Abstract
Purpose: This paper studied the effect of post-burn local hyperthermia on burn induced injury.
Methods: A second-degree burn injury was induced on the right and left flanks of Balb/c mice. Thirty-two burn wounds were divided into four groups. Opioid receptor blocking was done for groups 3 and 4 by intra-peritoneal administration of Naloxone (NLX) 30 min before the thermal injury. Local hyperthermia (45°C, 30 s) was applied only for the burn wounds of groups 2 and 4. Twenty-four hours after burn injury, the burned wounds were assessed for the level of iNOS (by immunohistochemistry) and the number of hair follicles (as an indicator of tissue injury).
Results: The wounds that received hyperthermia (group 2) had significantly more hair follicles (p < 0.001) compared to the control wounds (group 1). There was no significant difference between the number of hair follicles and acute inflammation of group 1 and group 3 (NLX + burn). Group 4 (NLX + burn + hyperthermia) had significantly fewer hair follicles compared to group 1 (p < 0.001), group 2 (p < 0.001) and group 3 (p < 0.001). The level of iNOS in groups 1, 3 and 4 was not significantly different but significantly more than group 2 (p < 0.001, p < 0.001 and p < 0.001, respectively).
Conclusions: The results showed that local hyperthermia after second degree burn decreased the tissue injury and iNOS expression. It is also concluded that endogenous opioid response may have a key role in the above mentioned effects of post-burn local hyperthermia.
Introduction
Many studies have shown that if the environmental temperature rises severely, cells grown in normal temperature will die rapidly. This phenomenon is called ‘heat shock’. If these cells are exposed to a sub-lethal temperature before being exposed to a lethal temperature, their resistance against heat shock will be increased due to the induction of the heat shock proteins (HSPs) at sub-lethal temperature. This phenomenon is regarded as ‘heat shock tolerance’ (thermo tolerance) Citation[1], Citation[2].
Other sub-lethal stresses can also induce stress tolerance, when the cells are exposed to them before being exposed to the lethal stresses Citation[1]. In addition to cells, tissues can also develop stress tolerance if they are exposed to a sub-lethal stress. Topping et al. Citation[3] have shown that if the skin is mildly warmed before being exposed to a high temperature, the thermal injury to the skin will be decreased as a result of the induction of HSPs. Also, if the myocardium undergoes a sub-lethal ischemia before undertaking a lethal ischemia, its resistance to the lethal ischemia will be increased Citation[4].
In addition to HSPs, opioids and opioid receptors also have a significant role in the stress tolerance of tissues Citation[5]. Opioids are peptides that are mainly produced in the nervous system and in less quantity by the cells of the immune system and their receptors are on both nerve cells and immune cells Citation[6–8]. Depending on the dose of administration, opioids may have both anti-inflammatory and pro-inflammatory effects Citation[9–11].
Skin burn can be regarded as a lethal heat shock to the skin. Cutaneous thermal injury initiates a pathophysiological response with a significant inflammatory component that involves several classes of chemical mediators. These mediators interact in a complex manner to cause the pain and secondary tissue damage associated with burn injury. There is some evidence to suggest that nitric oxide (NO) may contribute to the development of the inflammatory response after burn Citation[12–14].
Although NO can be derived from the acidic reduction of NO2 in some tissues, it is generally accepted that most NO is generated from the five-electron oxidation of L-arginine catalysed by the enzymes known as nitric oxide synthases (NOSs). These enzymes are classified in three groups according to the producing tissue (by endothelial cells, neural tissue and immune system) and also in two groups according to whether they are constitutively expressed (endothelial nitric oxide synthase: eNOS and neuronal nitric oxide synthase: nNOS) or induced in response to pathological stimuli by the immune system (iNOS) Citation[14]. Following the acute inflammation, increased iNOS is the main reason for NO synthesis Citation[14]. Therefore, in this condition, the amount of iNOS is an indicator for NO production.
