Abstract
In recent years high intensity focused ultrasound (HIFU) has received increasing interest as a non-invasive modality for the treatment of tumours of solid organs. Surgeons continue their quest to find the optimal technique whereby a diseased organ can be treated with a minimum of damage to the patient, while providing a comprehensive treatment to produce either cure or resolution of symptoms. Two of the areas in which HIFU is beginning to establish itself as a real therapeutic alternative, are in the treatment of abdominal and gynaecological disease. In this paper, we will review the literature available regarding the use of HIFU in the treatment of various organs: liver, kidney, pancreas, bladder, uterus and vulva.
Introduction
In recent years high intensity focused ultrasound (HIFU) has received increasing interest as a non-invasive modality for the treatment of tumours of solid organs. Doctors continue to search for the optimal technique to treat a diseased organ with a minimum of damage to the patient, while providing a comprehensive treatment aiming at either cure or resolution of symptoms. New technologies aimed at minimising the harm done to the patient, while optimising the clinician's ability to perform an effective procedure, are constantly being introduced into the therapeutic field. HIFU is one of these and could offer the ultimate answer, namely non-invasive tumour treatment.
HIFU as a concept is not new, but ever improving imaging modalities have meant that in the last decade the technology has advanced in leaps and bounds. With this rapid development, there have been a number of approaches to delivery, monitoring and technique. Many different devices exist, although only a handful have achieved sufficient levels of evidence to move from prototype to commercial product Citation[1–4]. Aiming at complete non-invasiveness, extracorporeal machines have been developed allowing delivery of the focused ultrasound from outside the body Citation[5–7]. In reality, anatomical and physical considerations mean that some organs cannot be approached extracorporeally, thus trans-rectal Citation[8], Citation[9], endoscopic Citation[10] and laparoscopic Citation[11] probes are increasingly providing alternative ways to deliver the energy. Both ultrasound guided Citation[12], Citation[13] and magnetic resonance guided Citation[14] devices are in widespread use, and it is becoming clear that both modalities will have their place in the management of a variety of diseases.
Two of the areas in which HIFU is beginning to establish itself as a real therapeutic alternative, are in the treatment of abdominal and gynaecological disease. In this paper, we will review the published literature relating to the various organs in these areas that can be treated using HIFU.
Liver
Hepatocellular carcinoma (HCC) is amongst the most common malignancies worldwide, and hepatic metastases are the most common cause of death in cancer patients. Currently surgery remains the only real hope for cure, although even after surgical resection of hepatic metastases survival rates are only 25–30% at 5 years. As a result, a non-invasive alternative to surgery such as HIFU would be of considerable benefit, and it is of no surprise that interest continues to develop in this field.
Animal liver has provided a useful experimental model for assessment of HIFU devices. Small animal models Citation[15], Citation[16] were used to establish the ability of HIFU to create lesions, while the thresholds for liver tissue destruction at varying exposure parameters were established in the 70s and 80s Citation[17]. Normal tissue investigations continued during the 1990s in the form of further small animal experiments, with the histological impact of HIFU Citation[18], the relationship between tissue depth and the required intensity levels Citation[19], and the effects of blood perfusion on tissue ablation being studied Citation[20]. At the same time a number of tumour models have been used to predict the effects of HIFU on HCC or discrete liver metastases in humans. HIFU has been seen to cause significant inhibition of growth of Morris hepatoma 3924A in rats Citation[21], Citation[22], and to destroy a large proportion of VX2 liver tumours in rabbits Citation[23], Citation[24], although less impressive results were seen in advanced disease states Citation[25]. Hooded Sarcoma N (HSN) fibrosarcoma has been used as a tumour model in rats with some success Citation[26] and Ter Haar et al. published a single case report of the successful HIFU treatment of a 4 cm × 2.5 cm × 2.5 cm hepatic lesion caused by metastatic melanoma in a cat Citation[27].
Equipments of varying designs have been used in large animal studies in a number of centres. Bush et al. Citation[28] used pig liver specimens to evaluate the acoustic properties of HIFU lesions, while Vaughan et al. Citation[29] performed a feasibility study in Large White pigs in vivo. Here it was demonstrated that ablative lesions could be accurately created either singly, or in arrays. Arefiev et al. Citation[30] in Lyon used an isolated, perfused porcine liver to study the feasibility of deep-focused tissue ablation in a controlled environment. In Chongqing, the study group used the livers of miniswine to demonstrate the accuracy of placement of lesions, and the relationships between intensity, time and lesion size, as well as to investigate the histological progression after treatment Citation[31–33].
There is an increasing body of work describing HIFU in the treatment of both primary HCC and secondary liver metastases in human clinical trials. In the early 1990s, Vallancien et al. Citation[34] treated two patients with solitary liver metastases prior to surgical resection, but in one there was no visible effect, and in the other there was extensive tissue laceration and patchy necrosis.
By the start of the millennium, the Chongqing group started to publish their clinical work. Wu et al. Citation[35] using the extra-corporeal Model JC HIFU device (HAIFU™ Technology Company, China to treat 68 patients with liver malignancies. They reported 30 cases where surgical excision followed HIFU ablation, in all cases the tumour was totally ablated Citation[12]. The group went onto report a series of 474 patients with HCC treated using the HAIFU device, although HIFU was often used in combination with other treatments such as trans-arterial chemo-embolisation (TACE) Citation[2], Citation[36].
HIFU ablation has also been used for palliation in patients with advanced-stage liver cancer. Li et al. Citation[37] reported a series of 100 patients with liver cancer who were treated with HIFU, including 62 patients with primary liver cancer and 38 with metastatic liver cancer. Following treatment, symptoms, such as pain and lethargy, were relieved in 87% of the patients.
