than 3 weeks in yellow, and activation that is greater at 3 weeks than 8 weeks in blue. This last map shows change in statistical maps as a function of time. [To view this figure in colour, please see the online version of this journal.]](/cms/asset/cd5f9c1b-b984-4407-8e4d-afcc617208e0/pcgn_a_875467_f0004_c.jpg)
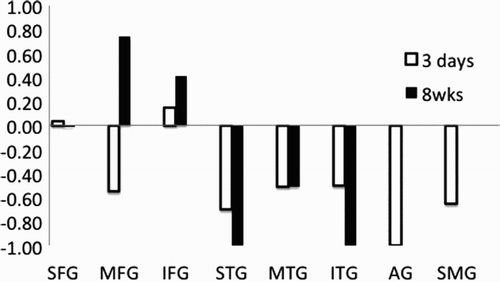
The oral word generation (word fluency) paradigm was used in fMRI imaging studies at Day 3 and Week 8. At Day 3, Participant 2 showed almost no left cortical activation in the language areas but showed activation in right frontal and temporal cortex and right supramarginal gyrus (areas homologous to regions that show activation in normal controls on this task (Prabhakaran et al., Citation2007), as well as bilateral subcortical activation (, Panel C). At Week 8, when his word generation had improved to normal level of fluency (), he showed significant activation in left frontal cortex as well as bilateral subcortical and cingulate gyri (, Panel D). The difference between the scans (Week 8 minus Day 3; , Panel E) shows increased activation (in yellow) in Broca's area and decreased activation (in blue) in several right-hemisphere areas and bilateral cerebellum. For word generation, his lateralization index shifted from strongly right lateralized to strongly left lateralized (from −.552 to .737) between Day 3 and Week 8 in middle frontal gyrus, and it shifted from slightly left lateralized to strongly left lateralized (from .147 to .412) in inferior frontal gyrus ().
Figure 5. Lateralization index for word generation in eight regions of interest (ROIs) at 3 days compared to 8 weeks in Participant 2. ROIs are: superior frontal gyrus (SFG), medial frontal gyrus (MFG), inferior frontal gyrus (IFG), superior temporal gyrus (STG), middle temporal gyrus (MTG), inferior temporal gyrus (ITG), supramarginal gyrus (SMG), and angular gyrus (AG).
Discussion of Case 2
In a previously published fMRI study of this same word generation task, we found that 20 healthy control participants showed activation in the following areas associated with the task: left posterior middle frontal gyrus, left posterior inferior frontal gyrus, left supramarginal gyrus, and left posterior inferior temporal gyrus, as well as right postcentral gyrus and right middle temporal lobe (Prabhakaran et al., Citation2007). In contrast, Participant 2 showed a near absence of significant activation in left language cortex ROI associated with word generation at Day 3. As Participant 2 showed no hypoperfusion of these areas, or structural disconnection to these areas, the absence of activation might be best explained by the phenomenon of diaschisis—dysfunction of one area (in this case, language cortex) caused by a remote lesion (in the thalamus in this case). Increased activation in left frontal ROI within language cortex at 8 weeks, confirmed by a substantial increase in the lateralization index in left middle and inferior frontal gyri, can be explained by recovery from diaschisis. This recovery from diaschisis may be responsible for his improvement in letter word fluency (word generation) from 5 to 17 words per minute.
Participant 3: Functional right-hemisphere homologous “substitution” acutely due to disconnection, followed by recovery from right-hemisphere substitution
Participant 3 is a 59-year-old right-handed male cable inspector with a history of long-standing hypertension who was speaking normally at 11 pm the night before admission, but awoke at 6 am with a right-sided facial weakness and difficulty speaking. He was evaluated in the emergency department the same day with perfusion and diffusion MRI, neurological examination, and language testing. His neurological examination revealed that he had fluent, grammatical language production, with occasional hesitations for word retrieval and mild upper motor neuron dysarthria. He was fully oriented, with normal short- and long-term memory. Language testing is shown in . He had minimal deficits in naming and comprehension. Cranial nerve examination was normal except for right upper motor neuron facial weakness. Motor examination showed normal mass, tone, and strength, with no ataxia. He had no sensory deficits. Stance and gait were normal.
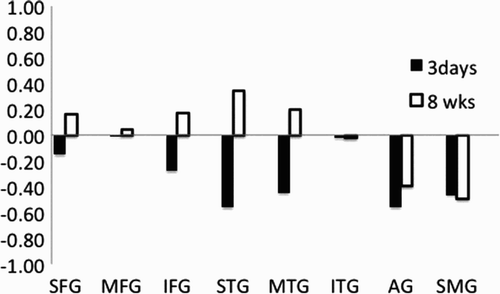
Clinical MRI at Day 1 confirmed an acute left posterior corona radiata stroke, with no cortical hypoperfusion (, Panel A). Research MRI, including fMRI study with a picture naming task, was acquired both Day 3 and Week 8. The fMRI study at Day 3 showed a right-dominant pattern of activation, with activation of the right-hemisphere homologue of Broca's area (as well as bilateral inferior temporal and occipital cortex), despite nearly normal language performance (, Panel B). fMRI acquired at Week 8 showed predominantly left Broca's area activation, as well as bilateral subcortical activation, associated with picture naming (, Panel C). The difference between the scans (Week 8 minus Day 3; , Panel D) shows increased activation (in yellow) in Broca's area and decreased activation (in blue) in the right homologue of Broca's area, right inferior temporal cortex, and bilateral fusiform. Lateralization index (LI) shifted from strongly right lateralized to left lateralized in superior and inferior frontal gyri (including Broca's area) and superior and middle temporal gyri, although the minimal activation in supramarginal gyrus and angular gyrus remained right lateralized (). For example, LI shifted from −.561 to .341 in superior temporal gyrus.
