Abstract
Purpose: We clarified the usefulness of mild temperature hyperthermia (MTH) in combination with the continuous administration of tirapazamine (TPZ) under reduced dose-rate irradiation (RDRI) using γ-rays.
Materials and Methods: SCC VII tumour-bearing mice received a continuous administration of 5-bromo-2′-deoxyuridine (BrdU) to label all proliferating (P) cells. Then, they received a 24 h continuous subcutaneous infusion of TPZ either with or without MTH under high dose-rate irradiation (HDRI) or RDRI using γ-rays. After the irradiation, the tumour cells were isolated and incubated with a cytokinesis blocker, and the micronucleus (MN) frequency in non-proliferating tumour cells without BrdU labeling (= quiescent (Q) cells) was determined using immunofluorescence staining for BrdU. The MN frequency in the total tumour cell populations was determined using tumours that were not pretreated with BrdU.
Results: The sensitivity of both the total and Q cell populations, especially the latter, was significantly reduced with RDRI compared with HDRI. TPZ increased the sensitivity of both populations, with a slightly more remarkable increase in Q cells. Further, MTH combined with TPZ raised the sensitivity of both the total and Q cell populations, especially the latter, under RDRI more markedly than under HDRI.
Conclusion: From the viewpoint of solid tumour control as a whole, including intratumour Q-cell control, the use of TPZ, especially in combination with MTH, is useful for suppressing the reduction in the sensitivity of tumour cells caused by the decrease in irradiation dose rate in vivo.
Introduction
Many cells in solid tumours are non-proliferating quiescent in situ but are still clonogenic Citation[1]. These quiescent (Q) tumour cell populations have been thought to be more resistant to irradiation because of their much larger hypoxic fractions and greater potentially lethal damage repair (PLDR) capacities than proliferating (P) tumour cells, mainly based on the characteristics of plateau-phase cultured cells in vitro Citation[1]. Employing our method for selectively detecting the response of intratumour Q cell populations in vivo, we have already shown that all these characteristics could be applied to Q state cells in solid tumours in vivo under conventional high dose-rate irradiation conditions Citation[2], Citation[3]. However, clinically, reduced dose-rate irradiation was found to spare normal tissue from radiation-induced damage resulting in a greater therapeutic gain Citation[4].
Meanwhile, the development of bioreductive agents that are particularly toxic to hypoxic cells is considered a promising approach to solving the problem of radio-resistant tumour hypoxia in cancer radiotherapy Citation[5]. Tirapazamine (TPZ), a lead compound in the development of a bioreductive hypoxic cytotoxin, in combination with radiation has been shown to be very useful for controlling solid tumours as a whole, especially for controlling hypoxia-rich Q tumour cell populations Citation[2], Citation[5]. Tumour hypoxia results from either limited oxygen diffusion (chronic hypoxia) or limited perfusion (acute hypoxia, transient hypoxia or ischemic hypoxia). Chronically hypoxic tumour cells existing at the rim of the oxygen diffusion distance can be killed by just a single administration of TPZ Citation[2]. Acutely hypoxic tumour cells occurring sporadically throughout solid tumours can be killed by TPZ during long-term continuous administration. In other words, the long-term continuous administration of TPZ can kill both chronically and acutely hypoxic tumour cells. Actually, continuously administered TPZ was reported to be very useful for sensitizing tumour cells in vivo Citation[6].
Mild temperature hyperthermia (MTH) has been reported to increase the response of tumours to radiation by improving oxygenation through an increase in tumour blood flow Citation[7]. Further, MTH was also shown to enhance the tumour response, especially of the intratumor quiescent (Q) cell population, to TPZ Citation[8].
Therefore, in this study, the usefulness of combining both MTH and the continuous administration of TPZ with conventionally employed high dose-rate or reduced dose-rate irradiation with low linear energy transfer (LET) radiation γ-rays was evaluated in terms of the responses of the total (= P + Q) and Q tumour cell populations Citation[2].