Inducible NOS inhibition has previously been reported to reduce micro-vascular leakage after burn Citation[15] and it has been suggested that iNOS inhibition may be of benefit in limiting the secondary tissue damage Citation[13].
Hair follicles are epidermal appendages that have an important role in the healing process after a burn injury Citation[16]. Tissue necrosis (including necrosis of hair follicles) progresses after burning and it is not limited to the time of the burn occurrence Citation[3]. If a treatment can prevent progressing of the necrosis after burn injury, it can prevent the rise in the number of the necrotic hair follicles after burning. Thus the hair follicles count 24 h after burn injury is used for the assessment of the skin injury Citation[3].
Although the protective effect of pre-treatment of a tissue by sub-lethal stress before lethal stress induced injury has already been shown Citation[3], Citation[4], to date, no study has been done on the effect of sub-lethal stress on a tissue after the lethal stress and its effect on decreasing the injury severity has not yet been reported. Such an effect is possible at least in theory because the progress of necrosis of tissues is controlled by several mechanisms.
The present study aimed at evaluating the effect of mild and local post-burn hyperthermia (as a sub-lethal stress) on burn necrosis (as a lethal stress) and investigating of the possible role of opioids in this phenomenon.
Materials and methods
Animals
Twenty-four male BALB/c mice (average weight of 20 g) aged 6 weeks were obtained from the Razi Institute (Karaj, Iran), of which eight animals were depilated using a standard depilatory cream on the right and left flanks and the remaining 16 mice were depilated on the right flanks. All procedures on animal have been conducted with approval by the animal care committee of Tarbiat Modarres University.
Drugs
Naloxone (NLX) (Opioid Receptor Antagonist) was purchased from Sigma (USA). Rabbit anti-iNOS antibody was obtained from BD Company (USA). Goat anti-rabbit antibody conjugated with FITC and propidium iodide were purchased from Sigma (USA).
Induction of burn injury
The mice were anaesthetized by ether inhalation and the burn wounds were induced using a specifically designed metal sheet () supplemented by a sensor to adjust the temperature (accuracy 0.1°C) (Control Laboratory, Engineering Faculty, Tarbiat Modarres University). Temperature was set at 80°C and the metal plate was adjusted to 80°C. The hairless area of the right and left sides was burned by contacting the heated metal sheet for 1 s so that a partial thickness second degree burn wound (70 mm2) was created. Therefore, right and left flanks of eight mice were burned and, for the remaining 16 mice that had received NLX, only the right flanks were burned.
Local hyperthermia
Local hyperthermia was done by the same method as applied for burning; at 45°C for 30 s, using a bigger metal sheet in size. Because it was found in pilot studies that heating the margin of wound had a better effect than heating the whole wound, there was a damper cover on the central zone of this metal sheet. Post-burn local hyperthermia was applied for the right side wounds of eight mice whose right and left flanks were burned and had not received NLX before the burn injury (group 2), while hyperthermia was not applied for the left side wounds of these mice (group 1). These mice received saline 0.9% (by intra-peritoneal injection) 30 min before the burn injury. Local hyperthermia was not applied for half of the mice that received NLX before the burn injury (group 3), but it was applied for the other half (group 4). Grouping of the burn wounds are shown in .
Hyperthermia was applied for the treated wounds 1 and 3 min after burn injury.
Table I. Grouping of the burn wounds.
Histological analysis
Haematoxylin-eosin staining
Twenty-four hours after burn injury, the central area of the wound together with 5 mm of the marginal tissue was harvested. The samples were fixed in a 10% buffered formalin solution and embedded in paraffin, then 4 µm-thick sections were cut and stained with haematoxylin and eosin (H & E). The sections were assessed for the number of hair follicles. The assessor was blind to the allocation of the sections. Evaluation of the number of hair follicles was done by the following method: hair follicles in the whole wound areas were counted in high-power fields (×40).