Figure 6. Panel A: DWI and PWI at Day 1 for Participant 3. Panel B: Activation associated with picture naming for Participant 3 at Day 3. Panel C: Activation associated with picture naming at 8 weeks. Functional magnetic resonance imaging (fMRI) activation maps are false discovery rate (FDR) corrected for multiple comparisons and are displayed with a threshold of p < .05. Panel D: A difference statistical map shows activation that is greater at 8 weeks than 3 weeks in yellow, and activation that is greater at 3 weeks than 8 weeks in blue. [To view this figure in colour, please see the online version of this journal.]
![Figure 6. Panel A: DWI and PWI at Day 1 for Participant 3. Panel B: Activation associated with picture naming for Participant 3 at Day 3. Panel C: Activation associated with picture naming at 8 weeks. Functional magnetic resonance imaging (fMRI) activation maps are false discovery rate (FDR) corrected for multiple comparisons and are displayed with a threshold of p < .05. Panel D: A difference statistical map shows activation that is greater at 8 weeks than 3 weeks in yellow, and activation that is greater at 3 weeks than 8 weeks in blue. [To view this figure in colour, please see the online version of this journal.]](/cms/asset/6871d33f-7d1e-438e-af29-e06003f1af44/pcgn_a_875467_f0006_c.jpg)
Figure 7. Lateralization index for picture naming in eight regions of interest (ROIs) at 3 days compared to 8 weeks in Participant 3. ROIs are: superior frontal gyrus (SFG), medial frontal gyrus (MFG), inferior frontal gyrus (IFG), superior temporal gyrus (STG), middle temporal gyrus (MTG), inferior temporal gyrus (ITG), supramarginal gyrus (SMG), and angular gyrus (AG).
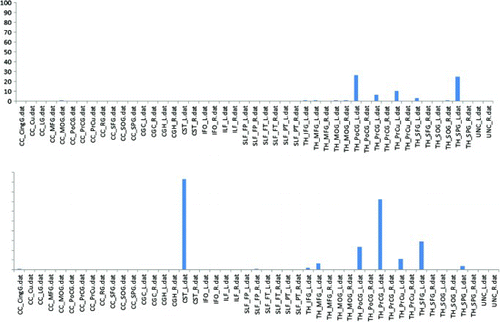
Structural MRI sequences acquired at the same time as the fMRI were used to determine which white matter tracts were disrupted in Participant 3. This analysis revealed two fibre tracts with greater than 30% disruption. The lesion disrupted 90% of the left thalamic–corticospinal (CST) white matter tract, and 72% of the left thalamic–precentral gyrus white matter tract. This structural “disconnection” between the thalamus and posterior frontal cortex (shown in , lower panel) may explain the reduced activation of the left posterior frontal cortical regions during tasks that normally engage these areas. In contrast, there was minimal structural disconnection (damage to these white matter tracts) in Participant 2, who had a lesion in the thalamus, as shown in , top panel.
Figure 8. Probabilistic fibre tract lesion disruption analysis. The bar graph illustrates the percentage of disruption to each white matter tract for Participant 2 (top panel) and Participant 3 (lower panel). CST_L, cortico spinal cord (left); PrCG_L = precentral gyrus (left).
shows a global analysis of predicted cortical function disruption, based on the degree of white matter tract disruption to the cortical region. In this analysis, the percentage of fibre tract disruption is normalized to the cortical region with the highest level of disruption. The results of this analysis are reported as colour-coded volumes, representing the percentage disruption. For Participant 3, the greatest disruption was in the white matter connections to the left frontal cortex, particularly the precentral gyrus. There was essentially no disruption of the white matter tracts to the inferior temporal cortex (purple), another area critical for naming. Based on this analysis, we would expect a reduction in the normal activation of left frontal cortex during naming. This reduction in normal left frontal activation associated with naming was seen at Day 3 (and was associated with a concomitant increase in activation of the right frontal cortex at Day 3). In contrast, there was normal activation of bilateral inferior temporal cortex at Day 3 (, Panel B) and Week 8 (, Panel C). The lateralization index was −.021 at both time points, consistent with nearly equal activation of both hemispheres in this area.
Figure 9. Global analysis of cortical function disruption in Participant 3. The degree of white matter tract disruption in each cortical region is determined from the intersection of the lesion region of interest (ROI) and a probabilistic fibre tract atlas. The results are presented as a percentage of total white matter tract disruption, normalized to the region of highest level of disruption, in Participant 3. [To view this figure in colour, please see the online version of this journal.]
![Figure 9. Global analysis of cortical function disruption in Participant 3. The degree of white matter tract disruption in each cortical region is determined from the intersection of the lesion region of interest (ROI) and a probabilistic fibre tract atlas. The results are presented as a percentage of total white matter tract disruption, normalized to the region of highest level of disruption, in Participant 3. [To view this figure in colour, please see the online version of this journal.]](/cms/asset/33e1119b-a01e-44e2-a815-d03288576087/pcgn_a_875467_f0009_c.jpg)
Discussion of Case 3
Participant 3 showed a reduction in the normal activation of the left frontal components of the language network (including Broca's area) and some associated activation of the right-hemisphere homologue, in the acute stage of stroke, along with bilateral inferior temporal cortex activation. His right frontal activation may have been an acute “right-hemisphere substitution”, which was compensatory, albeit not as proficient as the normal left-hemisphere network. Alternatively, the right-hemisphere activation may have been the normal degree of activation, or a release from inhibition by Broca's area (which did not show functional activation). This “reorganization” had the sequence described by Saur and colleagues (Citation2006), but a different time course. The right-hemisphere activation was seen acutely, and the shift back to the left cortex was seen subacutely. More importantly, the acute right-hemisphere activation was associated with nearly normal language performance in this case. As there was no lesion in Broca's area or other “language cortex”, there may have been subthreshold activation of Broca's area associated with naming, but there was greater (and significant) right-hemisphere activation associated with language. It is conceivable that this right-hemisphere activation was responsible for his rare errors, rather than his good performance.