Materials and methods
Mice and tumours
SCC VII squamous cell carcinomas (Department of Radiology, Kyoto University) derived from C3H/He mice were maintained in vitro in Eagle's minimum essential medium supplemented with 12.5% fetal bovine serum. Cells were collected from exponentially growing cultures, and 1.0 × 105 cells were inoculated subcutaneously into the left hind legs of 8- to 11-week-old syngeneic female C3H/He mice (Japan Animal Co., Ltd, Osaka, Japan). Fourteen days after the inoculation, each tumour had reached approximately 1 cm in diameter. At treatment, the body weight of the tumour-bearing mice was 22.1 (19.8–24.4) g (mean (95% confidence limit)). Mice were handled according to the Recommendations for Handling of Laboratory Animals for Biomedical Research, compiled by the Committee on Safety and Ethical Handling Regulations for Laboratory Animal Experiments, Kyoto University. All experimental procedures mentioned here were in accordance with institutional guidelines for the care and use of laboratory animals in research. Incidentally, the p53 of the SCC VII tumour cells is the wild type Citation[9].
Labeling with 5-bromo-2′-deoxyuridine (BrdU)
The combining size (the van der Waals radius) of an atom of bromide is very similar to that of the methyl group CH3. BrdU consequently is very similar to the normal DNA precursor thymidine, having a halogen substituted in place of the methyl group. Thus, 9 days after the tumour cell inoculation, mini-osmotic pumps (Durect Corporation, Cupertino, CA) containing BrdU dissolved in physiological saline (250 mg/ml) were implanted subcutaneously to label all P cells for 5 days. Administration of BrdU did not change the tumour growth rate. The tumours were 1 cm in diameter on treatment. The labelling index after continuous labeling with BrdU was 55.3 (50.8–59.8)% (mean (95% confidence limit)), and reached a plateau at this stage. Therefore, we regarded tumour cells not incorporating BrdU after continuous labeling as non-proliferating Q cells.
Treatment
After the labelling with BrdU, TPZ dissolved in physiological saline was administered at a dose of 224 millimoles/kg (40 mg/kg) continuously for 24 h by subcutaneously implanting mini-osmotic pumps (Durect Corporation, Cupertino, CA) containing TPZ dissolved in physiological saline. During the 24 h of continuous subcutaneous infusion of TPZ, or without TPZ administration, whole body irradiation to the tumour-bearing mice was carried out using γ-rays. In addition, MTH (40°C, 60 min) was also performed during the continuous administration of TPZ immediately before γ-ray irradiation.
γ-Ray irradiation was performed with a cobalt-60 γ-ray irradiator at a dose rate of 2.75 Gy/min as conventionally employed high dose-rate irradiation (HDRI) with tumour-bearing mice held in a specially constructed device with the tail firmly fixed with an adhesive tape. Reduced dose-rate irradiation (RDRI) was performed at a dose rate of 0.039 Gy/min by maintaining an appropriate distance between the cobalt-60 radiation source and the irradiated tumour-bearing mouse fixed within the specially constructed device. This dose rate is almost the minimum value available at our institute without contaminating secondary γ-rays. According to ICRU Report 58, high, middle, and low dose rates are defined as more than 0.2 Gy/min (= 12 Gy/h), 0.033 ∼ 0.2 Gy/min (= 2 ∼ 12 Gy/h), and less than 0.033 Gy/min (= 2 Gy/h), respectively Citation[10]. Thus, if the definition is applied, the employed irradiation corresponds to middle dose rate.
Concerning MTH, the tumors grown in the left hind legs of mice were heated at 40°C for 30 min by immersing the tumour-bearing foot in a water bath. The mouse was held in a specially constructed device with the tail and right leg firmly fixed with an adhesive tape. The left tumour-bearing leg was pulled down by a special sinker (approximately 45 g) which was affixed to the skin of the toe with Superglue (Arone-arufa, Konishi Co., Osaka, Japan). The mice were then placed on a circulating water bath maintained at the desired temperature. The mice were air-cooled during the heat treatment Citation[11]. Temperatures at the tumour center equilibrated within 3 to 4 min after immersion in the water bath and remained 0.2–0.3°C below the bath's temperature. The water bath's temperature was maintained at 0.3°C above the desired tumour temperature.