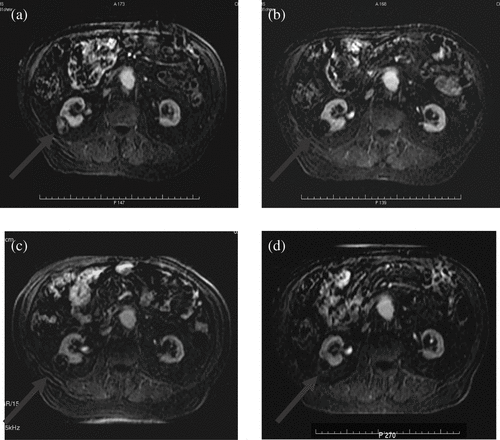
Illing et al. Citation[38] have reported interim results of a prospective, non-randomised clinical trial that is currently under way in Oxford, UK. In this trial they have again used the Model JC HIFU device with the aim of assessing its use in a Western population. Using either radiological images such as MRI and contrast ultrasound, or histological examination, they are evaluating the safety and effectiveness of HIFU in the treatment of metastatic liver cancer. These interim results show the outcome of 20 assessable patients and reveal that morbidity is low when compared with open or minimally invasive techniques. All patients who completed one treatment session with HIFU had some evidence of discrete ablation on follow-up ().
Figure 1. T1-weighted images, 1 min post IV Gadolinium. A patient with metastatic liver disease treated as part of the Oxford trial.
MRI-guided HIFU has generally been reserved for the treatment of uterine fibroids and breast adenomas. However, there is little doubt that this mode of treatment monitoring and delivery will have a role in the treatment of liver tumours. Indeed, at Imperial College, London they have recently started a prospective, non-randomised clinical trial to assess the safety and efficacy of the MRgFUS system (ExAblate 2000, InSightec, Haifa, Israel) in the treatment of liver tumours, they have treated a small number of patients to date with promising results Citation[39], Citation[40].
It is however in the treatment of liver disease that the sole randomised, controlled clinical trial of any clinical HIFU device has been published. This is a seminal paper in the establishment of this technology; it provides good evidence that randomised, controlled clinical trials can be performed with emerging technologies. Wu et al. Citation[41] took the current best practice in the treatment of HCC, namely TACE, and compared a matched group of patients who received TACE and with a group who received TACE and HIFU. Fifty patients were recruited, 26 patients underwent TACE alone and the remaining 24 patients underwent TACE followed within 2-4 weeks by HIFU ablation. The tumour size was 4-14 cm in diameter (mean 10.5 cm). The median survival times for patients were 11.3 months in the HIFU combined TACE group and 4 months in the TACE group (P = 0.0042). The 6-month survival rate of patients was 80.4–85.4% in the combined group and 13.2% in the TACE group (P = 0.0029), and the 1-year survival rate was 42.9% and 0%, respectively.
Kidney
Renal cell cancer (RCC) has the seventh highest incidence of all cancers in adults, accounting for approximately 3% of all solid malignancies; in the UK there are around 6000 new cases each year. At the time of diagnosis, 75% of tumours are organ confined and as such should be amenable to a local treatment. RCC is both chemo- and radio-resistant. As a result, the mainstay of treatment for tumours remains surgery, with 5-year survival rates greater than 80% after resection Citation[42]. Renal cancer surgery (either open or laparoscopic) is associated with both an inpatient stay of 3–6 days and significant risk. Risk-adjusted 30-day morbidity and mortality rates for all available surgical procedures have been shown to be comparable, with mortality rates of up to 2%, and morbidity rates at around 15%. A safer alternative to surgery would be a very attractive option for such patients.
The kidney has also been used as an animal model for HIFU ablation Citation[43–48]. In 1973, Linke et al. Citation[16] were the first to report successful kidney tissue ablation using HIFU. A 2.5 cm diameter piezo-ceramic plate fronted by an acoustic lens driven at 2 MHz was used to ablate rabbit kidney. The test animals were kept for over one year following HIFU exposure allowing assessment of the long term affects of HIFU on the kidney. The treated area of healthy kidney was replaced with a thin fibrous scar on gross histological analysis of the specimens.
Adams et al. Citation[44] used a modified 4MHz endo-rectal probe to treat implanted VX-2 tumours in rabbits’ kidneys as part of a two phase trial. The first phase involved ablation of implanted tumours at an open procedure, while in the second phase the implanted tumours were ablated trans-cutaneously. The kidneys were excised shortly after exposure and assessed histologically for ablation. The authors found areas of discrete renal damage in all nine treated tumours treated in the first phase. In the second phase, seven of nine tumours showed evidence of ablation, however in only two cases was ablation seen within the implanted tumours. Accurate targeting remained difficult.
In 1997, Watkin et al. Citation[47] used a large animal model to assess the feasibility of non-invasive renal tumour ablation. A 1.69 MHz extracorporeal HIFU transducer of 150 mm focal length was used to evaluate the time/exposure thresholds for kidney damage in ex vivo pig kidney. Eighteen porcine kidneys were then treated in vivo at a depth of 40 mm from the skin surface, with acute damage detected in thirteen (67%). The lesions appeared well circumscribed with a pale central area surrounded by a haemorrhagic rim.
Roberts et al. Citation[49] have recently performed a series of trans-cutaneous ablations in the 10 normal rabbit kidneys. This group has suggested that the non-thermal mechanical effects of ultrasound, i.e. cavitation, can be used to progressively homogenise tissue in controlled fashion with predictable results. To do this, they developed a focused annular array ultrasound system that delivered high intensity, short pulses of ultrasound to a targeted volume. They found that lesions created with 10 to 100 pulses produced scattered areas of damage characterised by focal haemorrhage and small areas of cellular injury in the targeted volume. While, lesions created with up to 1000 or 10 000 pulses demonstrated complete destruction of the targeted volume.
