Abstract
Purpose: Low-intensity ultrasound irradiation is a potential method for suppressing cancer cell proliferation, inducing apoptosis and delivering specific cytotoxic genes or drugs into tumors topographically in future cancer therapies. However, ultrasound attenuates rapidly in tissue and produces heat. Pulsed ultrasound is frequently used to minimize pain and possible thermal damage to the surrounding normal tissue during therapy, since it results in smaller temperature increases. This study compared three pulsed-ultrasound strategies for destroying cancer cells, measuring their induced temperature increases to determine the optimal pulsing parameters.
Materials and methods: We performed three types of experiment, involving ultrasound with (1) a fixed duty cycle of 50% with variable on- and off-times, (2) a fixed off-time with variable on-times, and (3) a fixed on-time with variable off-times.
Results: The results show that for different types of cultured cells (HeLa, HT-29, Ca9-22 and fibroblast) exposed to ultrasound of the same frequency (1 MHz) and energy, long pulses combined with off-times that are 5–10 times longer (on-/-off-times pairs of 5/25, 25/250, or 250/2500 ms/ms) cause significant cell destruction whilst avoiding temperature increases of more than 1.5°C. Furthermore, the correlation between the temperature increase and the percentage of surviving cells is low.
Conclusions: Pulsed ultrasound with a long on-time and an even longer off-time exerts a high cytotoxic effect but a smaller temperature increase compared with non-pulsed ultrasound. This indicates that the cytotoxic effects observed in the current study were not purely due to the thermal effects of the ultrasound.
Introduction
Using low-intensity ultrasound for cancer therapy and the induction of certain therapeutic bioeffects are rapidly developing fields. Low-intensity ultrasound has been shown to enhance the cytotoxic effect of chemotherapy agents on cancer cells Citation[1], Citation[2], suppress cancer cell proliferation Citation[3], induce apoptosis Citation[4], and facilitate the transport of genetic material across cell membranes for cancer gene therapy Citation[5], Citation[6].
The intensity of ‘low-intensity’ ultrasound is generally 3 W/cm2 or lower Citation[7], which is within the range of clinical therapeutic ultrasound used for physiotherapy. Some of the ultrasound-induced bioeffects may be attributable to the mechanical effects of ultrasound such as cavitation, rather than to thermal effects Citation[8]. Cavitation is typically generated through the activation of small dissolved gas nuclei in an acoustic pressure field. The absorption of ultrasound by tissue generates heat, and so pulsed ultrasound—which results in smaller temperature increases—is frequently used to minimize pain and thermal damage to the surrounding normal tissue whilst not sacrificing the non-thermal bioeffects.
In this study, we examined the cytotoxic effects of the following three pulsed-ultrasound strategies on cultured HeLa cells: (1) a fixed duty cycle of 50% with variable on- and off-times; (2) a fixed off-time with variable on-times; and (3) a fixed on-time with variable off-times. Three other adherent cell types, HT-29, Ca9-22 and fibroblast, were also tested using the third strategy to exam the variation of cell responses to ultrasound. The tested hypothesis was that the non-thermal effect of pulsed ultrasound alone is sufficient to destroy cultured cells. Optimal condition exists for inducing substantial cell destruction (>50%) with minimal temperature elevation (<1.5°C).
Experimental materials and methods
Cell preparation
All tested cells including HeLa (Human cervical epithelioid carcinoma), HT-29 (Human colon cancer cell), Ca9-22 (Human gingival cancer cell) and fibroblast were obtained from collaborating labs in the National Taiwan University Hospital. These cells were routinely cultured as a monolayer in culture flasks covered with Dulbecco's modified Eagle's medium (DMEM, GIBCO, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (GIBCO) and a 1% mixture of penicillin G, streptomycin, and amphotericin B (GIBCO) in an incubator at 5% CO2 at 37°C.