In contrast to Participant 2, the reduced left frontal activation acutely might have been explained by a structural disconnection (disruption of the thalamocortical tracts, including thalamus-precentral gyrus and thalamus-superior frontal gyrus, and a small percentage disruption of thalamus-inferior frontal gyrus). These tracts remained disrupted at 8 weeks. The normal left frontal area activation at 8 weeks (and shift to left lateralization in superior frontal gyrus and inferior frontal gyrus) may reflect recovery from structural disconnection, through either functional reconnection (other input from the thalamus to left frontal regions), or new structural connections through new dendritic connections. However, as there was only a small percentage disruption in white matter tracts to left Broca's area itself, we cannot be sure that a structural disconnection was responsible for the reduced activation in this area acutely (which recovered by 8 weeks). Recovery from diaschisis may also have contributed or could have been the primary mechanism of recovery of activation in left Broca's area. That is, left thalamus-to-precentral gyrus, and left thalamus-to-superior frontal gyrus tracts can only explain reduced activation of left precentral gyrus and superior frontal gyrus acutely (which increased at Week 8); the far less substantial disruption of thalamus-to-inferior frontal gyrus tracts may not account for the reduced activation of left inferior frontal gyrus (including Broca's area) acutely (which also increased at Week 8).
Participant 4 and Participant 5: The influence of individual variables that affect level of performance and “reorganization” of the language network
Participant 4 was a 57-year-old right-handed female lawyer with history of multiple myeloma and an ovarian mass who experienced word-finding difficulty two days before coming to the hospital. On the day of admission, she started having trouble formulating sentences and following complex directions, so her husband brought her to the hospital. She was found to be alert and oriented, with no trouble understanding conversations, but trouble understanding syntactically complex sentences. She made some semantic paraphasias in naming, but frequently attempted self-correction (e.g., cup → “tablespoon … mug”; hammer → “screw, no”). Her spontaneous speech was hesitant, with many pauses for word finding. She produced mostly short, simple, but grammatical sentences (e.g., “She is pouring juice. He is reading stuff … .He is flying a kite … .She is … .I guess … .I can't get it.”). She had a very mild apraxia of speech. She could repeat and read aloud words and sentences relatively well. Performance on language tests is reported in . She had mild ideomotor apraxia of the right hand. Cranial nerve examination showed no deficits. Motor examination was notable only for the mild apraxia. She had no sensory deficits. Stance and gait were normal. She had no ataxia.
Within the lexical system, Participant 4 had a relatively selective deficit in accessing oral and written lexical representations for output, or impaired linking of semantic representations to lexical representations through modality-independent lemma level representations. She also had a mild apraxia of speech and mild difficulty understanding and producing syntactically complex sentences.
Participant 5 was a 39-year-old right-handed female nurse with a history of sickle cell disease who drove herself to a community hospital after experiencing inability to speak appropriately. She was transferred to our hospital for further care. On admission, she was noted to be alert and oriented in all spheres. She was reported to have intact comprehension for conversation and directions. Her speech was halting and effortful, with numerous and varied off-target attempts to correctly articulate words, with particular trouble with polysyllabic words (e.g., eraser → “raser … duraser”). She had telegraphic speech, consisting mostly of single words separated by prolonged pauses. Naming errors included semantic paraphasias with attempts to self-correct (e.g., toothbrush → “brush … teeth … for teeth”; pencil → “book”). Memory could not be tested well because of her aphasia. She had no visual field deficits. Cranial nerve evaluation was notable for right upper motor neuron facial weakness. She had full strength throughout, except for right hip flexion, which was mildly weak. Her stance and gait were preserved, nevertheless. She had no sensory deficits. Cerebellar testing was normal.
Participant 5's language performance is reported in . She had multiple deficits, including: (a) apraxia of speech; (b) impairment in grammatical sentence production (but with relatively intact repetition of sentences, despite distorted phonemes, disrupted prosody, and self-corrected articulatory errors); (c) impairment in comprehension of syntactically complex sentences (such as following complex commands); and (d) a mild lexical–semantic impairment with occasional semantic errors in naming and word comprehension (when there was no context). She also had a superimposed spelling deficit that we did not fully characterize due to limited testing in the acute period. Her spelling to dictation was only 80% correct; she made deletions, substitutions, and transpositions of letters. In contrast, her oral reading was 100% correct other than articulatory distortions, although it was slow and effortful for items used in the fMRI task.
Structural MRI, including DWI and PWI, in the acute period of stroke showed very similar areas of acute infarct, involving the posterior inferior frontal cortex (approximately Broca's area) for both patients (, Panels A and B). For Participant 5, infarct volume (ADC < 600) was 28.75 cc; volume of hypoperfusion (TTP > 4-s delay relative to right hemisphere) was smaller than the infarct (0.63 cc), indicating no areas of hypoperfusion beyond the infarct. For Participant 4, infarct volume (apparent diffusion coefficient, ADC < 600) was almost identical at 27.64 cc; her volume of hypoperfusion was also smaller than the infarct (5.47 cc), again indicating no areas of hypoperfusion beyond the infarct.
Figure 10. Panel A: DWI (top) and PWI (bottom) at Day 1 for Participant 4. Panel B: DWI (top) and PWI (bottom) at Day 1 for Participant 5. Panel C: Functional magnetic resonance imaging (fMRI) activation associated with the orthographic task (retrieval of spelling) for Participant 4. Panel D: fMRI activation associated with the orthographic task (retrieval of spelling) for Participant 5. Panel E: Activation for Participant 4 minus activation for Participant 5, associated with the orthographic task. fMRI activation maps are false discovery rate (FDR) corrected for multiple comparisons and are displayed with a threshold of p < .05. Volumes with greater than 2 mm movement were censored out. [To view this figure in colour, please see the online version of this journal.]