Each irradiation group also included mice that were not pretreated with BrdU.
Immunofluorescence staining of BrdU-labeled cells and micronucleus (MN) assay
Immediately after irradiation, tumours were excised from the mice given BrdU, minced, and trypsinized. Tumour cell suspensions thus obtained were incubated for 72 h in tissue culture dishes containing complete medium and 1.0 µg/ml of cytochalasin-B to inhibit cytokinesis while allowing nuclear division, and the cultures were then trypsinized and cell suspensions were fixed. After the centrifugation of fixed cell suspensions, the cell pellet was resuspended with cold Carnoy's fixative (ethanol:acetic acid = 3:1 in volume). The suspension was then placed on a glass microscope slide and the sample was dried at room temperature. The slides were treated with 2 M hydrochloric acid for 60 min at room temperature to dissociate the histones and partially denature the DNA. The slides were then immersed in borax-borate buffer (pH 8.5) to neutralize the acid. BrdU-labelled tumour cells were detected by indirect immunofluorescence staining using monoclonal anti-BrdU antibody (Becton Dickinson, San Jose, CA) and fluorescein isothiocyanate (FITC)-conjugated antimouse IgG antibody (Sigma, St. Louis, MO). To observe the double staining of tumour cells with green-emitting FITC and red-emitting propidium iodide (PI), cells on the slides were treated with PI [2 µg/ml in phosphate-buffered saline (PBS)] and monitored under a fluorescence microscope.
When cell division is disrupted, or the chromosomes are broken or damaged by chemicals or radiation, then the distribution of genetic material between the two daughter nuclei during cell division is affected and pieces or entire chromosomes fail to be included in either of the two daughter nuclei. The genetic material that is not incorporated into a new nucleus forms its ‘micronucleus’. The MN frequency in cells not labeled with BrdU could be examined by counting the micronuclei in the binuclear cells that showed only red fluorescence. The MN frequency was defined as the ratio of the number of micronuclei in the binuclear cells to the total number of binuclear cells observed Citation[2].
The ratios obtained in tumours not pretreated with BrdU indicated the MN frequency at all phases in the total (P + Q) tumour cell population. More than 400 binuclear cells were counted to determine the MN frequency.
Clonogenic cell survival assay
The clonogenic cell survival assay was also performed in the mice given no BrdU using an in vivo-in vitro assay method. Tumours were disaggregated by stirring for 20 min at 37°C in PBS containing 0.05% trypsin and 0.02% ethylenediamine tetraacetic acid. The cell yield was 4.5 (3.4 − 5.6) × 107/g tumour weight. Appropriate numbers of viable tumour cells from the single cell suspension were plated on 60 or 100-mm tissue culture dishes, and, 12 days later, colonies were fixed with ethanol, stained with Giemsa, and counted. For the tumours that received no irradiation, the plating efficiencies for the total tumour cell populations and the MN frequencies for the total and Q cell populations are shown in . Surviving fraction (SF) is the ratio of colonies produced to cells plated, with a correction necessary for plating efficiency Citation[12].
Table I. Plating efficiencies and micronucleus frequencies at 0 Gy.
Three mice were used to assess each set of conditions and each experiment was repeated twice. To examine the differences between pairs of values, Student's t-test was used when variances of the two groups could be assumed to be equal; otherwise the Welch t-test was used. p-Values are from two-sided tests. p-Values <0.05 were considered significant.
Results
Overall, at 0 Gy, the MN frequencies for Q cells were significantly larger than those for the total cell poulations (p < 0.05) (). Whether combined with MTH or not, TPZ induced a significantly lower plating efficiency and significantly higher MN frequency in both the total and Q cell populations than each treatment without TPZ (P < 0.05). The combination with MTH caused a slightly lower plating efficiency and slightly higher MN frequency in both the populations than each treatment without MTH.