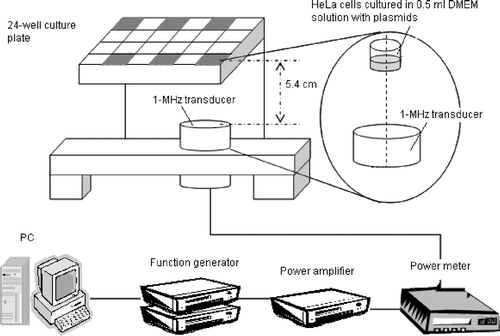
One day before the ultrasound exposure, the cultured cells were trypsinized and seeded at a concentration of 3.5 × 104 cells/well in six wells of 24-well culture plates (Corning Incorporation, NY, USA), as shown in . A similar experimental setup could be found in various in vitro studies of ultrasonic bioeffects, such as those on gene transfection or cell viability Citation[6], Citation[9].
Figure 1. Schematic of the experimental setup. HeLa cells were seeded at a concentration of 3.5 × 104 cells/well in the six wells of a 24-well plate shown in gray.
Four hours after the ultrasound exposure, the exposed cells were rinsed with phosphate-buffered saline (PBS) solution and cultured in 0.5 ml of DMEM solution. By this procedure, non-viable cells and cell debris were removed. Cells were cultured for another 24 h for apoptosis analysis, by which time most of the apoptotic cells had died. Viable cells were washed again, trypsinized and counted by the Trypan Blue dye exclusion method.
Ultrasound apparatus
Ultrasound setup
The ultrasound setup used in the experiments is also shown in . All experiments were performed in a tank containing degassed water at 35∼37°C. (O2 saturation in water of less than <3 ppm). The ultrasound field was generated by a 1-MHz plane piezoelectric transducer that was 3.8 cm in diameter and 20 cm in focal length (model A392S, Panametrics, Waltham, MA, USA). The 24-well plate was placed 5.4 cm above the transducer, and only one well at a time was exposed to ultrasound by aligning the center of the exposed well with the center of the transmitting transducer. The other wells with cultured cells were too far away from the main acoustic beam and thus no surface movement was found during sonication of the target well. The 5.4 cm distance was chosen to ensure that the culture well was within the near field of the acoustic field, in which the whole well could be included by the main acoustic beam. The plate was covered with its original to avoid contamination with the outside environment. It should be noted that standing waves may have been present in the experiment system since the wavelength and the depth of the culture medium being comparable. However, during ultrasound exposures, the surface of the culture medium displayed violent motion, which may have greatly reduced the possibility of standing waves.
Programs written by Labview (v. 5.1, National Instruments, Austin, TX, USA) were used to control the ultrasound exposure time, pulse repetition frequency (PRF), and output voltage of the function generators (model 271, Wavetek, San Diego, CA, USA; and model DS345, Stanford Research Systems, Sunnyvale, CA, USA). The output signals from the function generators were amplified by a power amplifier (model A100, Amplifier Research, Souderton, PA, USA) and monitored by a power meter (model 4421, BIRD Electronic Corporation, Solon, OH, USA). The input power to the transducer was set to 55 W. A needle hydrophone (Onda, Sunnyvale, CA, USA) was used to measure the ultrasound pressure. In order to avoid the influence of reflected waves, a 20-cycle short pulse was transmitted from the transducer to the needle hydrophone. Under the above conditions, the peak-to-peak pressure obtained was 0.361 MPa (the peak positive and negative pressures were 0.180 and 0.181 MPa, respectively). The attenuation of the 24-well plate was about 9%, and the average acoustic intensity at the bottom of the exposure well was calculated to be about 0.54 W/cm2. The total delivered energy was estimated to be about 100 J.
Temperature measurement
The temperature increase for each ultrasound exposure condition was measured using a k-type thermocouple (TC-2190, National Instruments). For the sham exposure group, the cultured cells were first placed in a water bath preheated to 42°C for 20 s, and then returned to 37°C. Four hours later, the cells in both the experimental and control groups were rinsed with PBS solution, cultured and quantified in the same manner as described in the previous section.
Experimental procedures
Fixed duty cycle of 50% with variable on- and off-times
The cytotoxic effect (assessed as the percentage of viable cells relative to controls at 28 h after ultrasound exposure) of the pulsed ultrasound on cultured HeLa cells was tested for the parameters listed in . For this set of experiments, the duty cycle was fixed at 50% (for a total ultrasound exposure of 20 s), and on- and off-times of 250, 25, 5, 2.5, 0.5, 0.25 and 0.125 ms were used. That is, the on-time, equaled the off-time, and ranged between 250 ms and 125 µs. After the viability test, a thermocouple was inserted into the medium at the center of the culture well without touching the bottom. Experiments were repeated in the absence of the culture layer to measure the maximal temperature change in the medium during the entire period of ultrasonic irradiation.