![Figure 10. Panel A: DWI (top) and PWI (bottom) at Day 1 for Participant 4. Panel B: DWI (top) and PWI (bottom) at Day 1 for Participant 5. Panel C: Functional magnetic resonance imaging (fMRI) activation associated with the orthographic task (retrieval of spelling) for Participant 4. Panel D: fMRI activation associated with the orthographic task (retrieval of spelling) for Participant 5. Panel E: Activation for Participant 4 minus activation for Participant 5, associated with the orthographic task. fMRI activation maps are false discovery rate (FDR) corrected for multiple comparisons and are displayed with a threshold of p < .05. Volumes with greater than 2 mm movement were censored out. [To view this figure in colour, please see the online version of this journal.]](/cms/asset/a821d3d7-0c6e-47e6-a7f8-29dbfae0fffc/pcgn_a_875467_f0010_c.jpg)
In the fMRI study of the orthographic task, three days after their strokes, Participant 4 performed with 100% accuracy, while Participant 5 performed with 80% accuracy outside of the scanner. They showed very different patterns of activation on fMRI. Participant 4 showed activation of perilesional areas around Broca's area, in addition to left fusiform gyrus, posterior superior temporal cortex, supramarginal gyrus, and angular gyrus. She also showed activation in some homologous right-hemisphere areas to a lesser degree (, Panel C), as do normal controls during a comparable orthographic task (Purcell, Turkeltaub, et al., 2011). The activation pattern of Participant 4 during this orthographic task was similar to that of normal controls during similar tasks that require access to the orthographic representation for spelling. That is, a meta-analysis of 11 fMRI studies of spelling identified left inferior frontal gyrus (including Broca's area), left inferior temporal gyrus/fusiform gyrus, left superior temporal gyrus/sulcus, and left supramarginal gyrus as the anatomical regions where activation was most consistently associated with “central processes” of spelling (activation of an orthographic representation) relative to “peripheral processes” such as letter formation (Purcell, Turkeltaub, et al., 2011). In contrast, Participant 5 showed minimal activation in the orthographic task (). In inferior frontal gyrus (IFG), Participant 4 had a lateralization index (LI) of .473 (strongly left lateralized), while Participant 5 did not have any significant activation in IFG or middle frontal gyrus.
Table 3. Number of voxels with activation in nine regions of interest in each hemisphere
In contrast, in an fMRI study of reading, on which Participant 5 performed with 100% accuracy, she showed a relatively normal pattern of activation of “language cortex”, including activation of perilesional areas around Broca's area, in addition to left fusiform gyrus and bilateral occipital cortex. That is, on this task, Participant 5 showed areas of activation seen in normal controls during similar reading tasks (Cohen et al., Citation2008; Turkeltaub et al., Citation2002; Vinckier et al., Citationin press), including bilateral IFG, superior frontal gyrus (SFG), superior temporal gyrus (STG), middle temporal gyrus (MTG), and fusiform gyrus, as shown in and . Participant 4 showed little activation in the reading task, possibly because it was too easy for her or she was not actually engaged in the task. We know that Participant 4 was able to activate these areas (because during the orthographic task requiring access to the spelling of the word, she activated many of the areas activated by Participant 5 and some normal controls during reading).
Figure 11. Activation associated with reading for Participant 5. Functional magnetic resonance imaging (fMRI) data are adjusted for multiple corrections and are presented with threshold of p < .05. [To view this figure in colour, please see the online version of this journal.]
![Figure 11. Activation associated with reading for Participant 5. Functional magnetic resonance imaging (fMRI) data are adjusted for multiple corrections and are presented with threshold of p < .05. [To view this figure in colour, please see the online version of this journal.]](/cms/asset/21c8ae39-d3cd-49c6-9bc9-8118bca4da23/pcgn_a_875467_f0011_c.jpg)
Discussion of Cases 4 and 5
The contrast between Participant 4 and Participant 5 shows that even in individuals with the nearly the same size and site of stroke, at the same time post onset of stroke, the same task (e.g., the orthographic task) may engage different areas of the brain. Participant 4 was able to recruit perilesional left-hemisphere “language network” (posterior inferior frontal, superior temporal, fusiform cortex, angular and supramarginal gyri) during the orthographic task requiring retrieval of the spelling of the word, which she performed with 100% accuracy. In contrast, Participant 5 showed no activation in left-hemisphere perilesional areas and minimal activation in the “language network” during the orthographic (spelling) task, which she performed less accurately, but did show activation of perilesional “language network” regions during the reading task, which she performed with 100% accuracy. These two cases illustrate that the areas that can assume the function of the damaged area (structure–function reorganization in response to a lesion) vary across language tasks and vary across individuals engaged in the same language task, even if they have very similar lesions. It is possible that individual variables that influence the task difficulty and accuracy for the patient (e.g., age or level of education or aptitude) might influence the area(s) of the brain responsible for assuming the function of the damaged part. Differences in the location or amount of affected white and grey matter might also result in differences in activation patterns across individuals, but were less likely to account for substantial differences between these cases, as their lesion volume and location were very similar.
LIMITATIONS
There are many limitations of our study, including some weaknesses in the fMRI paradigms that do not allow us to distinguish correct from incorrect responses in the scanner, differences in the exact timing of fMRI studies across patients, and limited analyses of the fMRI data. For example, we did not have adequate data to model each individual's hemodynamic response, which is often useful in patients with cerebrovascular disease (Bonakdarpour, Parrish, & Thompson, Citation2007; Fridriksson, Rorden, Morgan, Morrow, & Baylis, Citation2006). We also have relatively little longitudinal behavioural data for the participants, given the constraints of testing individuals at the acute stage and some attrition. Participant 4 died within 2 weeks of her stroke (from cancer), and Participant 5 moved out of the country. However, our purpose here was not to provide the definitive study on the mechanisms of aphasia recovery. Rather, we hoped to test some very specific hypotheses: that language recovery takes place through distinct mechanisms with different time courses; and that “reorganization” of structure–function relationships depends on the language task and level of performance, as well as the size and site of stroke and time post onset. We attempted to provide enough data to illustrate these points.