HIFU ablation of renal tumours in humans remains in the early stages of clinical trials. In the early 1990s, Vallancien et al. Citation[50] reported the first clinical feasibility study. They exposed eight patients to extracorporeal HIFU. They reported evidence of ablation in the treated areas following excision of the kidney, but encountered a high rate of skin burns with 10% of patients suffering this complication.
In a case report, Kohrmann et al. Citation[7] reported treating a patient with three renal tumours using a hand-held HIFU device. Two of the tumours reduced in size following ablation, however one remained unaffected. They suggested that the interposition of the ribs might have prevented successful ablation in the third tumour. Susani et al included two patients with renal tumours in a phase I trial Citation[51]. They claim accurate placement of lesions using the Sonablate device, but detail is sparse.
Marberger et al. Citation[52] reported a series of sixteen patients who had renal tumours treated with HIFU. In 14 patients a 10 mm3 volume of renal tumour was treated with HIFU and this was followed by immediate surgical resection of the kidney. In nine patients, areas of acute tissue necrosis were seen, although the lesions only measured between 15 and 35% of the original targeted volume. Two patients were treated with curative intent; however, both had incomplete ablation with residual disease visible on follow-up magnetic resonance imaging (MRI).
The Chongqing group has also looked into kidney ablation. Wu et al. Citation[53] have described a series of thirteen patients with renal tumours who have received HIFU treatment. They only comment on the ten patients who were treated with palliative intent, the three receiving HIFU with curative intent were not analysed. They showed that nine out of ten patients described a reduction in tumour related pain, while haematuria resolved in seven out of eight cases.
Hacker et al. Citation[54] described no major side effects following ablation of tissue in 43 kidneys, porcine and human, using an experimental handheld extracorporeal technology. However, technical success was mixed and the authors concluded that further work was required in the dosage and application of this system.
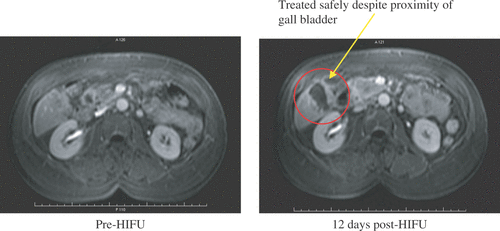
Illing et al. Citation[38] have published interim results of a prospective, non-randomised clinical trial to evaluate the safety and effectiveness of HIFU in the treatment of small kidney tumours currently underway in Oxford, UK (). They are looking at the histological outcome in resected tumours in one arm of the trial, while in the second arm they are following the ablated tumours up with contrast-enhanced MRI. Numbers published were low, but evidence of ablation was seen in 5 out of 10 patients treated with extracorporeal HIFU ().
Pancreas
Pancreatic carcinoma is the fourth leading cause of cancer-related deaths in the United States and the Western world. In 2002, 30 300 new cases were diagnosed for the United Sates, with 29 700 associated deaths Citation[55]. The late onset of symptoms means that nearly 80% of patients have unresectable disease on diagnosis. There remains no effective modality for the treatment of patients with locally advanced disease, chemo-radiation is the current best practice Citation[56] although a great deal of debate remains on this issue. The median survival time is 6–10 months for patients with locally advanced pancreatic cancer and 3–6 months for patients with metastatic disease.
Again, Wu et al. Citation[57] have published the only series describing the treatment of patients with pancreatic cancer. Eight patients with advanced pancreatic cancer were treated with ultrasound-guided HIFU for palliation. The tumour size ranged from 4.5 to 8 cm in diameter (mean 5.89 cm), and with most located in the pancreatic body and tail. In this small series, the treatment proved to be safe and the results were impressive. Following HIFU, pre-existing severe back pain of presumed malignant origin disappeared in every patient. Follow-up imaging with MRI, showed reduced or absent tumour blood supply in the treated regions and there was significant shrinkage of the ablated tumours. Symptomatic relief was universal, imaging showed a significant reduction in ablated tumour size and there appeared to be a degree of survival benefit in all the treated patients.
There are high levels of interest in HIFU for the treatment of pancreatic cancer with many centres undertaking trials at this time. At the recent meeting of the International Symposium of Therapeutic Ultrasound, there were three papers presented on this topic. These are outlined as follows:
Hwang et al. Citation[58] have used the extracorporeal YDME FEP-BY02 Tumour Therapy System to develop a large animal model to assess the feasibility, safety and efficacy of extracorporeal HIFU therapy with targeted treatment of the pancreas in preparation for human clinical trials in patients with advanced pancreatic cancer. They concluded that the common swine appears to be an appropriate model to evaluate the feasibility, safety, and efficacy of performing in vivo HIFU therapy directed at the pancreas.
Xiong et al. Citation[59] used a large animal model to investigate the feasibility and safety of HIFU in treating the pancreases, and then went on to evaluate the efficacy and feasibility of HIFU in the clinical treatment of pancreas cancer in humans. HIFU treatments were performed in 12 mongrel pig pancreases in vivo. Following the animal studies, 62 patients with advanced pancreas cancer were treated with HIFU. In the animal studies, coagulative necrosis was identified in all the cases; however, where low power HIFU had been used microstructure damage and apoptosis were only identified on electron microscopy. In the clinical treatment pain was relieved in 87% of those treated. The median survival was 11.2 months for stages II and III disease and 5.6 months for those with metastatic disease.
Choi et al. Citation[60] treated seven consecutive patients with unresectable pancreatic cancer from January 2006 to May 2006 using the JC Model. Three patients had complete necrosis of the gland on follow-up MRI, while all the patients had improved pain scores and performance status. One patient received grade-3 burns, and no deaths were reported.
Bladder
Bladder cancer is the fifth most common malignancy in Europe and the fourth most common malignancy in the United States. It affects one in 4000 people and accounts for 5% of all diagnosed cancers Citation[61]. Currently the superficial form of the disease is managed by endoscopic resection of the tumour, often followed by the instillation into the bladder of chemotherapeutic agents. Due to the tendency of bladder cancer to recur repeated cystoscopies and resections are often required. The management of superficial bladder cancer is already minimally invasive; however, HIFU could offer an alternative.