Immunohistochemistry
The tissues were formalin-fixed, paraffin-embedded, cut into 4 µm thick sections and mounted on glass slides coated with poly-L-lysine. The sections were deparaffined in xylene and dehydrated in 100, 96 and 70% ethanol (each for 5 min) and finally brought to PBS. The sections were incubated in blocking buffer (0.1 M PBS containing 0.5% normal goat serum, 0.2% Triton X-100) for 1 h and thereafter in primary antibody for 12 h at 4°C. The primary antibody used in this study was mouse monoclonal anti-iNOS (1: 2000 R&D Systems). The adjacent sections served as negative controls and were processed using identical procedures, except for incubation without the primary antibody. The IgG/FITC goat anti-rabbit (1/1000) was added as a secondary antibody and incubated at 25°C for 30 min. Nuclear counterstaining was done with propidium iodide. After staining, images were captured using a fluorescent microscope (Nikon, Japan) and digital camera (Nikon, Japan). Measurements of fluorescence intensity were done using ImageJ software (NIH image). The measurement involved three steps: (1) 480 × 640 pixel RGB colour images were acquired with a background fluorescence (non-specific fluorescence) set at a constant level by adjusting the gain of the digital camera; (2) colour images were converted to an 8-bit gray scale (fluorescence intensity from 0–255) using ImageJ; and (3) mean intensity in the region of was calculated from pixels with intensity values >50, a value selected to exclude all background fluorescence. For comparisons among different groups, the mean fluorescence intensity of group 1 (burn without any treatment) was assigned a value of 1 and the values for other groups were scaled accordingly and designated normalized fluorescence intensities Citation[17].
Statistical methods
The hair follicle number and relative fluorescence intensity of wounds of group 1 and those of their corresponding wounds in group 2 were compared by paired Student t-test, while the other comparisons of these parameters among the different groups were done by independent Student t-test.
Results
Number of hair follicles
The number of hair follicles of the studied groups 24 h after burn injury is shown in . The numbers of hair follicles in the wounds of group 2 were significantly higher than their corresponding wounds in group 1 (p < 0.001). The difference between the number of hair follicles between group 1 and group 3 was not significant.
After 24 h, the burn wounds of group 4 had changed into severe third degree burn wounds () and the number of hair follicles was zero (). The differences between the number of hair follicles in group 4 and those of groups 1, 2 and 3 were statistically significant (p < 0.001, p < 0.001 and p < 0.001, respectively).
Figure 2. Mean of the number of hair follicles in the burn wounds of the four groups. Comparison between group 1 and group 2 was done by paired Student t-test while independent student t-test was applied for other comparisons. The numbers of hair follicles in the wounds of group 2 were significantly higher than their corresponding wounds in group 1. The difference between the number of hair follicles between group 1 and group 3 was not significant. The differences between the number of hair follicles in group 4 and those of groups 1, 2 and 3 were statistically significant. Group 1: burn without any treatment, Group 2: burn + hyperthermia, Group 3: NLX + burn, Group 4: NLX + burn + hyperthermia. NLX: Naloxone, ***p < 0.001.
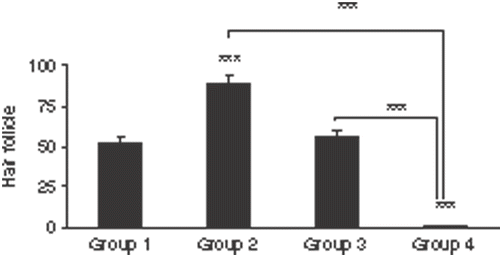
Figure 3. Histological sections of the central area of the burned wounds in four groups. (a) Histological section of burned wound of group 1 (burn without any treatment), 24 h after the burn injury. There were no hair follicles in the central area of the wound. (H & E, 10×). (b) A burn wound of group 2 (burn + hyperthermia), 24 h after the burn injury. There were a few hair follicles in the central area of the wound (H & E, 10×). (c) A burn wound of group 3 (NLX + burn), 24 h after the burn injury. There were no hair follicles in the central area of the wound. (H & E, 10×). (d) A burn wound of group 4 (NLX + burn + hyperthermia), 24 h after the burn injury. The burned wound had changed into a severe third degree burn wound. There were no hair follicles in the central area of the wound. (H & E, 10×).