SUMMARY AND CONCLUSIONS
This series of brief case studies has illustrated distinct mechanisms of recovery during the first year after stroke. All mechanisms are most effective if the normal left-hemisphere “language network” can be restored. Sometimes the left-hemisphere language network can be restored, if a component of it was hypoperfused (and the area can be reperfused, as in the case of Participant 1). Sometimes the left-hemisphere language network can be restored if a component was dysfunctional due to diaschisis (caused by a remote lesion functionally connected to the component), and there is recovery from diaschisis, as in the case of Participant 2. Recovery from diaschisis may occur through reestablishment of a balance in inhibitory and excitatory interactions between subcortical and cortical structures or between hemispheres. The left-hemisphere language network can also be restored after structural disconnection between critical components of the language network, as in cases like Participant 3. It is unlikely that there was development of new white matter tracts, because such tracts would take longer than 2 months to develop, if in fact they do develop after injury to the brain. However, it is plausible that new direct or indirect synaptic connections between thalamus and frontal cortex (not visible on structural imaging) facilitated recovery of the normal activation of left frontal cortex at 2 months post stroke in Participant 3.
We also showed that individuals with similar lesions in language network, in terms of volume and location, might show different levels of performance acutely and different patterns of structure–function reorganization. That is, different areas of the brain were engaged in the orthographic task that required retrieval of spelling. In the cases of Participant 4 and Participant 5 it was not possible to recover the normal language network because there was infarct in Broca's area. However, they each showed perilesional activation in the language network in at least one task they could perform accurately. However, the study of Participant 3 shows that accurate performance of a language task is not always associated with a normal left-dominant pattern of activation, as Participant 3's naming performance, outside the scanner, was accurate, even when he showed a right-dominant pattern of activation.
Thus, we confirmed our hypotheses. First, there are distinct mechanisms of recovery of language after acute focal lesions, which may have different time courses. Secondly, which areas “take over” for damaged components of the language network depends on the language task and individual factors that affect level of performance of the task, in addition to size and site of stroke and time from onset.
We gratefully acknowledge these mechanisms of support and the participation of the participants.
This publication was made possible by National Institutes of Health (NIH) [grant number R01 DC 05375], [grant number R01 DC 03681], [grant number P50 DC012283] from National Institute on Deafness and Other Communication Disorders (NIDCD) to A.H. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of this institute. Additional financial support was received from the Philips Corporation as a part of the “Multimodal, Functional-MRI Study of Recovery Following Stroke” exhibit.
REFERENCES
- Astrup, J. S. L., Branston, N. M., & Lassen, N. A. (1977). Cortical evoked potential and extracellular K+ and H+ at critical levels of brain ischemia. Stroke, 8, 51–57. doi: 10.1161/01.STR.8.1.51
- Barwood, C. H., Murdoch, B. E., Whelan, B. M., Lloyd, D., Riek, S., O'Sullivan, J. D.,…Wong, A. (2011). Improved language performance subsequent to low-frequency rTMS in patients with chronic non-fluent aphasia post-stroke. European Journal of Neurology, 18, 935–943. doi: 10.1111/j.1468-1331.2010.03284.x
- Beeson, P. M., Rapcsak, S. Z., Plante, E., Chargualaf, J., Chung, A., Johnson, S. C., & Trouard, T. P. (2003). The neural substrates of writing: A functional magnetic resonance imaging study. Aphasiology, 17, 647–665. doi: 10.1080/02687030344000067
- Berthier, M. L., Garcia-Casares, N., Walsh, S. F., Nabrozidis, A., Ruiz de Mier, R. J., Green, C.,…Pulvermuller, F. (2011). Recovery from post-stroke aphasia: Lessons from brain imaging and implications for rehabilitation and biological treatments. Discovery Medicine, 12, 275–289.
- Bonakdarpour, B., Parrish, T. B., & Thompson, C. K. (2007). Hemodynamic response function in patients with stroke-induced aphasia: Implications for fMRI data analysis. NeuroImage, 36, 322–331. doi: 10.1016/j.neuroimage.2007.02.035
- Cappa, S. F. (2011). The neural basis of aphasia rehabilitation: Evidence from neuroimaging and neurostimulation. Neuropsychological Rehabilitation, 21, 742–754. doi: 10.1080/09602011.2011.614724
- Cappa, S. F., Perani, D., Grassi, F., Bressi, S., Alberoni, M., Franceschi, M.,…Fazio, F. (1997). A PET follow-up study of recovery after stroke in acute aphasics. Brain and Language, 56, 55–67. doi: 10.1006/brln.1997.1737
- Cohen, L., Dehaene, S., Vinckier, F., Jobert, A., & Montavont, A. (2008). Reading normal and degraded words: Contribution of the dorsal and ventral visual pathways. NeuroImage, 40, 353–366. doi: 10.1016/j.neuroimage.2007.11.036
- Cox, R. W. (1996). AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, 29, 162–173. doi: 10.1006/cbmr.1996.0014
- Crinion, J., & Price, C. J. (2005). Right anterior superior temporal activation predicts auditory sentence comprehension following aphasic stroke. Brain, 128, 2858–2871. doi: 10.1093/brain/awh659
- Dabul, B. (2000). Apraxia battery for adults-2. Austin: Pro-Ed.