Chartier-Kastler et al. Citation[62] showed in lab-based studies on cell cultures that HIFU could kill tumour cells, although the exact mechanisms of cell death were felt to be different from those in vivo. In 1994, Vaughan et al. Citation[29] first used a pig model to demonstrate the technical feasibility of bladder tumour ablation. HIFU lesions were created in the bladder walls of five pigs. Watkin et al. Citation[63] went onto to expand this study in 25 pigs, again proving feasibility with successful lesion creation in their bladder walls.
Vallancien et al. Citation[48] have published the only clinical data on bladder tumour ablation. They first performed a feasibility study on five patients with superficial bladder tumours. They cystoscoped each patient during the same episode, noting the disappearance of tumour in two cases, and coagulative necrosis in the remainder. They went on to carry out a phase II trial on 25 patients with previous low grade, superficial bladder tumours, and a single papillary recurrence, which of these had to be visible on ultrasound Citation[6]. Two-thirds were tumour free at one year, and no invasion or metastasis was observed with follow-up of 3–21 months.
However, these preliminary studies although demonstrating technical feasibility showed the technique to be time consuming and provided no improvement on current techniques for superficial bladder tumour treatment. As such HIFU for bladder cancer has not been pursued any further at this time.
Uterine fibroids
Uterine fibroids are the most common pelvic tumours in women and are a significant cause of morbidity for women of reproductive age. Although the majority are asymptomatic, approximately 25% are associated with pelvic pain, menorrhagia, dysmenorrhoea, dyspareunia, pressure-related symptoms such as pelvic fullness, urinary frequency and infertility. Larger leiomyomas are more likely to be symptomatic. It is thought that a reduction in leiomyoma size is associated with an improvement in symptoms Citation[64]. HIFU is at the forefront of the management of this disease, offering a non-invasive option for treatment.
Pre-clinical work looking at the treatment of uterine fibroids was published by Vaezy et al. in 2000 Citation[65]. They used 60 female athymic nude mice inoculated with 3 × 106 to 5 × 106 ELT-3 cells, a uterine fibroid tumour cell line, to demonstrate the ability of HIFU to ablate induced uterine fibroid tumours. A single HIFU treatment resulted in an average reduction in tumour volume of 91% within 1 month of the treatment. Histological analysis of tumours treated with HIFU showed coagulation necrosis and nuclear fragmentation of tumour cells.
Keshavarzi et al. Citation[66] continued this when they applied intra-operative HIFU to uterine fibroid tumours in rats. Thirty-five tumours in 27 Eker rats that had spontaneous in situ uterine fibroids were randomly assigned into two groups receiving HIFU (n = 29) or sham (n = 6) treatments. More than half of the tumours in the HIFU treatment group showed significant tumour volume reduction. The average tumour volume in the sham treatment group increased 40-fold. Gross and histological analysis showed coagulative necrosis of tumour cells in the HIFU treatment group.
Köhrmann et al. Citation[67] were the first to report ablation of uterine fibroids in human clinical trials. They used an extracorporeal transducer to treat 15 patients in phase I study, and three patients in a subsequent phase II trial, but follow-up data is absent and as such few conclusions can be made.
Zhou et al. Citation[68] used ultrasound-guided HIFU device to treat sixteen patients with uterine fibroids. Clinical symptoms, such as menorrhagia and pelvic pain, were reduced or completely alleviated in twelve patients after HIFU treatment. Follow-up imaging showed a reduction of blood perfusion and subsequent significant tumour shrinkage within the treated tumour in 14 of 16 patients. However, partial damage of the sciatic nerve was observed in four patients, though it recovered completely during follow-up period including both normal sensation and motion of leg and buttock.
Stewart et al. Citation[69] reported the first series of patients treated with MRgFUS. They treated 55 women with uterine fibroids with HIFU. Overall there was comparable efficacy to embolisation, and there were minimal complications. Tempany et al. Citation[70] continued this work looking at the feasibility and safety. They concluded that MR imaging-guided focused ultrasound causes thermo-coagulation and necrosis in uterine leiomyomas and is feasible and safe, without serious consequences. This study also looked closely at the role of thermometry, and they stated that MR thermometry was successful in all sonications and cases.
Following the earlier safety and feasibility studies described Citation[69], Citation[70], Hindley et al. Citation[71] have gone on to report a phase III efficacy trial. This series of 108 women with uterine fibroids, treated using MR-guided HIFU, showed a significant symptomatic improvement in 79.3% patients. No severe complications were recorded after HIFU treatment. Of note, the only published case report of full thickness skin burns is described in patient receiving MR-guided HIFU for uterine fibroids Citation[72]. However, several other series have recorded grade-3 burns as a complication.
Stewart et al. Citation[73] reported another series of 109 women who under went MR-guided HIFU ablation of uterine leiomyomas. A multicentre clinical trial, this study has recruited pre-menopausal women with symptomatic uterine leiomyomas. Using a validated questionnaire, in 71% of the women there was targeted symptom reduction at 6 months, and 51% reached this at 12 months. The authors concluded that this treatment results in short-term symptom reduction for women with symptomatic uterine leiomyomas with an excellent safety profile.
Vulva and cervix
Vulvar and cervical dystrophies are one of the most common groups of chronic skin diseases in women Citation[74]. Local approaches, such as hormone and herbal medications and laser and microwave exposures, are still the fundamental treatment options but have a high recurrence rate Citation[75–77]. The excision of a focal region or simple vulvectomy is effective for a period after the procedure, but the recurrence rate is high (50%), and the vulvar scar shrinks to seriously affect the sexual life of the patient Citation[78]. Once again, a non-invasive but effective treatment that HIFU could offer may revolutionise the management of this disease.