Table I. Parameters for experiments involving a fixed duty cycle of 50% with variable on- and off-times.
Fixed off-time of 25 ms with variable on-times
To determine the effect of the on-time on the destruction of cultured HeLa cells, a second set of experiments was performed with the off-time fixed at 25 ms and the on-time ranging between 0.5 and 250 ms (). The delivered acoustic energy was kept constant by adjusting the total experiment time, with a shorter experiment time used for a longer pulse length (on-time). The maximal temperature change was also measured as above.
Table II. Parameters for experiments involving a fixed off-time (25 ms) with variable on-times.
Fixed on-time with variable off-times
To determine the effect of the off-time on the destruction of cultured cells, and the variation of responses for different cell types to ultrasound, a third set of experiments was performed with pairs of a fixed on-time and an off-time that was either the same or 10 times longer for four different types of cultured cells: HeLa, HT-29, Ca9-22 and fibroblast. The on-times used were 0.25, 2.5, 5, 25 and 250 ms, and the corresponding off-times for on-time of 0.25 ms were 0.25 ms and 2.5 ms. For the group of 2.5 ms on-time, the off-times were thus set at either 2.5 or 25 ms. Details are listed in . The maximal temperature change was also measured as above.
Table III. Parameters for experiments involving a fixed on-time with variable off-times.
Cell viability assay
At either 4 h or 28 h after the ultrasound treatment, exposure cells were harvested by trypsinization. For the Trypan Blue dye exclusion assay, 20 µl of cell suspension was mixed with equal volume of 0.4% (w/v) Trypan Blue dye solution (Sigma, St. Louis, MO, USA). The stained cells were counted manually using a Burker Turk hemocytometer chamber and light microscopy. After staining, dead cells took up the blue stain of Trypan Blue, whereas live cells excluded the dye. Cell viability was estimated by the percentage of the number of the unstained in comparison with the untreated control. All the data were obtained from at least three independent experiments.
Apoptosis analysis
Evaluation of cell apoptosis using FITC-labeled annexin V and PI double staining is a rapid and objective method to study the effect of pulsed ultrasound on cell lines. The annexin V-FITC kit (BioVision Inc., Mountain View, CA, USA) used in our study for apotosis analysis of cells receiving ultrasound exposure is based on the observation that soon after initiating apoptosis, cells translocate the membrane phosphatidyl-serine (PS) from the inner face of the plasma membrane to the cell surface. Annexin V, a 35–36 kDa Ca2+-dependent phospholipid-binding protein, has a high affinity for PS expression on the cell membrane as an endpoint indicator of early apoptosis Citation[10]. At 4 h and 28 h after treatment of the ultrasound exposure, cells were collected by centrifugation at 300 × g for 5 min. The pellets were resuspended in the 1X binding buffer (490 µl), and Annexin V-FITC (5 µl) and propidium iodide (5 µl) were added to each sample and mixed gently. After incubation at room temperature for 5 min in the dark, cells were analyzed with a flow cytometer (Epics XL-MCL, Beckman Coulter, Miami, FL, USA).
Data analysis was performed with EXPO32 ADC Analysis software (Beckman Coulter, Miami, FL, USA). The annexin V-FITC+/PI− cells are considered as early apoptotic cells, while cells which are both annexin V-FITC+/PI+ are considered as late apoptotic or secondary necrosis. Therefore, the total apoptotic cell count was the addition of annexi V-FITC+/PI− cells and annexin V-FITC+/PI+.
Statistical analysis
The data are presented as mean and standard deviation values. All analyses were performed with Student's two-tailed t-test, and an ANOVA with Fisher's least-significant-difference method was used for multiple comparisons. Differences with a probability value of p < 0.05 were considered significant.