- DeLeon, J., Gottesman, R. F., Kleinman, J. T., Newhart, M., Davis, C., Heidler-Gary, J.,…Hillis, A. E. (2007). Neural regions essential for distinct cognitive processes underlying picture naming. Brain, 130, 1408–1422. doi: 10.1093/brain/awm011
- Fridriksson, J. (2010). Preservation and modulation of specific left hemisphere regions is vital for treated recovery from anomia in stroke. Journal of Neuroscience, 30, 11558–11564. doi: 10.1523/JNEUROSCI.2227-10.2010
- Fridriksson, J., Moser, D., Bonilha, L., Morrow-Odom, K. L., Shaw, H., Fridriksson, A.,…Rorden, C. (2007). Neural correlates of phonological and semantic-based anomia treatment in aphasia. Neuropsychologia, 45, 1812–1822. doi: 10.1016/j.neuropsychologia.2006.12.017
- Fridriksson, J., Rorden, C., Morgan, P. S., Morrow, K. L., & Baylis, G. C. (2006). Measuring the hemodynamic response in chronic hypoperfusion. Neurocase, 12, 146–150. doi: 10.1080/13554790600598816
- Hamilton, R. H., Sanders, L., Benson, J., Faseyitan, O., Norise, C., Naeser, M.,…Coslett, H. B. (2010). Stimulating conversation: Enhancement of elicited propositional speech in a patient with chronic non-fluent aphasia following transcranial magnetic stimulation. Brain and Language, 113, 45–50. doi: 10.1016/j.bandl.2010.01.001
- Heiss, W. D., Karbe, H., WeberLuxenburger, G., Herholz, K., Kessler, J., Pietrzyk, U., & Pawlik, G. (1997). Speech-induced cerebral metabolic activation reflects recovery from aphasia. Journal of the Neurological Sciences, 145, 213–217. doi: 10.1016/S0022-510X(96)00252-3
- Heiss, W. D., Kessler, J., Thiel, A., Ghaemi, M., & Karbe, H. (1999). Differential capacity of left and right hemispheric areas for compensation of poststroke aphasia. Annal of Neurology, 45, 430–438. doi: 10.1002/1531-8249(199904)45:4<430::AID-ANA3>3.0.CO;2-P
- Hillis, A. E. (2002). Does the right make it right? Questions about recovery of language after stroke. Annals of Neurology, 51, 537–538. doi: 10.1002/ana.10211
- Hillis, A. E., Kane, A., Tuffiash, E., Ulatowski, J. A., Barker, P. B., Beauchamp, N. J., & Wityk, R. J. (2001). Reperfusion of specific brain regions by raising blood pressure restores selective language functions in subacute stroke. Brain and Language, 79, 495–510. doi: 10.1006/brln.2001.2563
- Hillis, A. E., Kleinman, J. T., Newhart, M., Heidler-Gary, J., Gottesman, R., Barker, P. B.,…Chaudhry, P. (2006). Restoring cerebral blood flow reveals neural regions critical for naming. Journal of Neuroscience, 26, 8069–8073. doi: 10.1523/JNEUROSCI.2088-06.2006
- Hillis, A. E., Ulatowski, J. A., Barker, P. B., Torbey, M., Ziai, W., Beauchamp, N. J.,…Wityk, R. J. (2003). A pilot randomized trial of induced blood pressure elevation: Effects on function and focal perfusion in acute and subacute stroke. Cerebrovascular Disease, 16, 236–246. doi: 10.1159/000071122
- Hillis, A. E., Wityk, R. J., Tuffiash, E., Beauchamp, N. J., Jacobs, M. A., Barker, P. B., & Selnes, O. A. (2001). Hypoperfusion of Wernicke's area predicts severity of semantic deficit in acute stroke. Annals of Neurology, 50, 561–566. doi: 10.1002/ana.1265
- Karbe, H., Kessler, J., Herholz, K., Fink, G. R., & Heiss, W. D. (1995). Long-term prognosis of poststroke aphasia studied with positron emission tomography. Archive of Neurology, 52, 186–190. doi: 10.1001/archneur.1995.00540260092022
- Kertesz, A. (1982). The Western aphasia battery. Philadelphia: Grune & Stratton.
- Kertesz, A., & Munoz, D. G. (1997). Primary progressive aphasia. Clinical Neuroscience, 4, 95–102. doi: 10.1016/S0967-5868(97)90058-8
- Kiran, S. (2012). What is the nature of poststroke language recovery and reorganization? ISRN Neurol, 2012, 786–872. doi:10.5402/2012/786872. Epub 2012 Dec 23. doi: 10.5402/2012/786872
- Leff, A. P., Scott, S. K., Rothwell, J. C., & Wise, R. J. (2001). The planning and guiding of reading saccades: A repetitive transcranial magnetic stimulation study. Cerebral Cortex, 11, 918–923. doi: 10.1093/cercor/11.10.918
- Lin, D. D., Kleinman, J. T., Wityk, R. J., Gottesman, R. F., Hillis, A. E., Lee, A. W., & Barker, P. B. (2009). Crossed cerebellar diaschisis in acute stroke detected by dynamic susceptibility contrast MR perfusion imaging. AJNR American Journal of Neuroradiology, 30, 710–715. doi: 10.3174/ajnr.A1435
- Marcotte, K., Adrover-Roig, D., Damien, B., de Preaumont, M., Genereux, S., Hubert, M., & Ansaldo, A. I. (2012). Therapy-induced neuroplasticity in chronic aphasia. Neuropsychologia, 50, 1776–1786. doi: 10.1016/j.neuropsychologia.2012.04.001
- Martin, P. I., Naeser, M. A., Ho, M., Doron, K. W., Kurland, J., Kaplan, J.,…Pascual-Leone, A. (2009a). Overt naming fMRI pre- and post-TMS: Two nonfluent aphasia patients, with and without improved naming post-TMS. Brain and Language, 111, 20–35. doi: 10.1016/j.bandl.2009.07.007
- Martin, P. I., Naeser, M. A., Ho, M., Treglia, E., Kaplan, E., Baker, E. H., & Pascual-Leone, A. (2009b). Research with transcranial magnetic stimulation in the treatment of aphasia. Current Neurology and Neuroscience Report, 9, 451–458.