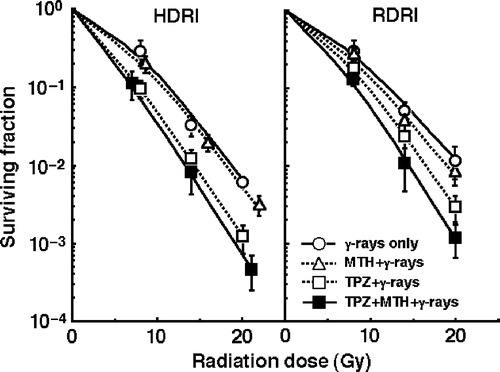
shows the clonogenic cell survival curves after γ-ray irradiation with HDRI (left panel) or RDRI (right panel) in combination with TPZ and/or MTH or without TPZ or MTH. As a whole, RDRI showed significantly larger SFs than HDRI under all conditions. Namely, more radiation-induced DNA damages were repaired during long-term RDRI than with HDRI, resulting in more loss of clonogenic tumour cells after HDRI than with RDRI. Further, this increase was effectively suppressed by TPZ, especially in combination with MTH. MTH alone suppressed the increase very slightly.
Figure 1. The clonogenic cell survival curves after γ-ray irradiation with conventional high dose-rate irradiation (HDRI) (left panel) or reduced dose-rate irradiation (RDRI) (right panel) in combination with tirapazamine (TPZ) and/or mild temperature hyperthermia (MTH) or without TPZ or MTH. TPZ was given by continuous subcutaneous administration. Bars represent 95% confidence limits.
and show the normalized MN frequencies after γ-ray irradiation with HDRI (left panel) or RDRI (right panel) in combination with TPZ and/or MTH or without TPZ or MTH in the total () and Q () tumour cell populations. For baseline correction, we used the normalized MN frequency to exclude the effect of the induced MN frequency by TPZ and/or MTH in non-irradiated control tumours. The normalized MN frequency was the MN frequency in the irradiated tumours minus that in the non-irradiated tumours. All MN frequencies were actual values obtained under each set of conditions.
Figure 2. The normalized micronucleus frequencies after γ-ray irradiation with conventional high dose-rate irradiation (HDRI) (left panel) or reduced dose-rate irradiation (RDRI) (right panel) in combination with tirapazamine (TPZ) and/or mild temperature hyperthermia (MTH) or without TPZ or MTH in the total tumour cell population. TPZ was given by continuous subcutaneous administration. Bars represent 95% confidence limits.
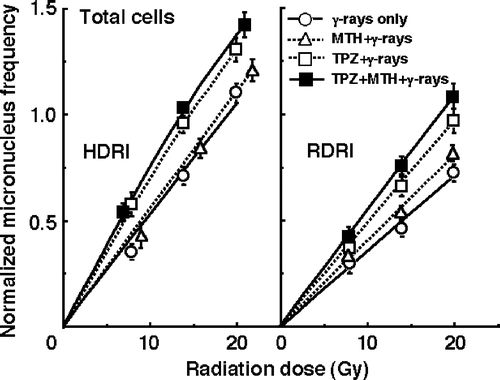
Figure 3. The normalized micronucleus frequencies after γ-ray irradiation with conventional high dose-rate irradiation (HDRI) (left panel) or reduced dose-rate irradiation (RDRI) (right panel) in combination with tirapazamine (TPZ) and/or mild temperature hyperthermia (MTH) or without TPZ or MTH in the quiescent tumour cell population. TPZ was given by continuous subcutaneous administration. Bars represent 95% confidence limits.
