Abstract
Purpose: Hyperthermia induced by electrothermal needle (ETN) and electrochemical therapy (ECT) were combined (HECT) and the anti-tumor effect was evaluated.
Methods: Mice with Sarcoma180 were randomized into four different treatment groups: Control, ECT alone, Hyperthermia alone and HECT.
Results: The tumours in the HECT group were completely destroyed and they did not recur within a period of 10 days after treatment. However, tumour recurrence was found in six mice in the Hyperthermia group and five mice in the ECT group.
Conclusion: The HECT is a potentially effective way to treat solid malignant tumours.
Introduction
Electrochemical therapy (ECT), a local ablative technique, has been an additional method to treat malignant tumours Citation[1], Citation[2]. It involves using destructive electrolysis induced by low-level direct electric current passing through a pair of electrodes implanted in the tumour. This creates a large pH change, which leads to cell death Citation[3]. In China more than 10 000 patients with various malignant tumours have been treated with ECT over the past 15 years Citation[4], Citation[5]. However, ECT is a slow process and typical treatment time is 3–4 h Citation[6]. In order to enhance the efficacy of ECT, this study developed a new technique called hyperthermal and electrochemical therapy (HECT), using ECT and hyperthermia in combination. It also developed a hyperthermal and electrochemical device (HECD) based on the model of an electrothermal needle (ETN) which was devised by Sun and Chen Citation[7], Citation[8] to induce interstitial hyperthermia.
In a previous study Citation[9], the destructive effect of ECT combined with hyperthermia on normal rat livers was greatest and HECT was demonstrated to have a synergistic effect. This experiment further evaluated the anti-tumour effect of HECT on mouse Sarcoma180.
Materials and methods
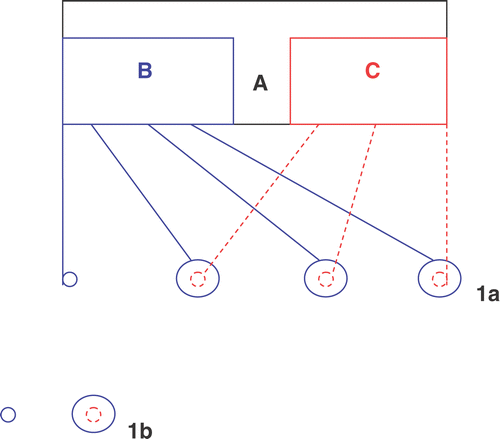
The patented HECD was designed by Sun Citation[10] ().
Figure 1. The structure draft of HECD (a) and the arrangement of the group of electrodes (b). (a) There are two Parts: ECT generator (B) and hyperthermia generator (C) in the HECD (A). One anode (small blue circle) and three cathodes (big blue circle) are connected with ECT generator, while one ETN (small red circle) connected with hyperthermia generator is inserted in each cathode. (b) Two electrodes were inserted into the tumour tissue in the longitudinal direction from the edge of the tumour and a 0.5 cm distance was kept between them. In the ECT group one anode and one cathode were used; in the Hyperthermia group an ETN was used; in the HECT group ECT was induced by one anode and one cathode and hyperthermia was produced by an ETN.
The suspension of Sarcoma180 cells (2–4 × 105 cells/0.2 ml) was transplanted subcutaneously into the right femoral region of 52 female ICR mice, weighing 15–20 g. Ellipsoidal tumours, 1.0–1.2 cm in length, had formed day 10 after transplantation. Forty mice bearing Sarcoma180 were randomized into four groups (n = 10 in each group). A group of electrodes were implanted in the tumour (). They were then given the following treatments: (1) Control group: No therapy was given; (2) ECT group: ECT was induced with a current of 30 ∼ 35 mA for 15 min. The dosage was 27 ∼ 31.5 coulombs; (3) Hyperthermia group: Hyperthermia was induced with 520 mA for 15 min; the temperatures were maintained at 56°C ± 1°C at the cathode and 42°C ± 1°C at the edge of the tumour; (4) HECT group: ECT and hyperthermia were induced simultaneously. The dosage given and the time applied were the same as groups 2 and 3.