- Martin, P. I., Naeser, M. A., Theoret, H., Tormos, J. M., Nicholas, M., Kurland, J.,…Pascual-Leone, A. (2004). Transcranial magnetic stimulation as a complementary treatment for aphasia. Seminars in Speech and Language, 25, 181–191. doi: 10.1055/s-2004-837247
- Meinzer, M., Beeson, P. M., Cappa, S., Crinion, J., Kiran, S., Saur, D.,…Thompson, C. K. (2013). Neuroimaging in aphasia treatment research: Consensus and practical guidelines for data analysis. Neuroimage, 73, 215–224. doi: 10.1016/j.neuroimage.2012.02.058
- Mori, S., Oishi, K., Jiang, H., Jiang, L., Li, X., Akhter, K.,…Mazziotta, J. (2008). Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage, 40, 570–582. doi: 10.1016/j.neuroimage.2007.12.035
- Musso, M., Weiller, C., Kiebel, S., Muller, S. P., Bulau, P., & Rijntjes, M. (1999). Training-induced brain plasticity in aphasia. Brain, 122, 1781–1790. doi: 10.1093/brain/122.9.1781
- Mylius, V., Zouari, H. G., Ayache, S. S., Farhat, W. H., & Lefaucheur, J. P. (2012). Stroke rehabilitation using noninvasive cortical stimulation: Aphasia. Expert Review of Neurotherapeutics, 12, 973–982. doi: 10.1586/ern.12.76
- Naeser, M. A., Martin, P. I., Nicholas, M., Baker, E. H., Seekins, H., Helm-Estabrooks, N.,…Pascual-Leone, A. (2005). Improved naming after TMS treatments in a chronic, global aphasia patient--case report. Neurocase, 11, 182–193. doi: 10.1080/13554790590944663
- Newhart, M., Ken, L., Kleinman, J. T., Heidler-Gary, J., & Hillis, A. E. (2007). Neural networks essential for naming and word comprehension. Cognitive and Behavioral Neurology, 20, 25–30. doi: 10.1097/WNN.0b013e31802dc4a7
- Ohyama, M., Senda, M., Kitamura, S., Ishii, K., Mishina, M., & Terashi, A. (1996). Role of the nondominant hemisphere and undamaged area during word repetition in poststroke aphasics. A PET activation study. Stroke, 27, 897–903. doi: 10.1161/01.STR.27.5.897
- Olea Medical (2012). Olea Sphere (Version 2.2) [Computer software]. Cambridge, MA: Author.
- Olsen, T. S., Bruhn, P., & Oberg, R. G. (1986). Cortical hypoperfusion as a possible cause of ‘subcortical Aphasia’. Brain, 109, 393–410. doi: 10.1093/brain/109.3.393
- Parker Jones, O., Green, D. W., Grogan, A., Pliatsikas, C., Filippopolitis, K., Ali, N.,…Price, C. J. (2012). Where, when and why brain activation differs for bilinguals and monolinguals during picture naming and reading aloud. Cerebral Cortex, 22, 892–902. doi: 10.1093/cercor/bhr161
- Poline, J. B., Vandenberghe, R., Holmes, A. P., Friston, K. J., & Frackowiak, R. S. J. (1996). Reproducibility of PET activation studies: Lessons from a multi-center European experiment—EU concerted action on functional imaging. Neuroimage, 4, 34–54. doi: 10.1006/nimg.1996.0027
- Postman-Caucheteux, W. A., Birn, R. M., Pursley, R. H., Butman, J. A., Solomon, J. M., Picchioni, D.,…Braun, A. R. (2010). Single-trial fMRI shows contralesional activity linked to overt naming errors in chronic aphasic patients. Journal of Cognitive Neuroscience, 22, 1299–1318. doi: 10.1162/jocn.2009.21261
- Prabhakaran, V., Raman, S. P., Grunwald, M. R., Mahadevia, A., Hussain, N., Lu, H.,…Hillis, A. E. (2007). Neural substrates of word generation during stroke recovery: The influence of cortical hypoperfusion. Behavioural Neurology, 18, 45–52. doi: 10.1155/2007/430402
- Price, C. J., & Crinion, J. (2005). The latest on functional imaging studies of aphasic stroke. Current Opinion in Neurology, 18, 429–434. doi: 10.1097/01.wco.0000168081.76859.c1
- Purcell, J. J., Napoliello, E. M., & Eden, G. F. (2011). A combined fMRI study of typed spelling and reading. Neuroimage, 55, 750–762. doi: 10.1016/j.neuroimage.2010.11.042
- Purcell, J. J., Turkeltaub, P. E., Eden, G. F., & Rapp, B. (2011). Examining the central and peripheral processes of written word production through meta-analysis. Frontiers in Psychology, 2, 239. doi: 10.3389/fpsyg.2011.00239
- Rapp, B., & Lipka, K. (2011). The literate brain: The relationship between spelling and reading. Journal of Cognitive Neuroscience, 23, 1180–1197. doi: 10.1162/jocn.2010.21507
- Raymer, A. M., Foundas, A. L., Maher, L. M., Greenwald, M. L., Morris, M., Rothi, L. J. G., & Heilman, K. M. (1997). Cognitive neuropsychological analysis and neuroanatomic correlates in a case of acute anomia. Brain and Language, 58, 137–156. doi: 10.1006/brln.1997.1786
- Reineck, L. A., Agarwal, S., & Hillis, A. E. (2005). “Diffusion-clinical mismatch” is associated with potential for early recovery of aphasia. Neurology, 64, 828–833. doi: 10.1212/01.WNL.0000152983.52869.51
- Rochon, E., Leonard, C., Burianova, H., Laird, L., Soros, P., Graham, S., & Grady, C. (2010). Neural changes after phonological treatment for anomia: An fMRI study. Brain and Language, 114, 164–179. doi: 10.1016/j.bandl.2010.05.005
- Saur, D., Lange, R., Baumgaertner, A., Schraknepper, V., Willmes, K., Rijntjes, M., & Weiller, C. (2006). Dynamics of language reorganization after stroke. Brain, 129, 1371–1384. doi: 10.1093/brain/awl090
- Sebastian, R., & Kiran, S. (2011). Task-modulated neural activation patterns in chronic stroke patients with aphasia. Aphasiology, 25, 927–951. doi: 10.1080/02687038.2011.557436
- Segaert, K., Menenti, L., Weber, K., Petersson, K. M., & Hagoort, P. (2012). Shared syntax in language production and language comprehension--an FMRI study. Cerebral Cortex, 22, 1662–1670. doi: 10.1093/cercor/bhr249
- Szaflarski, J. P., Allendorfer, J. B., Banks, C., Vannest, J., & Holland, S. K. (2013). Recovered vs. not-recovered from post-stroke aphasia: The contributions from the dominant and non-dominant hemispheres. Restorative Neurology and Neuroscience, 31, 347--360.