Model-CZF FU therapeutic device (Chongqing Haifu Tech Co., Ltd. China) has been used extensively in China to treat superficial gynaecological diseases. Over 2500 cases including chronic cervicitis, lichen sclerosis, and vulva condyloma acuminatum have been treated Citation[79]. The focused ultrasound therapy transducer uses frequencies ranging from 5 to 8 MHz and the transducer is 12 mm in diameter. The applicator directly contacts the skin using water as the coupling medium.
The only published data on the Model-CZF FU therapeutic device is by Li et al. Citation[80]. They describe a prospective randomised phase II study in which a total of 76 patients (45 with squamous hyperplasia and 31 with lichen sclerosis) were treated with focused ultrasound therapy from 1999 to 2002. Before and after the treatment, the therapeutic responses were evaluated based on changes in clinical symptoms and signs such as vulvar pruritis, a burning or stimulating sensation, skin coarsening, skin depigmentation, leukoplakia, and atrophy of the nymphae or clitoris. After the ultrasound treatment, clinical symptoms were dramatically improved with a total response rate of 95%. They reported that after one month, symptoms of vulvar pruritis had improved and that the elasticity of the skin and mucosa had become normal and looked pink and pigmented. Three to 6 months after treatment, the atrophied labia minora started to grow and pigmentation of the skin became normal. In the 2-year follow-up, 49 of 76 (65%) cases were cured; twenty- three improved and in only six cases did symptoms persist. This approach appears to be a new promising treatment method, although further studies are still needed.
Conclusion
HIFU offers a treatment option for several abdominal and gynaecological diseases. There are many centres around the world performing vital research and clinical trials that may bring HIFU into the mainstream of medical management. However, more mature evidence is needed; the medical community requires the proof that this technology is not only safe but that it can provide a long lasting and complete treatment for both benign and malignant disease.
In the light of this, the technology continues to develop. The optimum imaging modality is much debated Citation[81] and many aspects of dosimetry need further research to facilitate the more effective application of HIFU Citation[82], Citation[83]. In the laboratory, the systemic and local effects of HIFU have already shown great promise, and in terms of clinical technique there is still much work to be done in verifying current devices Citation[84].
There is clearly a place for HIFU in the management of abdominal and gynaecological disease. These common and serious problems affect many thousands of people every year and if HIFU can offer an option to even a small proportion of these patients then it is vital to continue pushing the technology forward. HIFU provides a non-invasive therapeutic option that once perfected will add a useful extra string to the clinicians bow.
References
- Gianfelice D, Khiat A, Amara M, Belblidia A, Boulanger Y. MR imaging-guided focused US ablation of breast cancer: Histopathologic assessment of effectiveness—initial experience. Radiology 2003; 227: 849–855
- Wu F, Wang ZB, Chen WZ, Zou JZ, Bai J, Zhu H, Li KQ, Xie FL, Jin CB, Su HB, et al. Extracorporeal focused ultrasound surgery for treatment of human solid carcinomas: Early Chinese clinical experience. Ultrasound Med Biol 2004; 30: 245–260
- Madersbacher S, Kratzik C, Szabo N, Susani M, Vingers L, Marberger M. Tissue ablation in benign prostatic hyperplasia with high-intensity focused ultrasound. Eur Urol 1993; 23(Suppl 1)39–43
- Chaussy C, Thuroff S. High-intensity focused ultrasound in prostate cancer: Results after 3 years. Mol Urol 2000; 4: 179–182
- Visioli AG, Rivens IH, Ter Haar GR, Horwich A, Huddart RA, Moskovic E, Padhani A, Glees J. Preliminary results of a phase I dose escalation clinical trial using focused ultrasound in the treatment of localised tumours. Eur J Ultrasound 1999; 9: 11–18
- Vallancien G, Harouni M, Guillonneau B, Veillon B, Bougaran J. Ablation of superficial bladder tumors with focused extracorporeal pyrotherapy. Urology 1996; 47: 204–207
- Kohrmann KU, Michel MS, Gaa J, Marlinghaus E, Alken P. High intensity focused ultrasound as noninvasive therapy for multilocal renal cell carcinoma: Case study and review of the literature. J Urol 2002; 167: 2397–2403
- Thuroff S, Chaussy C, Vallancien G, Wieland W, Kiel HJ, Le Duc A, Desgrandchamps F, De La Rosette JJ, Gelet A. High-intensity focused ultrasound and localized prostate cancer: Efficacy results from the European multicentric study. J Endourol 2003; 17: 673–677
- Uchida T, Sanghvi NT, Gardner TA, Koch MO, Ishii D, Minei S, Satoh T, Hyodo T, Irie A, Baba S. Transrectal high-intensity focused ultrasound for treatment of patients with stage T1b-2n0m0 localized prostate cancer: A preliminary report. Urology 2002; 59: 394–398, discussion 398–399