Results
Fixed duty cycle of 50% with variable on- and off-times
The mean percentage of surviving cells kept the same as the on-time decreased from 250 to 25 ms, and then increased gradually for on-/off-times from 25 to 0.125 ms, with it increasing substantially for on-/off-times from 0.25 to 0.125 ms (40% to more than 100%, t-test; p < 0.05, n ≥ 6, ), even though the acoustic energy was constant under all these conditions. These results indicated that pulses longer than 0.125 ms were necessary to destroy significant numbers of cultured HeLa cells.
Figure 2. Effect of the PRF (fixed duty cycle with variable on-/off-times) on the percentage of viable cultured HeLa cells. Significant cell destruction compared with controls (p < 0.05, two-tailed t-test) is evident for PRFs between 2 and 2000 Hz. The temperature did not differ significantly among the groups for PRF equal to 2000 and 4000 Hz (#, p > 0.05, two-tailed t-test), but significantly difference in cell survival was found between them (*, p < 0.05, two-tailed t-test).
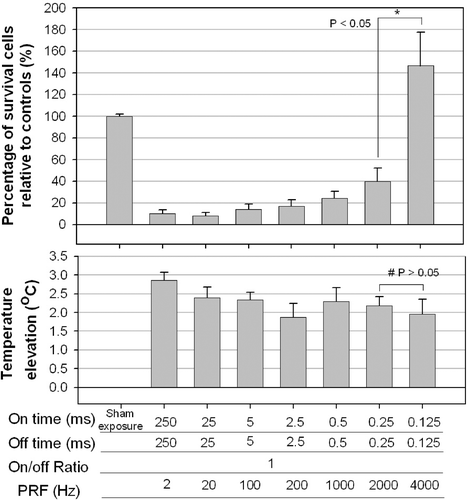
The temperature elevations for different on- and off-times are shown in the lower plot of . No difference was found among on-/off-times of 250/250, 25/25, 5/5, 2.5/2.5, 0.5/0.5, 0.25/0.25 and 0.125/0.125 ms/ms (ANOVA: p > 0.05, n = 6). Continuous ultrasonic irradiation for 10 s induced a significant 0.3–0.7°C higher temperature elevation, except for the on-/off-times of 250/250 ms/ms (data not shown).
Fixed off-time of 25 ms with variable on-times
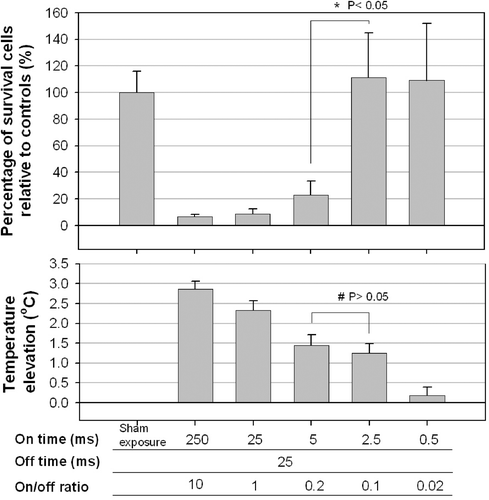
In the experiments with a fixed off-time of 25 ms and variable on-times (from 0.5 to 250 ms; i.e. on-/off-time ratios from 10 to 0.02), there was no significant cell destruction for on-times up to 2.5 ms. However, a sudden decrease in the percentage of surviving cells was found for on-times of 5 ms or longer (). Moreover, the temperature decreased as the on-time decreased (lower plot of ), with 0.5-ms pulses failing to induce a significant temperature rise compared with controls.
Figure 3. Effect of the on-time (and variable off-times) on the percentage of viable HeLa cells. Significant cell destruction compared with controls (p < 0.05, two-tailed t-test) is evident for on-times of 5, 25 and 250 ms. No significant cell destruction is evident for on-time of 0.5 and 2.5 ms. However, the temperature decreased with the on-time. Significant difference was found in cell destruction (*) between on-times of 5 and 2.5, but not in temperature elevation (#).
Fixed on-time with variable off-times
The above results suggest that the on-time greatly influenced the cell survival. To examine the effect of the off-time on the ultrasound-induced cytotoxicity in different types of cultured cells, five paired experiments were performed using the parameters listed in . In each pair of experiments the on-time was fixed, and the off-time was either the same or 10 times longer.