- Szelies, B., Herholz, K., Pawlik, G., Karbe, H., Hebold, I., & Heiss, W. D. (1991). Widespread Functional-Effects of Discrete Thalamic Infarction. Archives of Neurology, 48, 178–182. doi: 10.1001/archneur.1991.00530140072019
- Thiel, A., Herholz, K., Koyuncu, A., Ghaemi, M., Kracht, L. W., Habedank, B., & Heiss, W. D. (2001). Plasticity of language networks in patients with brain tumors: A positron emission tomography activation study. Annals of Neurology, 50, 620–629. doi: 10.1002/ana.1253
- Thompson, C. K., den Ouden, D. B., Bonakdarpour, B., Garibaldi, K., & Parrish, T. B. (2010). Neural plasticity and treatment-induced recovery of sentence processing in agrammatism. Neuropsychologia, 48, 3211–3227. doi: 10.1016/j.neuropsychologia.2010.06.036
- Thompson, C. K., Riley, E. A., den Ouden, D. B., Meltzer-Asscher, A., & Lukic, S. (2013). Training verb argument structure production in agrammatic aphasia: Behavioral and neural recovery patterns. Cortex, 49, 2358–2376. doi: 10.1016/j.cortex.2013.02.003
- Thulborn, K. R., Carpenter, P. A., & Just, M. A. (1999). Plasticity of language-related brain function during recovery from stroke. Stroke, 30, 749–754. doi: 10.1161/01.STR.30.4.749
- Turkeltaub, P. E., Eden, G. F., Jones, K. M., & Zeffiro, T. A. (2002). Meta-analysis of the functional neuroanatomy of single-word reading: Method and validation. Neuroimage, 16, 765–780. doi: 10.1006/nimg.2002.1131
- Vallar, G., Perani, D., Cappa, S. F., Messa, C., Lenzi, G. L., & Fazio, F. (1988). Recovery from aphasia and neglect after subcortical stroke—neuropsychological and cerebral perfusion study. Journal of Neurology Neurosurgery and Psychiatry, 51, 1269–1276. doi: 10.1136/jnnp.51.10.1269
- Vinckier, F., Cohen, L., Oppenheim, C., Salvador, A., Picard, H., Amado, I., Krebs, M. O., & Gaillard, R. (in press). Reading impairment in schizophrenia: Dysconnectivity within the visual system. Neuropsychologia [e-pub ahead of print].
- Von Monakow, C. (1914). Die lokalisation im grosshirn und der abbau der funktion durch kortikale herde. Wiesbaden: J. F. Bergmann.
- Warburton, E., Price, C. J., Swinburn, K., & Wise, R. J. (1999). Mechanisms of recovery from aphasia: Evidence from positron emission tomography studies. Journal of. Neurology, Neurosurgery & Psychiatry, 66, 155–161. doi: 10.1136/jnnp.66.2.155
- Warren, J. E., Crinion, J. T., Lambon Ralph, M. A., & Wise, R. J. (2009). Anterior temporal lobe connectivity correlates with functional outcome after aphasic stroke. Brain, 132, 3428–3442. doi: 10.1093/brain/awp270
- Weiduschat, N., Thiel, A., Rubi-Fessen, I., Hartmann, A., Kessler, J., Merl, P.,…Heiss, W. D. (2011). Effects of repetitive transcranial magnetic stimulation in aphasic stroke a randomized controlled pilot study. Stroke, 42, 409–415. doi: 10.1161/STROKEAHA.110.597864
- Weiller, C., Isensee, C., Rijntjes, M., Huber, W., Muller, S., Bier, D.,…Diener, H. C. (1995). Recovery from Wernicke's aphasia: A positron emission tomographic study. Annals of Neurology, 37, 723–732. doi: 10.1002/ana.410370605
- Yetkin, F. Z., Swanson, S., Fischer, M., Akansel, G., Morris, G., Mueller, W., & Haughton, V. (1998). Functional MR of frontal lobe activations: Comparison with Wada language results. American Journal of Neuroradiology, 19, 1095–1098.
- Zhang, Y. J., Zhang, J. Y., Oishi, K., Faria, A. V., Jiang, H. Y., Li, X.,…Mori, S. (2010). Atlas-guided tract reconstruction for automated and comprehensive examination of the white matter anatomy. Neuroimage, 52, 1289–1301. doi: 10.1016/j.neuroimage.2010.05.049