- Prat F, Chapelon JY, Arefiev A, Cathignol D, Souchon R, Theilliere Y. High-intensity focused ultrasound transducers suitable for endoscopy: Feasibility study in rabbits. Gastrointest Endosc 1997; 46: 348–351
- Paterson RF, Barret E, Siqueira TM, Jr, Gardner TA, Tavakkoli J, Rao VV, Sanghvi NT, Cheng L, Shalhav AL. Laparoscopic partial kidney ablation with high intensity focused ultrasound. J Urol 2003; 169: 347–351
- Wu F, Chen WZ, Bai J, Zou JZ, Wang ZL, Zhu H, Wang ZB. Pathological changes in human malignant carcinoma treated with high-intensity focused ultrasound. Ultrasound Med Biol 2001; 27: 1099–1106
- Madersbacher S, Kratzik C, Susani M, Marberger M. Tissue ablation in benign prostatic hyperplasia with high intensity focused ultrasound. J Urol 1994; 152(6 Pt 1)1956–1960, discussion 1960–1961
- Hynynen K, Pomeroy O, Smith DN, Huber PE, McDonnold NJ, Kettenbach J, Baum J, Singer S, Jolesz FA. MR imaging-guided focused ultrasound surgery of fibroadenomas in the breast: A feasibility study. Radiology 2001; 219: 176–185
- Taylor KJ, Connolly CC. Differing hepatic lesions caused by the same dose of ultrasound. J Pathol 1969; 98: 291–293
- Linke CA, Carstensen EL, Frizzell LA, Elbadawi A, Fridd CW. Localized tissue destruction by high-intensity focused ultrasound. Arch Surg 1973; 107: 887–891
- Frizzell LA. Threshold dosages for damage to mammalian liver by high intensity focused ultrasound. IEEE Transactions on Ultrasonics, Ferroelectrics and Frequency Control 1988; 35: 578
- Ter Haar G, Robertson D. Tissue destruction with focused ultrasound in vivo. Euro Urol 1993; 23: 8–11
- Sibille A, Prat F, Chapelon JY, Abou el Fadil F, Henry L, Theillere Y, Ponchon T, Cathignol D. Extracorporeal ablation of liver tissue by high-intensity focused ultrasound. Oncology 1993; 50: 375–379
- Chen L, Ter Haar G, Hill CR, Dworkin M, Carnochan P, Young H, Bensted JP. Effect of blood perfusion on the ablation of liver parenchyma with high-intensity focused ultrasound. Phys Med Biol 1993; 38: 1661–1673
- Moore WE, Lopez R-M, Mathews DE, Sheets PW, Etchison MR, Hurwitz AS, Chalian AA, Fry FJ, Vane DW, Grosfeld JL. Evaluation of high-intensity therapeutic ultrasound irradiation in the treatment of experimental hepatoma. J Paediatr Surg 1989; 24: 30–33
- Yang R, Reilly CR, Rescorla FJ, Faught PR, Sanghvi NT, Fry FJ, Franklin TD, Jr, Lumeng L, Grosfeld JL. High-intensity focused ultrasound in the treatment of experimental liver cancer. Arch Surg 1991; 126: 1002–1009, discussion 1009–1010
- Sibille A, Prat F, Chapelon JY, Abou el Fadil F, Henry L, Theilliere Y, Ponchon T, Cathignol D. Characterization of extracorporeal ablation of normal and tumor-bearing liver tissue by high intensity focused ultrasound. Ultrasound Med Biol 1993; 19: 803–813
- Prat F, Centarti M, Sibille A, Abou el Fadil FA, Henry L, Chapelon JY, Cathignol D. Extracorporeal high-intensity focused ultrasound for VX2 liver tumors in the rabbit. Hepatology 1995; 21: 832–836
- Kong F, Wu F, Bai J, et al. Intraoperative high-intensity focused ultrasound in treatment of advanced experimental liver cancer. Chinese J Ultrasonog 1999; 8: 251–254
- Ter Haar G, Rivens I, Chen L, Riddler S. High intensity focused ultrasound for the treatment of rat tumours. Phys Med Biol 1991; 36: 1495–1501
- Ter Haar G, Clarke RL, Vaughan MG, Hill CR. Trackless surgery using focused ultrasound: Technique and case report. Minimal Invasiv Ther 1991; 1: 13–19
- Bush NL, Rivens I, Ter Haar GR, Bamber JC. Acoustic properties of lesions generated with an ultrasound therapy system. Ultrasound Med Biol 1993; 19: 789–801
- Vaughan MG, Ter Haar GR, Hill CR, Clarke RL, Hopewell JW. Minimally invasive cancer surgery using focused ultrasound: A preclinical, normal tissue study. Brit J Radiol 1994; 67: 267–274
- Arefiev A, Prat F, Chapelon JY, Tavakkoli J, Cathignol D. Ultrasound-induced tissue ablation: Studies on isolated, perfused porcine liver. Ultrasound Med Biol 1998; 24: 1033–1043
- Wang ZB, Wu F, Wang ZL, Zhang Z, Zou JZ, Liu C, Liu YG, Cheng G, Du YH, He ZC, et al. Targeted damage effects of high intensity focused ultrasound (HIFU) on liver tissues of Guizhou Province miniswine. Ultrason Sonochem 1997; 4: 181–182
- Bai J, Wu F, Wang Z-B, et al. Localised lesion to normal miniswine liver with high-intensity focused ultrasound and dose-effect relation. Chinese J Ultrasonog 1999; 8: 247–250
- Ruan X, Du Y, Kong F. Pathological regression following localised ablation of liver tissue of 28 miniswine with high-intensity focused ultrasound. Chin J Exp Surg 1999; 16: 263–264
- Vallancien G, Harouni M, Veillon B, Mombet A, Prapotnich D, Brisset JM, Bougaran J. Focused extracorporeal pyrotherapy: Feasibility study in man. J Endourol 1992; 6: 173–181
- Wu F, Chen W, Bai J. Effect of high-intensity focused ultrasound on patients with hepatocellular cancer - preliminary report. Chinese J Ultrasonog 1999; 8: 213–216