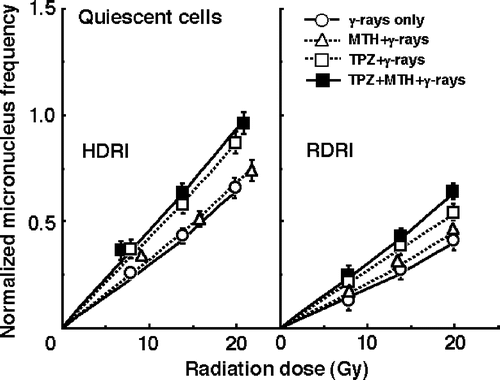
RDRI induced significantly lower normalized MN frequencies than HDRI under all conditions, especially in Q cells. Namely, more radiation-induced DNA damages were repaired during long-term RDRI and in Q cells than with HDRI and in total cells, respectively. Further, this decrease in the normalized MN frequency was effectively suppressed by TPZ, especially in combination with MTH. MTH alone suppressed the decrease very slightly in both total and Q cells.
To evaluate the effect of TPZ and/or MTH on the surviving fractions in the total cell population and the normalized MN frequencies in the total and Q cell populations, an enhancement ratio (ER) was calculated using the data given in through (). The values in Q cells and under RDRI were larger than those in the total cell population and under HDRI, respectively. In both cell populations, under both HDRI and RDRI, the values increased in the following order; with MTH only < with TPZ only < with both TPZ and MTH.
Table II. The effect* of TPZ** and/or MTH*** on each end-point.
To assess the effects of TPZ and/or MTH on the change in the SFs and the normalized MN frequency caused by reducing the dose rate, their effects on the repair in the SF and the normalized MN frequency under RDRI compared with HDRI were calculated using the data given in through (). With or without TPZ and/or MTH treatment, the values in Q cells were higher than those in the total cell population. In both the total and Q cell populations, the values were reduced in the following order; with MTH only > with TPZ only > with both TPZ and MTH. Further, the extent of this reduction with the TPZ and/or MTH treatment was slightly more remarkable in the Q cells than in the total cell population.
Table III. The effect* of TPZ** and/or MTH# on the repair from radiation-induced damage.
Discussion
In contrast to normal tissues, most solid tumours have regions of low oxygen (hypoxia), low pH, and low levels of glucose Citation[5]. So far, we have already shown the usefulness of TPZ in combination with γ-ray (low LET) irradiation with a conventionally employed high-dose rate, in terms of the cytocidal effects on both the total and Q cell populations in solid tumours by employing our method for selectively detecting the response of intratumour Q cell populations in vivo Citation[2]. Meanwhile, acute hypoxic areas can develop throughout a tumour depending on the causative factors such as vessel plugging by blood cells or circulating tumour cells, the collapse of vessels in regions of high tumour interstitial pressure, or spontaneous vasomotor activity in normal tissue vessels incorporated into the tumour which subsequently affects flow in downstream tumour microvessels, and can occur sporadically in large areas of a solid tumour. Thus, when continuously administered, TPZ can produce DNA breaks in larger areas of solid tumours where acute hypoxia has occurred during a long-term administration period than a single administration Citation[6]. Further, MTH was shown to enhance the response, especially of Q cells, to TPZ in spite of no apparent toxicity by MTH itself probably because of a higher dose distribution of TPZ in intermediately hypoxic areas derived mainly from chronic hypoxia through the increase in tumor blood flow induced by MTH Citation[8]. Therefore, through the current study, we tried to reveal the effectiveness of the combined use of continuously administered TPZ and/or MTH with a reduced dose-rate long-term γ-ray irradiation during which radiation-induced DNA damages are partially repaired, compared with a conventionally employed high dose-rate γ-ray irradiation.