iNOS expression
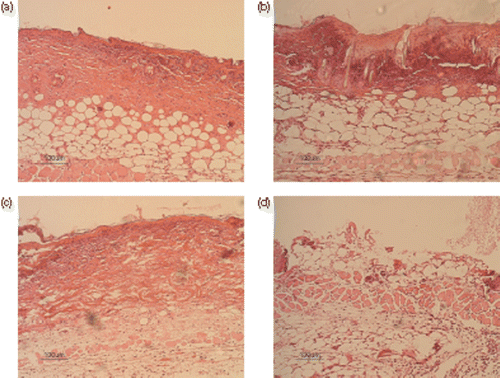
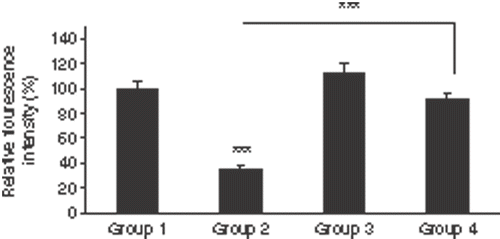
To investigate the level of iNOS, burned wound sections were labelled with anti-iNOS antibody (). shows the relative fluorescence intensities of different groups. The wounds of groups 1 and 4 showed a significant higher relative fluorescence intensities than group 2 (p < 0.001 and p < 0.001, respectively). There was no significant difference in the fluorescence intensity between group 1 and groups 3 and 4.
Figure 4. Inducible NOS immunohistochemistry 24 h after the skin burn injury. The sections were nuclear counterstained with propidium iodide. (a1) A burned skin section of left flank of one mouse (group 1: burn without any treatment). (a2) Nuclear counterstaining of the same section. (b1) A burned skin section of right flank of the same mouse. Hyperthermia was done for the burned skin. (group 2). (b2) Nuclear counterstaining of the same section. (c1) A burned skin section of the right flank of a mouse. NLX has been administered for the mouse 30 min before burn injury (group 3). (c2) Nuclear counterstaining of the same section. (d1) A burned skin section of right flank of a mouse. NLX has been administered for the mouse 30 min before the burn injury and hyperthermia has been done for the burned skin. (group 4). (d2) Nuclear counterstaining of the same section. NLX: Naloxone.
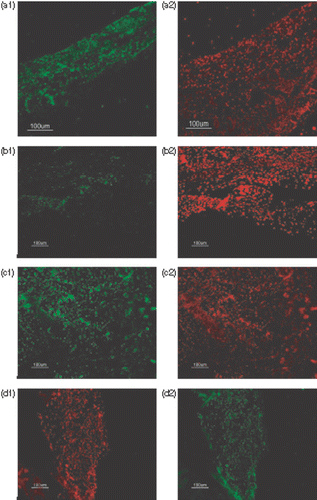
Figure 5. Mean of the relative fluorescence intensities of immunohistochemistry staining of the sections by anti-iNOS antibody in the four groups. Comparison between group 1 and group 2 was done by paired student t-test while independent student t-test was applied for other comparisons. The wounds of groups 1 (burn without any treatments) and 4 (NLX + burn + hyperthermia) showed significant higher relative fluorescence intensities than group 2 (burn + hyperthermia). Opioid receptors of group 3 (NLX + burn) had been blocked but there was no significant difference in the fluorescence intensity between groups 1 and 3. Also there was no significant difference between groups 1 and 4 (n = 8 mice/group, mean fluorescence intensity of group 1 normalized to 100%). ***p < 0.001.