- Wu F, Wang Z, Chen W, Zou J. Proceedings of the 2nd International Symposium on Therapeutic Ultrasound. Extracorporeal High-Intensity Focused Ultrasound for treatment of solid carcinomas: Four-year Chinese clinical experience, M Andrew, L Crum, S Vaezy. University of Washington, Seattle 2003; 34–43
- Li CX, Xu GL, Jiang ZY, Li JJ, Luo GY, Shan HB, Zhang R, Li Y. Analysis of clinical effect of high-intensity focused ultrasound on liver cancer. World J Gastroenterol 2004; 10: 2201–2204
- Illing RO, Kennedy JE, Wu F, Ter Haar GR, Protheroe AS, Friend PJ, Gleeson FV, Cranston DW, Phillips RR, Middleton MR. The safety and feasibility of extracorporeal high-intensity focused ultrasound (HIFU) for the treatment of liver and kidney tumours in a Western population. Br J Canc 2005; 93: 890–895
- Salio M, Cerundolo V. Viral immunity: Cross-priming with the help of TLR3. Curr Biol 2005; 15: R336–R339
- Gedroyc WM. Magnetic resonance guided focused ultrasound (MRgFUS) treatment of liver tumours. 6th International Symposium on Therapeutic Ultrasound, CC Coussios. AIP, Oxford 2006
- Wu F, Wang ZB, Chen WZ, Zou JZ, Bai J, Zhu H, Li KQ, Jin CB, Xie FL, Su HB. Advanced hepatocellular carcinoma: Treatment with high-intensity focused ultrasound ablation combined with transcatheter arterial embolization. Radiology 2005; 235: 659–667
- Reddan DN, Raj GV, Polascik TJ. Management of small renal tumors: an overview. Am J Med 2001; 110: 558–562
- Chapelon JY, Margonari J, Theillere Y, Gorry F, Vernier F, Blanc E, Gelet A. Effects of high-energy focused ultrasound on kidney tissue in the rat and the dog. Eur Urol 1992; 22: 147–152
- Adams JB, Moore RG, Anderson JH, Strandberg JD, Marshall FF, Davoussi LR. High-intensity focused ultrasound ablation of rabbit kidney tumors. J Endourol 1996; 10: 71–75
- Frizzell LA, Linke CA, Carstensen EL, Fridd CW. Thresholds for focal ultrasonic lesions in rabbit kidney, liver, and testicle. IEEE Trans Biomed Eng 1977; 24: 393–396
- Tu G, Qiao T-Y, He S, et al. An experimental study on high-intensity focused ultrasound in the treatment of VX2 rabbit and kidney tumours. Chinese J Urol 1999; 20: 456–458
- Watkin NA, Morris SB, Rivens IH, Ter Haar GR. High-intensity focused ultrasound ablation of the kidney in a large animal model. J Endourol 1997; 11: 191–196
- Vallancien G, Chartier-Kastler E, Chopin D, Veillon B, Brisset JM, Andre-Bougaran J. Focussed extracorporeal pyrotherapy: Experimental results. Eur Urol 1991; 20: 211–219
- Roberts WW, Hall TL, Ives K, Wolf JS, Jr, Fowlkes JB, Cain CA. Pulsed cavitational ultrasound: A noninvasive technology for controlled tissue ablation (histotripsy) in the rabbit kidney. J Urol 2006; 175: 734–738
- Vallancien G, Chartier-Kastler E, Harouni M, Chopin D, Bougaran J. Focused extracorporeal pyrotherapy: Experimental study and feasibility in man. Semin Urol 1993; 11: 7–9
- Susani M, Madersbacher S, Kratzik C, Vingers L, Marberger M. Morphology of tissue destruction induced by focused ultrasound. Eur Urol 1993; 23(Suppl 1)34–38
- Marberger M, Schatzl G, Cranston D, Kennedy JE. Extracorporeal ablation of renal tumours with high-intensity focused ultrasound. BJU Int 2005; 95(Suppl 2)52–55
- Wu F, Wang ZB, Chen WZ, Bai J, Zhu H, Qiao TY. Preliminary experience using high intensity focused ultrasound for the treatment of patients with advanced stage renal malignancy. J Urol 2003; 170(6 Pt 1)2237–2240
- Hacker A, Michel MS, Marlinghaus E, Kohrmann KU, Alken P. Extracorporeally induced ablation of renal tissue by high-intensity focused ultrasound. BJU Int 2006; 97: 779–785
- Jemal A, Thomas A, Murray T, Thun M. Cancer statistics, 2002. CA Cancer J Clin 2002; 52: 23–47
- Whittington R, Neuberg D, Tester WJ, Benson AB, 3rd, Haller DG. Protracted intravenous fluorouracil infusion with radiation therapy in the management of localized pancreaticobiliary carcinoma: A phase I Eastern Cooperative Oncology Group Trial. J Clin Oncol 1995; 13: 227–232
- Wu F, Wang ZB, Zhu H, Chen WZ, Zou JZ, Bai J, Li KQ, Jin CB, Xie FL, Su HB. Feasibility of US-guided high-intensity focused ultrasound treatment in patients with advanced pancreatic cancer: Initial experience. Radiology 2005; 236: 1034–1040
- Hwang JH, Yak-Nam W, Starr F, Xinbo F, Marla P, Warren C, Mitchell S. The development of a large animal model to evaluate the feasibility, safety, and efficacy of extracorporeal HIFU therapy of the pancreas. 6th International Symposium on Therapeutic Ultrasound, CC Coussios. AIP, Oxford 2006
- Xiong L, Huang X, Yao S, Yu J, Hwang J, Wang X, Fei X. An experimental study of high intensity focused ultrasound on pig's pancreas and results of early human clinical experience in pancreatic cancer. Coussios CC, editor. Proceedings of 6th International Symposium on Therapeutic Ultrasound. AIP, Oxford 2006