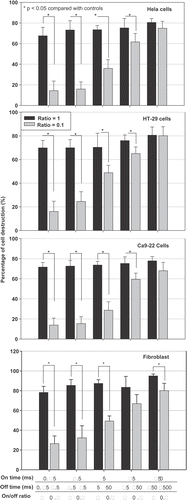
As shown in (28 h after sonocation) and (at 4 h after sonication), short on-times and long off-times (on-/off-times of 0.25/2.5, 2.5/25, and 5/50 ms/ms) did not result in significant cell destruction compared with controls. Equal on- and off-times all induced significant cell destruction and a temperature increase of 2.7–3.4°C. A long on-time (25 or 250 ms) and an off-time that was 10 times longer also resulted in significant cell destruction, but the temperature increase remained less than 1.5°C.
Figure 4. Effect of the off-times (pairs of a fixed on-time with an off-time that was either the same or 10 times longer) on the percentage of viable HeLa cells at 28 h after sonication. Significant cell destruction but with a temperature increase of less than 1.5°C compared with controls (p < 0.05, two-tailed t-test) is evident for on/-off-times of 25/250 and 250/2500 ms/ms were found.
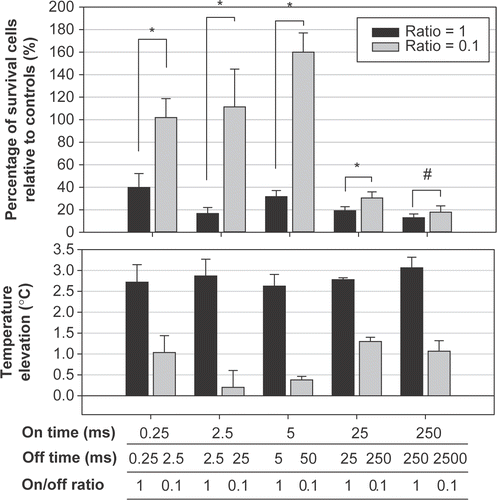
Figure 5. Effect of the fixed on-times but varied off-times on the survival ratio of different types of cultured cells at 4 h after sonication. * The survival ratio is significantly different from controls (p < 0.05, two-tailed t-test). Control: cells without ultrasound exposure. Each column represents the average of three trials of the combined result from nine samples per trial.
Variation in responses of different cell types to ultrasound exposure
As shown in , all tested cell types (HeLa, HT-29, Ca9-22 and fibroblast) exhibited similar trend and amount of cell destruction after ultrasound exposure, no matter what the exposure parameters were.
Induction of cell apoptosis by pulsed ultrasound exposure
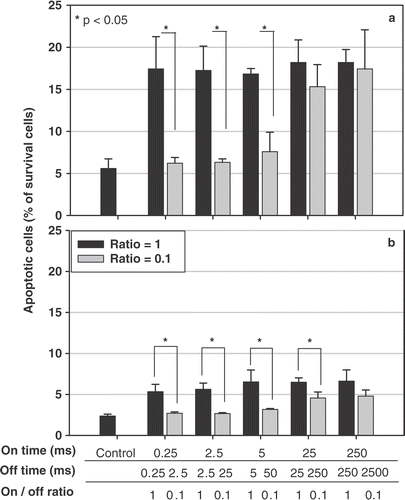
Pulsed ultrasound treatment at 0.54 W/cm2 1 MHz US using a fixed on-time with an off-time either the same or 10 times longer results in apoptotic cell death (). Signicantly more HeLa cells undergoing apoptosis were found at 4 h () after ultrasound exposure than those at 28 h (). Short on-times and 10-times longer off-times (on-/off-times of 0.25/2.5, 2.5/25, and 5/50 ms/ms) induced significantly less cell apoptosis compared with that of equal on- and off-times (on-/off-times of 0.25/0.25, 2.5/2.5, and 5/5 ms/ms).