Discussion
Conventional treatment of burns has involved the application of cold Citation[18], while the application of hyperthermia as a treatment protocol is surprising and constitutes a relatively new approach, particularly if employed immediately after the burn.
Recent studies have shown that hyperthermia quickens the healing of non-burn wounds Citation[19–21]. Also, application of hyperthermia (as a sub-lethal heat shock) before skin burn (as a lethal shock) has been shown as a useful protocol in decreasing the tissue damages Citation[3]. The present work investigated the effect of post-burn local hyperthermia on reduction of the second-degree burn induced damages.
The results of this study indicated the presence of moderate hair follicle injury and high iNOS expression in group 1 (burn without any treatment). Group 2 (burn + mild hyperthermia) showed mild hair follicle injury and low iNOS expression. Group 3 (NLX + burn) showed moderate hair follicle injury and high iNOS expression (similar to group 1) and finally group 4 (NLX + burn + local hyperthermia) showed a very severe hair follicle injury and high iNOS expression.
In this study, several considerations have been taken into account, which can collectively provide an acceptable survey on the efficacy of post-burn local hyperthermia. These include:
First, post-burn local hyperthermia could reduce the increase in number of the necrotic hair follicles after burning. Similar results have been obtained by Topping et al. Citation[3], who showed the same effect of local hyperthermia before thermal injury. They used hair follicle number as an indicator for skin thermal injury.
Secondly, it was found that post-burn local hyperthermia decreased the expression of iNOS. In group 1 (burn without any treatment) and group 3 (NLX + burn) iNOS expression was significantly more than in group 2 (burn + local hyperthermia), while tissue damage was significantly higher in groups 1 and 3 compared with group 2. It has been postulated that production of large amounts of inflammatory mediators like NO can increase tissue damage Citation[13], Citation[14]. Therefore, diminished production of NO due to decreased expression of iNOS may be one of the causes of less tissue damage following post-burn local hyperthermia.
Thirdly, use of heat in non-burn wound healing has been demonstrated to increase blood flow and, hence, to accelerate healing in this kind of wound Citation[18], Citation[22–24]. In this study, a short term (30 s, twice) hyperthermia after burn injury was applied, which seems to be insufficient to increase the blood flow in the burn wound area. Therefore, the mechanism of post-burn hyperthermia in decreasing the burn induced tissue damage is apparently not through increasing the blood flow. Considering the short time period of hyperthermia, a neurological mechanism for the effects of post-burn hyperthermia might be more acceptable. Opioids are neuropeptides whose role in stress tolerance has already been shown Citation[5]. They are also able to modulate the inflammation process and iNOS expression Citation[25–27]. Therefore, the next step examined the role of opioids in post-burn hyperthermia.
When opioid receptors were blocked by systemic administration of NLX, post-burn hyperthermia did not decrease tissue damage, but even led to its increase (group 4). This finding agreed with the results of a previous study that showed a protective effect for the administration of morphine on stress-induced ulcers Citation[28]. It seems that stimulation of opioid receptors is a key mechanism in the effects of post-burn hyperthermia on decreasing the burn-induced damage. It seems that in the absence of the endogenous opioid response, the post-burn hyperthermia strategy changes into a harmful lethal heat shock that increases the tissue damage.
Forthly, expression of iNOS in group 4 (NLX + burn + hyperthermia) was significantly more than group 2 (burn + hyperthermia) but was not significantly different from group 1 (burn without any treatment). Lysle and How Citation[26] have shown that morphine-6 beta-glucuronide administration results in a pronounced reduction in lipopolysaccharide (LPS)-induced expression of iNOS in spleen, lung and liver tissue. Therefore, one can suppose that opioid response has an important role in the reduction of iNOS expression due to post-burn local hyperthermia.