It has been thought that decreasing the dose rate reduces late effects in normal tissue much more than it decreases tumour control. Thus, the ‘therapeutic ratio’ increases as the dose rate decreases, because the therapeutic ratio is equal to the ratio of tumour control to normal tissue complications. Further, the difference between early and late effects for low dose-rate radiotherapy, as well as itself improving the therapeutic ratio, allows the delivery of a complete treatment in a short time, allowing the effects of tumour repopulation to be minimized. In other words, decreasing the dose rate increases the therapeutic ratio, limited only by tumour cell repopulation Citation[4]. This is the primary rationale for low dose-rate radiotherapy using low LET radiation. However, this rationale does not take into account the response of Q tumour cells. The current study showed that lowering the dose rate decreases the effect on Q cells more markedly than it reduces the effect on the total cell population (). Therefore, considering the Q-cell response, it follows that the therapeutic ratio does not always increase when the dose rate is reduced. Thus, there is a need to overcome this disadvantageous phenomenon.
In this study, the enhancing effect of TPZ with or without MTH was more remarkable in Q cells and under RDRI than in the total cell population and under HDRI, respectively (). This finding is advantageous for increasing the sensitivity of tumour cells, especially radio-resistant Q-cells, to reduced radiation dose rate long-term γ-ray irradiation. Actually, TPZ suppressed the decrease in the sensitivity of tumour cells by reducing the radiation dose rate to a considerable extent, in both the total and Q cell populations (). When administered continuously, TPZ can produce DNA breaks in larger areas of solid tumours where not only chronic hypoxia exists but also acute hypoxia has occurred during long-term administration than a single administration which mainly kills chronically hypoxic tumour cells Citation[6]. According to our previous report Citation[3], in the SCC VII tumour, the hypoxic fraction (HF) of the total cell population includes a large acutely HF and small chronically HF. In contrast, the HF of Q cells is made up of a large chronically HF and small acutely HF. Consequently, when MTH was combined with TPZ before irradiation, more TPZ could be distributed in intermediately hypoxic areas derived mainly from chronic hypoxia in solid tumours through the increase in tumor blood flow induced by MTH, resulting in greater enhancing effects preferably on chronic hypoxia-rich Q cell populations. Anyway, the use of TPZ, especially in combination with MTH, together with RDRI, is very useful for suppressing the decrease in the sensitivity of tumour cells, especially of radio-resistant Q tumour cells, by decreasing the irradiation dose rate.
MTH has been reported to provide a number of other clinical advantages, including immunostimulatory affects against tumours Citation[13]. Since the elevated temperature inhibits repair of sublethal damage by low dose-rate irradiation (LDRI), MTH is more beneficial when combined with LDRI than with HDRI Citation[14]. In an in vivo study, fractionated MTH given concomitantly with LDRI significantly delayed tumour growth without increasing the adverse effects Citation[15]. Thus, we also have a plan to detect the response of Q-cell population to MTH in simultaneous combination with RDRI in the near future.
Solid tumours, especially human tumours, are thought to contain a high proportion of Q cells Citation[16]. The presence of these cells is probably due, in part, to hypoxia and the depletion of nutrition in the tumour core, and this is another consequence of poor vascular supply Citation[16]. This might promote the formation of micronuclei in Q tumour cells (). We have reported that Q cell populations have less sensitivity, greater PLDR capacities, and a larger HF than P cell populations in solid tumours in vivo Citation[2]. Actually, also in this study, Q cells showed significantly less sensitivity than the total cell population under all irradiation conditions (p < 0.05), although the difference in the sensitivity between total and Q cell populations was slightly reduced with TPZ or both TPZ and MTH (). This means that more Q cells survive after radiation therapy than P cells. Consequently, the control of Q cells has a great impact on the outcome of radiation therapy. Meanwhile, in pre-clinical and clinical studies, a severe neuropathy (both peripheral and central) involving muscle cramping and hearing loss was the dose-limiting toxicity and precluded safe and efficacious use in combination with radiotherapy Citation[17]. In terms of the tumour cell-killing effect as a whole, including intratumour Q cell control, following elucidation of normal tissue toxicity, the use of both TPZ and MTH which are more toxic to hypoxia-rich radio-resistant Q cells than the total cell population, can be regarded as a promising modality as a combined treatment with conventional low LET radiation.
Table IV. Dose ratios* for quiescent tumour cells relative to the total tumour cell population.