- Choi IB, Hahn ST, Jung SE, Lee JH, Han JY, Cho SH, Choi BO, Kang YN, Bong JK, An SA. High intensity focused ultrasound in patient with pancreas cancer. 6th International Symposium on Therapeutic Ultrasound. Oxford, UK: AIP; 2006, CC Coussios, In press
- Sengupta N, Siddiqui E, Mumtaz FH. Cancers of the bladder. J R Soc Health 2004; 124: 228–229
- Chartier-Kastler E, Chopin D, Vallancien G. The effects of focused extracorporeal pyrotherapy on a human bladder tumor cell line [647 V]. J Urol 1993; 149: 643–647
- Watkin NA, Morris SB, Rivens IH, Woodhouse CR, Ter Haar GR. A feasibility study for the non-invasive treatment of superficial bladder tumours with focused ultrasound. Br J Urol 1996; 78: 715–721
- Pron G, Bennett J, Common A, Wall J, Asch M, Sniderman K. The Ontario Uterine Fibroid Embolization Trial. Part 2. Uterine fibroid reduction and symptom relief after uterine artery embolization for fibroids. Fertil Steril 2003; 79: 120–127
- Vaezy S, Fujimoto VY, Walker C, Martin RW, Chi EY, Crum LA. Treatment of uterine fibroid tumors in a nude mouse model using high-intensity focused ultrasound. Am J Obstet Gynecol 2000; 183: 6–11
- Keshavarzi A, Vaezy S, Noble ML, Paun MK, Fujimoto VY. Treatment of uterine fibroid tumors in an in situ rat model using high-intensity focused ultrasound. Fertil Steril 2003; 80(Suppl 2)761–767
- Kohrmann KU, Michel MS, Fruhauf J, Volzz J, Back W, Gaa J, Alken P. High intensity focused ultrasound for non-invasive tissue ablation in the kidney, prostate and uterus. J Urol 2000; 163(Suppl 4)156
- Zhou Y, Nie Y, Li Y. A preliminary study of high intensity focused ultrasound ablation for patients with uterine leiomyma. Proceedings of 3rd International Symposium on Therapeutic Ultrasound 2003; 157–160
- Stewart EA, Gedroyc WM, Tempany CM, Quade BJ, Inbar Y, Ehrenstein T, Shushan A, Hindley JT, Goldin RD, David M, et al. Focused ultrasound treatment of uterine fibroid tumors: Safety and feasibility of a noninvasive thermoablative technique. Am J Obstet Gynecol 2003; 189: 48–54
- Tempany CM, Stewart EA, McDannold N, Quade BJ, Jolesz FA, Hynynen K. MR imaging-guided focused ultrasound surgery of uterine leiomyomas: A feasibility study. Radiology 2003; 226: 897–905
- Hindley J, Gedroyc WM, Regan L, Stewart E, Tempany C, Hynyen K, McDannold N, Inbar Y, Itzchak Y, Rabinovici J, et al. MRI guidance of focused ultrasound therapy of uterine fibroids: Early results. AJR Am J Roentgenol 2004; 183: 1713–1719
- Leon-Villapalos J, Kaniorou-Larai M, Dziewulski P. Full thickness abdominal burn following magnetic resonance guided focused ultrasound therapy. Burns 2005; 31: 1054–1055
- Stewart EA, Rabinovici J, Tempany CM, Inbar Y, Regan L, Gostout B, Hesley G, Kim HS, Hengst S, Gedroyc WM. Clinical outcomes of focused ultrasound surgery for the treatment of uterine fibroids. Fertil Steril 2006; 85: 22–29
- Ayhan A, Yuce K, Urman B, Gokoz A. Vulvar dystrophies: An evaluation. Aust N Z J Obstet Gynaecol 1989; 29(3 Pt 1)250–252
- Carli P, Bracco G, Taddei G, Sonni L, De Marco A, Maestrini G, Cattaneo A. Vulvar lichen sclerosus. Immunohistologic evaluation before and after therapy. J Reprod Med 1994; 39: 110–114
- Bracco GL, Carli P, Sonni L, Maestrini G, De Marco A, Taddei GL, Cattaneo A. Clinical and histologic effects of topical treatments of vulval lichen sclerosus. A critical evaluation. J Reprod Med 1993; 38: 37–40
- Cattaneo A, Bracco GL, Maestrini G, Carli P, Taddei GL, Colafranceschi M, Marchionni M. Lichen sclerosus and squamous hyperplasia of the vulva. A clinical study of medical treatment. J Reprod Med 1991; 36: 301–305
- Abramov Y, Elchalal U, Abramov D, Goldfarb A, Schenker JG. Surgical treatment of vulvar lichen sclerosus: A review. Obstet Gynecol Surv 1996; 51: 193–199
- Wang ZCLWC. Focused Ultrasound Therapy: Clinical Application in the Treatment of Gynecologic Superficial Diseases. Proceedings of 6th International Symposium of Therapeutic Ultrasound, CC Coussios. AIP, Oxford 2006
- Li C, Bian D, Chen W, Zhao C, Yin N, Wang Z. Focused ultrasound therapy of vulvar dystrophies: A feasibility study. Obstet Gynecol 2004; 104(5 Pt 1)915–921
- Hynynen K, McDannold N. MRI guided and monitored focused ultrasound thermal ablation methods: A review of progress. Int J Hyperthermia 2004; 20: 725–737
- McDannold N, Tempany CM, Fennessy FM, So MJ, Rybicki FJ, Stewart EA, Jolesz FA, Hynynen K. Uterine leiomyomas: MR imaging-based thermometry and thermal dosimetry during focused ultrasound thermal ablation. Radiology 2006; 240: 263–272
- Lafon C, Zderic V, Noble ML, Yuen JC, Kaczkowski PJ, Sapozhnikov OA, Chavrier F, Crum LA, Vaezy S. Gel phantom for use in high-intensity focused ultrasound dosimetry. Ultrasound Med Biol 2005; 31: 1383–1389
- Gray R, Leslie T, Civale J, Kennedy J, Ter Haar G. A Comparison of Real-time Feedback and Tissue Response to Ultrasound-guided High Intensity Focused Ultrasound (HIFU) Ablation using Scanned Track Exposure Regimes. Proceeding 6th International Symposium Therapeutic Ultrasound 2006. AIP, Oxford 2006