However, the endogenous opioid response was blocked in group 3 by the administration of NLX. There was no difference between this group and group 1 (burn without any treatment). This finding is in line with the results of Brennum et al. Citation[29]. They showed that endogenous opioid response was not activated following the induction of hyperalgesia by a burn injury. These finding also agree with the findings of Uluoglu et al. Citation[30], who showed that NLX did not change the indices of gastric ulceration induced by ethanol and restraint-cold-stress. The reason seems to be the non-induction of endogenous opioid response during the primary burn injury. Therefore, blockage of endogenous opioid receptors does not affect tissue damage and iNOS expression following the original burn injury.
With due attention to the significant differences between the treated and the non-treated burn wounds in the number of follicles and iNOS expression, it can be concluded that post-burn local hyperthermia decreased the tissue damage and suppressed the iNOS expression in the second degree burn wounds.
Although the above mentioned results suggest that endogenous opioid response has an important role in the effect of post-burn local hyperthermia, further studies are required to reveal that whether the activation of endogenous opioids in the central nervous system or those in the peripheral tissues are responsible for this effect. In addition, the role of the stress proteins like heat shock proteins in post-burn local hyperthermia needs to be investigated.
Since a burn occurs due to a lethal hyperthermia (lethal heat shock) and post-burn local hyperthermia (a slight and non lethal hyperthermia) helps to eliminate the burn induced injury, one can consider the reduced injury due to the post-burn hyperthermia as one kind of heat shock tolerance, but since the tolerance takes place after the heat shock (burn), the term ‘post-heat shock tolerance’ seems suitable for it.
In conclusion, the present study demonstrates that post-burn local hyperthermia reduced the tissue damage and decreased iNOS expression in the second-degree burn wounds 24 h after the burn injury. In addition, these findings suggest that the effects of post-burn local hyperthermia may be mediated via the endogenous opioid peptides and receptors. The authors are continuing to explore the means by which post-burn hyperthermia reduces necrosis. These somewhat unexpected results suggest that there may be more effective means of treating burns than those currently in use.
Acknowledgement
This work has been supported by a research grant from Tarbiat Modarres University. We thank Professor Richard A. Lockshin for editing this paper.
References
- Ellis RJ, Laskey RA, Lorimer GH. Molecular chaperones. Chapman & Hall, London 1993
- Lindquist S. The heat shock response. Ann Rev Biochem 1986; 55: 1151–1191
- Topping A, Gualt D, Grobbelaar A, Green C, Sanders R, Sibbons P, Linge C. Successful reduction in skin damage resulting from exposure to the normal-mode ruby laser in an animal model. Br J Plast Sur 2001; 54: 144–150
- Sommerschild HT, Kirkeboen KA. Preconditioning—endogenous defence mechanisms of the heart. Acta Anaesthesiol Scand 2002; 46: 123–137
- Sonneborn JS, Gottsch H, Cubin E, Oeltgen P, Thomas P. Alternative strategy for stress tolerance: Opioids. J Gerontol A Biol Sci Med Sci 2004; 59: 433–440
- Way WL, Fields HL, Schumacher MA. Opioid analgesics & antagonists. Basic and clinical pharmacology, 8th ed, BG Katzurg. McGraw–Hill Co., New York 2001; 512–548
- Stein C, Hassan AH, Przewlocki R, Gramsch C, Peter K, Herz A. Opioids from immunocytes interact with receptors on sensory nerves to inhibit nociception in inflammation. Proc Natl Acad Sci USA 1990; 87: 5935–5939