Figure 6. Ultrasound induces apoptotic cell death as a function of time, determined by flow cytometer. Hela cell line was sonicated using five paired parameters (on-off ratios of 1 and 0.1, with different on- and off-times). Four (a) and 28 (b) hours after the ultrasound exposure, the relative percentage of cell apoptosis, including both early apoptosis and secondary necrotic cells, were determined using the FITC-labeled annexin V kit. * : p < 0.05 (n = 3).
Discussion
The current study shows that 1-MHz ultrasound can exert significantly cytotoxic effects on cultured cells in vitro at intensity levels within those used in clinical physiotherapy. Short pulse lengths or on-times (≤5 ms) were less effective in inducing cell destruction, except when a comparable short off-time was used (e.g. an on-/off-time ratio of 1). However, when a very short on-/off-time combination (e.g. 125/125 µl/µl) was used, the destruction efficiency did not differ from the sham group.
Long pulses (i.e. with long on-times) were more effective than short pulses, but they also induced higher temperature increases. Therefore, to avoid excessive temperature build-up, a long rest period (long off-times) should be used. This strategy resulted in a relatively low temperature increase without sacrificing the destruction efficacy. The on-/off-times pairs of 25/250 and 250/2500 ms/ms appeared to be optimal in the current experimental setup.
The first strategy (i.e. constant on-/off-time ratio with variable on- and off-times (which result in variable PRFs) has previously been used to study the effect of gene transfer and cell viability Citation[5]. Tata et al. Citation[5] showed that prostate cancer cell survival is strongly dependent on the PRF. They found that the cytotoxicity and also the gene transfer efficiency were maximal at the lowest tested PRF (10 Hz), or the longest on-/off-times pair. Their results are consistent with our first strategy on cell viability. However, no temperature comparison was made in their report.
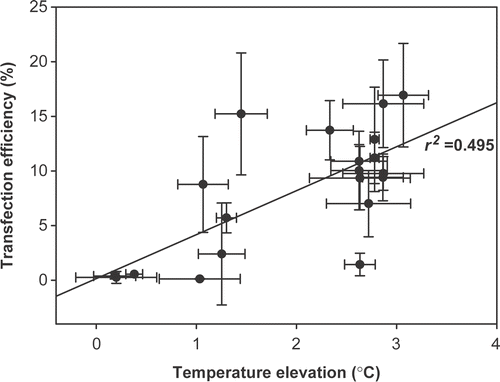
In our experiments the destruction of cells was not purely due to the thermal effects of ultrasound, as indicated by the low correlation (r2 = 0.42) between the temperature increase and the viability of the cells (). Therefore, nonthermal effects such as cavitation might be the major contributors to the cytotoxic effects on the cancer cells observed in this study. Cavitation is well known to exert transient or permanent membrane damage that subsequently induces macromolecular exchanges or cell death Citation[7], Citation[11]. Possible explanations for why a fixed power level but different pulsed-ultrasound strategies resulted in completely different effects on cancer cell destruction included: (1) that a certain duration of ultrasound is necessary to create cell membrane ‘fatigue’ that results in consequent breakage; and (2) that a certain pulse length is necessary for the development of sufficient cavitation to compromise the cell membrane and induce subsequent cell death.
Figure 7. The correlation between the maximal temperature elevation and the percentage of surviving cells relative to controls.
In a study employing voltage-clamp techniques and Xenopus oocyte model, the transmembrane current dropped during the on-time (which was the direct result of decreased membrance resistance due to pore formation), and gradually recovered during the off-time of ultrasound sonication Citation[12]. A longer on-time produced a larger drop in transmembrane current and thus more severe membrane damage, and a shorter off-time did not allow enough time for membrane repair. Therefore, a longer on-time and shorter off-time (i.e. a higher duty cycle) enhances cell porosity even for the same total effective ultrasound exposure time. The averaging of energy over a longer total experimental time helps to avoid pores becoming too large and the formation of too many pores, which leads to irreversible sonoporation.
Studies into the relationship between the ultrasonic PRF and inertial cavitation demonstrate that for a constant delivered energy, a higher PRF (shorter off-time) generates more inertial cavitation and related bioeffects (including hemolysis Citation[13]) owing to more bubbles surviving through the off-time of the ultrasonic irradiation.