Tumour size was measured daily after the treatment for a total of 10 days, according to the following formula Citation[11]: Tumour volume = 1/12 × (π × length2 × width).
Tumour tissues of the 12 remaining mice were histologically examined by light microscopy and transmission electron microscopy at 0, 6, 24 and 72 h after treatment in three treatment groups. In addition, mice without recurrent tumour were further determined by histological examination.
The study protocol was approved by the Committee on Animal Research of Health Bureau of Zhejiang Province, PR China. All procedures described complied with Interdisciplinary Principles and Guidelines for the Use of Animals in Research, Marketing and Education.
The data was analysed by Kruskal-Wallis test to determine if tumour volumes varied in the four groups. If the results showed significant difference among the four groups, the Nemenyi test was used to further compare in two related groups.
Results
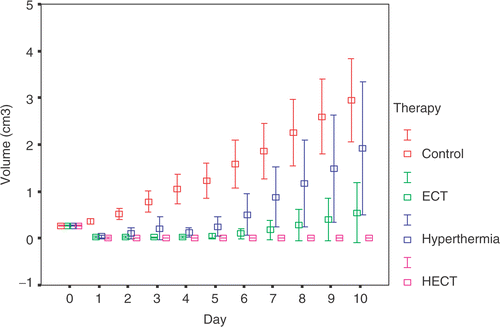
Data analysis ()
In the HECT group, there was a notable anti-tumour effect on mice with Sarcoma180. Almost all of the tumours had disappeared day 1 after treatment. On the third day, necrotic tissues remained in the primary lesions. Scar tissues had formed in the right femoral region of the mice with no tumour recurrence on day 10. However, there was tumour recurrence around the circumference of the necrotic tissue in six mice in the Hyperthermia group and five mice in the ECT group during the first 4 days after treatment. In addition, No malignant cell was histologically found in the 19 mice without tumour recurrence on day 10 (10 in the HECT group, five in the ECT group and four in the Hyperthermia group).
Figure 2. The changes of tumour volumes in four groups during the 10 days after treatment. The results were means with 95% confidence intervals. Because the result of the Kruskal-Wallis test showed the significance of the difference among the four groups, the Nemenyi test was chosen to further compare in two related groups. In the HECT group there was a significant difference in the recurrence of tumours compared to the other groups (p < 0.01). The recurreent tumour volumes in the Hyperthermia and ECT groups showed notable differences in contrast to the control group (p < 0.01). The capacity of tumour inhibition in the ECT group was significantly stronger than in the Hyperthermia group (p < 0.05).
Microscopic findings (data not shown)
In the early period of HECT, both coagulative and colliquative necroses occurred in the tumour. Abundant neutrophils and some lymphocytes infiltrated the necrotic tissue and the surrounding subcutaneous capillaries were dilated. After 72 h of treatment, tumour cells were not detected. At this stage predominantly lymphocytes and only a few neutrophils were found in subcutaneous tissue. Both coagulative and colliquative necroses were also found in the ECT group, whereas only coagulative necrosis was observed in the Hyperthermia group. After 72 h of treatment, a few remaining tumour cells were found in the tumour of some mice in the two groups.
Findings of transmission electron microscopy in the HECT group (data not shown)
Apart from the tumour necrosis, it was found that the mitochondria were swollen and the cristae had disappeared. The rough endoplasmic reticula were dilated, degranulated and cracked to form alveoli. The chromatins were condensed and abutted on their nuclear envelops. Finally, pyknotic nucleuses were formed.
Discussion
A solid tumour 1 cm in diameter can be completely destroyed only when the dosage is more than 100 coulombs Citation[12]. The hyperthermal effect induced by one ETN is limited to an area of 1 cm in diameter, resulting in ineffective killing of peripheral tumour cells Citation[13]. Wojcicki et al. Citation[14] demonstrated the inhibitory effects of intra-lesional ECT on the growth of advanced mouse mammary carcinomas and fibrosarcomas transplanted subcutaneously. However, this method did not result in a complete cure of the tumour-bearing animals. Maintz et al. Citation[15] examined the feasibility and effectiveness of applying direct-current therapy in liver metastases of colorectal carcinomas in an animal model. The study demonstrated an anti-tumour effect of ECT and its complete regression was 54%. However, it was found that combining ECT (27–31.5 coulombs) with hyperthermia at 42°C could completely destroy mice sarcoma 1 cm in diameter and no recurrence was determined by histological examination on day 10 after treatment.