- Wybran J, Appelboom T, Famaey JP, Govaerts A. Suggestive and evidence for receptors for morphine and methionine enkephalin on normal blood T lymphocytes. J Immunol 1979; 123: 1068–1070
- Peng X, Mosser DM, Adler MW, Rogers TJ, Meissler JJ, Jr, Eisenstein TK. Morphine enhances interleukin-12 and the production of other pro-inflammatory cytokines in mouse peritoneal macrophages. J Leukoc Biol 2000; 68: 723–728
- Cabot PJ, Cramond T, Smith MT. Morphine has a dual concentration-dependent effect on K(+)-evoked substance P release from rat peripheral airways. Pulm Pharmacol Ther 1997; 10: 215–21
- Sanchez IO, Weber RJ, Rice KC, Zhang X, Padilla CR, Guerra RT, Vazquez JLM, Flores RJ. Chemotaxis of human and rat leukocytes by the deltaselective non-peptidic opioid SNC 80. MG Rev Latinoam Microbiol 2003; 45: 16–23
- Shakespeare P. Burn wound healing and skin substitutes. Burns 2001; 27: 517–522
- Rawlingson A, Shendi K, Greenacre SA, England TG, Jenner AM, Poston RN, Halliwell B, Brain SD. Functional significance of inducible nitric oxide synthase induction and protein nitration in the thermally injured cutaneous microvasculature. Am J Path 2003; 162: 1373–1380
- Rawlingson A. Nitric oxide, inflammation and acute burn injury. Burns 2003; 29: 631–640
- Sozumi T. The role of nitric oxide in vascular permeability after a thermal injury. Ann Plast Surg 1997; 39: 272–277
- Dyer C, Roberts SD. Thermal trauma. Nurs Clin North Am 1990; 25: 85–117
- Baffert F, Thurston G, Rochon-Duck M, Le T, Brekken R, McDonald DM. Age-related changes in vascular endothelial growth factor dependency and angiopoietin-1-induced plasticity of adult blood vessels. Circ Res 2004; 94: 984–992
- Davies JWL. Prompt cooling of burned areas: A review of benefits and the effector mechanisms. Burns 1982; 9: 1–6
- Lee ES, Caldwell MP, Talarico PJ, Kuskowski MA, Santilli SM. Use of noncontact radiant heat bandage and Staphylococcus aureus dermal infections in ovine model. Wound Repair Regen 2000; 8: 899–903
- Santilli SM, Valusek PA, Robinson C. Use of a noncontact radiant heat bandage for the treatment of chronic venous stasis ulcers. Adv Wound Care 1999; 12: 899–903
- Nanbu NP, Wakabayashia T, Yamashita R, Hayashi H, Hisano S, Oshikaa T. Heat treatment enhances healing process of experimental pseudomonas corneal ulcer. Ophth Res 2004; 36: 218–225
- Johansen KS, Berger EM, Repine JE. Effect of temprature on polymorphonuclear leucocyte function. Acta Pathol Microbiol Immunol Scand 1983; 91: 355–359
- Ikeda T, Tayefeh F, Sessler DI, Kurz A, Plattner O, Petschnigg B, Hopf HW, West J. Local radiant heating increases subcutaneous oxygen tension. Am J Surg 1998; 175: 33–37
- Price P, Bale S, Crook H, Harding KG. The effect of a radiant heat dressing on pressure ulcers. J Wound Care 2000; 9: 201–205
- Lysle DT, Carrigan KA. Morphine-6beta-glucuronide modulates the expression of inducible nitric oxide synthase. Inflammation 2001; 25: 267–275
- Lysle DT, How T. Endogenous opioids regulate the expression of inducible nitric oxide synthase by splenocytes. JPET 1999; 288: 502–508
- Lysle DT, How T. Heroin modulates the expression of inducible nitric oxide synthase. Immunopharmacology 2000; 46: 181–192
- Cho CH, Wu KK, Wu S, Wong TM, So WHL, Liu ESL, Chu KM, Shin VY, Ye YN, Wong BCY. Morphine as a drug for stress ulcer prevention and healing in the stomach. Eur J Pharm 2003; 460: 177–182
- Brennum J, Kaiser F, Dahl JB. Effect of naloxone on primary and secondary hyperalgesia induced by the human burn injury model. Acta Anaesth Scand 2001; 45: 954
- Uluoglu C, Guney Z, Kilinc M, Bozkurt S, Ercan ZS. The effects of captopril and naloxone on restraint-cold-stress- and ethanol-induced gastric lesions in rats. Gen Pharmacol 1998; 30: 701–704