The difference of the apoptotic cell ratios for different pulsing conditions might be explained by the concept of membrane damage-and-repair. The on-time was the time for ultrasound to induce damages on the cell membrances. Longer on-time may result in larger extent of cell damages. The off-time was the ultrasound-free period allowing cells to repair their membrane damages. Longer off-time was important because it allowed enough time for repair, thus preventing further lysis. However, complete repair could not quarantine the survival of all cells. Part of the sonicated cells may experience insults other than membrane damages, and resulted in apoptosis Citation[14].
In conclusion, the greater destruction of cultured cells by ultrasound requires a longer on-time, but this is limited by the associated greater increase in temperature. In the present study the optimal compromise was for a long on-time and an off-time that was 10 times longer (to allow time for heat dissipation). However, if certain beneficial bioeffects and cell viability are essential (e.g. for gene transfer or drug delivery), different strategies such as a shorter on-time (but still sufficient off-time) might be necessary.
Acknowledgements
This work was supported in part by grant no. 92-2312-B-002-019 from the National Science Council of Taiwan. The authors sincerely thank Dr Pei-Jen Lou who is now one of the authors and Wei-Shiung Yang for technical assistance and informative discussions. We also thank the Secondary Core Laboratory of the Department of Medical Research, National Taiwan University Hospital, for technical support.
References
- Tachibana K, Uchida T, Tamura K, Eguchi H, Yamashita N, Ogawa K. Enhanced cytotoxic effect of Ara-C by low intensity ultrasound to HL-60 cells. Cancer Lett 2000; 149: 189–194
- Mohamed MM, Mohamed MA, Fikry NM. Enhancement of antitumor effects of 5-fluorouracil combined with ultrasound on Ehrlich ascites tumor in vivo. Ultrasound Med Biol 2003; 29: 1635–1643
- Hrazdira I, Skorpikova J, Dolnikova M. Ultrasonically induced alterations of cultured tumour cells. Eur J Ultrasound 1998; 8: 43–49
- Lagneaux L, de Meulenaer EC, Delforge A, Dejeneffe M, Massy M, Moerman C, et al. Ultrasonic low-energy treatment: A novel approach to induce apoptosis in human leukemic cells. Exp Hematol 2002; 30: 1293–1301
- Tata DB, Dunn F, Tindall DJ. Selective clinical ultrasound signals mediate differential gene transfer and expression in two human prostate cancer cell lines: LnCap and PC-3. Biochem Biophys Res Commun 1997; 234: 64–67
- Greenleaf WJ, Bolander ME, Sarkar G, Goldring MB, Greenleaf JF. Artificial cavitation nuclei significantly enhance acoustically induced cell transfection. Ultrasound Med Biol 1998; 24: 587–595
- Yu T, Wang Z, Mason TJ. A review of research into the uses of low level ultrasound in cancer therapy. Ultrason Sonochem 2004; 11: 95–103
- Kondo T, Kano E. Enhancement of hyperthermic cell killing by non-thermal effect of ultrasound. Int J Radiat Biol Relat Stud Phys Chem Med 1987; 51: 157–166
- Kim HJ, Greenleaf JF, Kinnick RR, Bronk JT, Bolander ME. Ultrasound-mediated transfection of mammalian cells. Hum Gene Ther 1996; 7: 1339–1346
- van Heerde WL, de Groot PG. Reutelingsperger CPM. The complexity of the phospholipid binding protein annexin V. Thromb Haemost 1995; 73: 172–179
- Deng CX, Sieling F, Pan H, Cui J. Ultrasound-induced cell membrane porosity. Ultrasound Med Biol 2004; 30: 519–526
- Pan H, Zhou Y, Izadnegahdar O, Cui J, Deng CX. Study of sonoporation dynamics affected by ultrasound duty cycle. Ultrasound Med Biol 2005; 31: 849–856
- Chen WS, Brayman AA, Matula TJ, Crum LA, Miller MW. The pulse length-dependence of inertial cavitation dose and hemolysis. Ultrasound Med Biol 2003; 29: 739–748
- Feril LB, Jr, Kondo T, Takaya K, Riesz P. Enhanced ultrasound-induced apoptosis and cell lysis by a hypotonic medium. Int J Radiat Biol 2004; 80: 165–175