Shou et al. Citation[16] investigated the inhibitory effects of different treatments, including ECT, hyperthermia induced by water bath and ECT combined with hyperthermia, on growth of L78 human lung cancer cells. It was demonstrated that there was no significant difference in L78 growth inhibition between the control group and the hyperthermia group in which the temperature was increased to 41°C. However, when hyperthermia was combined with ECT, the inhibition became prominent in comparison with other groups. They believed ECT could enhance the sensitivity of L78 to hyperthermia. Their results also supported the synergistic effect of the combination treatment.
Potential mechanisms for the anti-tumour effect of HECT are discussed as follows:
When the temperature is raised the movement of molecules in tissue will accelerate, which could decrease the resistance of tissue to the electric current. Therefore, it could enhance the electrolytic reaction induced by ECT. According to Van’t Hoff's rule: K(t+10)/Kt = 2 ∼ 4, the rate of chemical reaction is increased by 2–4-fold for each 10°C increment in temperature.
Hyperthermia can damage the stability of cell membranes and increase membrane permeability. It then becomes easier to destroy the tissue cells and inhibit sub-lethal cell repair due to reactive oxygen species, hypochlorous acid and chlorine gas produced by the anode and hydrogen produced by the cathode.
Electrolytic reaction induced by ECT could lead to release of chlorine, oxygen and hydrogen ions at the anode and hydrogen gas and sodium hydroxide at the cathode. The region surrounding the anode therefore becomes very acidic (around pH 2) and the region surrounding the cathode becomes strongly alkalotic (pH 12). This change will increase the thermosensitivity of the tumour cells and enhance the anti-tumour effect of hyperthermia. Gerweck et al. Citation[17] showed that the sensitizing effect took place over a range of temperatures (41–44°C) and increased with decreasing pH. The effect was particularly pronounced at 42°C. Maintaining tissue culture cells at low pH after heat was also found to increase the cytocidal effects of hyperthermia. They also found that when pH was decreased to as low as 6.7, the development of thermotolerance was inhibited in cultures exposed continuously to temperatures in the range of 42.0–42.5°C. The degree of inhibition was directly related to the extra-cellular pH of the culture media Citation[18]. In the study of Hahn and Shiu Citation[19], the thermosensitivity of the mammalian cells growing at pH 7.4 was dramatically increased when the pH was dropped to 6.5 and the temperature was increased to 42°C. The thermosensitivity of other cells cultured at pH 6.8 showed considerable change when exposed to similar conditions. This implies that the thermosensitivity of the tumours exposed to low pH environments long-term can be promoted only when there is a dramatic reduction in pH.
The direct cytotoxic effect of hyperthermia is improved when hypoxia, low pH and malnutrition are induced in the cells. After ECT, the perfusion and oxygenation of fibrosarcoma are significantly decreased Citation[20]. This is possibly due to micro-thrombus accumulation in the anode area and capillary blockage in the cathode area Citation[12], which leads to low pH and malnutrition in the tumour.
In conclusion, HECT induced by HECD was demonstrated to be a potentially effective way to treat cancer. The findings from this research provide a theoretical and an experimental basis for the development of a new approach to cancer treatment.
Acknowledgements
We thank Ms Wei Shi and Ms Xiuyang Li for skillful support in some parts of this work. We also thank Miss Tracia Loo, Dr Angela Fu and Dr Vincent Wong for translating and correcting this paper.