Acknowledgements
This study was supported, in part, by a Grant-in-aid for Scientific Research (C) (18591380) from the Japan Society for the Promotion of Science.
References
- Vaupel P, Kelleher DK, Hoeckel M. Oxygenation status of malignant tumors: Pathogenesis of hypoxia and significance for tumor therapy. Semin Oncol 2001; 28(Suppl 8)29–35
- Masunaga S, Ono K. Significance of the response of quiescent cell populations within solid tumors in cancer therapy. J Radiat Res 2002; 43: 11–25
- Masunaga S, Ono K, Abe M. The detection and modification of the hypoxic fraction in quiescent cell populations in murine solid tumors. Br J Radiol 1993; 66: 918–926
- Hall EL. Repair of radiation damage and the dose-rate effect. Radiobiology for the radiologist6th, EL Hall. Lippincott Williams & Wilkins, Philadelphia 2006; 60–84
- Masunaga S, Ono K, Hori H. Exploiting tumor hypoxia in the treatment of solid tumors. Jpn J Hyperthermic Oncol 2001; 17: 13–22
- Masunaga S, Nagasawa H, Uto Y, Hori H, Suzuki M, Nagata K, Kinashi Y, Ono K. The usefulness of continuous administration of hypoxic cytotoxin combined with mild temperature hyperthermia, with reference to effects on quiescent tumor cell populations. Int J Hyperthermia 2005; 21: 305–318
- Griffin RJ, Okajima K, Ogawa A, Song CW. Radiosensitization of two murine tumours with mild temperature hyperthermia and carbogen breathing. Int J Radiat Biol 1999; 75: 1299–1306
- Masunaga S, Ono K, Hori H, Kinashi Y, Suzuki M, Takagaki M, Kasai S, Nagasawa H, Uto Y. Modification of tirapazamine-induced cytotoxicity in combination with mild Hyperthermia and/or nicotinamide: Reference to effect on quiescent tumour cells. Int J Hyperthermia 1999; 15: 7–16
- Masunaga S, Ono K, Suzuki M, Nishimura Y, Kinashi Y, Takagaki M, Hori H, Nagasawa H, Tsuchiya I, Sadahiro S, et al. Radiosensitization effect by combination with paclitaxel in vivo including the effect on intratumor quiescent cells. Int J Radiat Oncol Biol Phys 2001; 50: 1063–1072
- International Commision on Radiation Units and Measurements. ICRU Report 58, Dose and volume specification for reporting interstitial therapy. ICRU. Bethesda, (Maryland) 1997
- Nishimura Y, Ono K, Hiraoka M, Masunaga S, Jo S, Shibamoto Y, Sasai K, Abe M, Iga K, Ogawa Y. Treatment of murine SCC VII tumors with localized hyperthermia and temperature-sensitive liposomes containing cisplatin. Radiat Res 1990; 122: 161–167
- Hall EL. Cell survival curves. Radiobiology for the radiologist6th, EL Hall. Lippincott Williams & Wilkins, Philadelphia 2006; 30–46
- Milani V, Noessner E, Ghose S, Kuppner M, Ahrens B, Scharner A, Gastpar R, Issels RD. Heat shock protein 70: Role in antigen presentation and immune stimulation. Int J Hyperthermia 2002; 18: 563–575
- Wang Z, Armour EP, Corry PM, Martinez A. Elimination of dose-rate effects by mild hyperthermia. Int J Radiat Oncol Biol Phys 1992; 24: 965–973
- Ryu S, Brown SL, Kolozsvary A, Kim JH. Increased tumour response of a murine fibrosarcoma to low temperature hyperthermia and low dose rate brachytherapy. Int J Hyperthermia 1996; 12: 635–643
- Vaupel P. Tumor microenvironmental physiology and its implications for radiation oncology. Semin Radiat Oncol 2004; 14: 197–275
- Rosenberg A, Knox S. Radiation sensitization with redox modulators: A promising approach. Int J Radiat Oncol Biol Phys 2006; 64: 343–354