References
- Wu G, Zhou X, Huang M. Electrochemical therapy and implanted ports treatment for unresectable carcinoma of body and tail of pancreas. Zhonghua Wai Ke Za Zhi 2001; 39: 596–598, (in Chinese)
- Liu J, Wei Y, Luo F, Lu S, Peng Y, Lei S, Zhao X, Yan Y. Cell cycle analysis and pathological changes of malignant tumors treated with electrochemical therapy. Hua Xi Yi Ke Da Xue Xue Bao 2000; 31: 104–6, (in Chinese)
- Nordenstrom BEW. Survey of mechanisms in electrochemical treatment (ECT) of cancer. Eur J Surg Supp 1994; 574: 93–109
- Nilsson E, von Euler H, Berendson J, Thorne A, Wersall P, Naslund I, Lagerstedt AS, Narfstrom K, Olsson JM. Electrochemical treatment of tumours. Bioelectrochemistry 2000; 51: 1–11
- Xin Y. Organisation and spread of electrochemical therapy in China. Eur J Surg Suppl 1994; 574: 25–29
- Wemyss-Holden SA, Dennison AR, Berry DP, Maddern GJ. Local ablation for unresectable liver tumor: Is thermal best?. J Hepatobiliary Pancreat Surg 2004; 11: 97–106
- Sun CJ, Chen GF. Primary trial of inhibiting effect of electrothermal needles therapy in mice bearing transplantable S180. J Zhejiang Med Univ 1986; 15: 107–108, (in Chinese)
- Sun CJ, Chen GF. Observation of long term effectiveness of electrothermal needles therapy in the treatment of oral cancers. J Pract Oncol 1992; 7: 232–341, (in Chinese)
- Xie L, Sun CJ, Zhao SF. A new local ablation for unresectabler liver tumor: Effect of electrothermal & electrochemical therapy on rat liver. JZUS 2006; 7: 654–659
- Sun CJ. Tumor electrochemical-therapeutic device using electrothermal needles. US patent 6,712,840, 30 March 2004
- Ohno S, Siddik ZH, Baba H, Stephens LC, Strebel FR, Wondergem J, Khokhar AR, Bull JM. Effect of carboplatin combined with whole body hyperthermia on normal tissue and tumor in rats. Cancer Res 1991; 51: 2994–3000
- Song ZY, Xin YL, Xu BN. Experimental research on ECT of malignant tumors. Electrochemical therapy in the treatment of cancer, 1st ed, YL Xin. People's Health Press, Beijing 1995; 97–102, (in Chinese)
- Ye XH, Sun CJ, Chen GF. Inhibition effectiveness research of mice with transplanted S180 treated with hyperthermia by electrothermal needles in combination with chemotherapy. J Pract Oncol 1998; 13: 354–355, (in Chinese)
- Wojcicki M, Drozdzik M, Olewniczak S, Opolski A, Wietrzyk J, Radzikowski C, Kaczmarek B, Wrzesinski M, Romanowski M, Kaminski M, Zielinski S. Antitumor effect of electrochemical therapy on transplantable mouse cancers. Med Sci Monit 2000; 6: 498–502
- Maintz D, Fischbach R, Schafer N, Schafer H, Gossmann A, Kugel H. Results of electrochemical therapy of colorectal liver metastases in rats followed up by MRI. Invest Radiol 2000; 35: 289–294
- Shou YN, Xin YL, Zhao TD, Gao FY. Experimental study of effect of direct electric current combined with hyperthermia on human lung cancer cells. J N Bethune Univ Med Sci 1998; 24: 466–468, (in Chinese)
- Gerweck LE, Gillette EL, Dewey WC. Killing of Chinese hamster cells in vitro by heating under hypoxic or aerobic conditions. Eur J Cancer 1974; 10: 691–693
- Gerweck LE, Jennings M, Richards B. Influence of pH on the response of cells to single and split doses of hyperthermia. Cancer Res 1980; 40: 4019–4024
- Hahn GM, Shiu EC. Adaptation to low pH modifies thermal and thermo-chemical responses of mammalian cells. Int J Hyperthermia 1986; 2: 379–387
- Jarm T, Cemazar M, Steinberg F, Streffer C, Sersa G, Miklavcic D. Perturbation of blood flow as a mechanism of anti-tumour action of direct current electrotherapy. Physiol Meas 2003; 24: 75–90