Abstract
Hyperthermia induces tumor cell death by a spectrum of tumor tissue changes. As whole-body hyperthermia (WBH) can cause cardiovascular complications, especially when cardiotoxic cytostatic agents are administered, invasive cardiovascular monitoring during WBH is necessary. WBH requires a great deal of expenditure and bears the risk of severe toxicity. Furthermore cardiovascular stress, alterations of cardiac index and systemic vascular resistance are major problems during WBH. The purpose of this prospective study was to evaluate cardiovascular changes in patients undergoing WBH under general anesthesia using transesophageal echocardiography (TEE) with special focus on left ventricular function.
Methods: Hemodynamic parameters were measured with standard monitoring and TEE at defined time points in 20 patients (ASA III) undergoing WBH: M37 (baseline, body temperature: 37°C) after induction of anesthesia, M39 during warming up (39°C), M41.8 at plateau level (41.8°C), M38 during cooling period (38°C).
Results: Invasive monitoring and TEE measurements showed signs of hyperdynamic circulation with significant increase of the heart rate (73.6 ± 13.7 min−1 (M37), 104.6 ± 13.0 min−1 (M41.8)) and significant decrease of mean blood pressure (74.9 ± 15.3 mmHg (M37), 65.3 ± 11.2 mmHg (M41.8)). Cardiac index (CI) nearly doubled and stroke volume index (SVI) increased significantly from M37 to M41.8. Cardiac contractility, fractional area change (FAC) and ejection fraction (EF) increased. At M38 CI, SVI, FAC and EF showed a tendency to decrease compared to M41.8 but remained elevated compared to M37.
Conclusion: Patients undergoing WBH showed typical signs of hyperdynamic circulation without impairment of left ventricle which could be monitored excellently by TEE. We recommend using TEE especially in patients with an increased cardiac risk.
Introduction
It has been shown that an increase of body temperature can enhance the effectiveness of oncological treatments Citation[1]. Previous studies reported synergistic effects of combined hyperthermia and chemotherapy and/or irradiation Citation[1]. Hyperthermia with an increase of tissue temperatures ranging from 40 to 44°C potentially induces tumor cell death by a variety of molecular, metabolic, cellular and tumor tissue changes Citation[2]. So far, whole-body hyperthermia (WBH) is not approved for standard oncological treatment. It has been used in clinical studies including patients with advanced tumor stages.
Most patients with malignancies are of older age with co-existing cardiopulmonary diseases. Furthermore, some chemotherapeutic agents are cardiotoxic and WBH can have adverse effects on the cardiopulmonary system Citation[3–6]. Therefore, intensive monitoring during this procedure is required in order to detect and treat hemodynamic complications as soon as possible. Especially for patients with coronary artery disease, tachycardia is potentially dangerous as it shortens the diastolic time. In consequence an imbalance between supply and demand of oxygen may result, which may lead to wall motion abnormalities and negative effects on cardiac output with subsequent cardiac depression. Kerner et al. could demonstrate that WBH induces cardiovascular stress but that it can be performed safely using general anesthesia Citation[7]. To monitor WBH treatment the authors used invasive cardiovascular monitoring including a pulmonary artery catheter Citation[7]. Due to the potential complications associated with the use of PA catheters, up to 13% Citation[8], we renounced the use of a pulmonary artery catheter. Transesophageal echocardiography (TEE) might be a promising alternative to monitor cardiovascular function during WBH especially since it has already been demonstrated that the results of cardiac monitoring using a PA catheter correspond with results of cardiac monitoring using TEE Citation[9]. Additionally, TEE enables the monitoring of wall motion abnormalities, volume status of the patients, preload and afterload, and to evaluate left ventricular function especially in the perioperative setting and in the ICU.
In order to achieve more insights of cardiovascular changes during WBH we designed a prospective study to evaluate cardiovascular alterations with special focus on left ventricular function during this procedure by the use of TEE.
Patients and methods
Patients
After approval of the local ethical committee and informed consent, twenty adult patients (13 men and 7 women) (ASA III) were included in this prospective study within 18 months. Patients with metastatic colorectal carcinomas (n = 14), pleural mesothelioma (n = 2), ovarian carcinoma (n = 2), histiocytoma (n = 1) and with bronchial carcinoma (n = 1) were investigated. Exclusion criteria consisted of history of angina pectoris or myocardial infarction, arterial hypertension, cardiac arrhythmias, pulmonary embolism, cerebrovascular disease, severe diseases of the liver, kidneys, esophagus, stomach and brain metastases, and patients with ASA IV-V classification (American Society of Anesthesiologists). Patients on beta-blocker medication were excluded from the study because of potentially inadequate response of the heart rate during increase of body core temperature. Pre-treatment evaluation included intensive physical examination, chest-X-ray, hematology and blood chemistry, pulmonary function test and 12-lead electrocardiography.
Anesthesia
Patients received 0.1 mg/kg midazolam (Dormicum®, Roche AG, Grenzach-Wyhlen, Germany) before being transferred to the intensive care unit where the procedure took place. WBH was performed under general anesthesia. After standard monitoring, (five-lead electrocardiogram including ST analysis (ECG), non-invasive blood pressure measurement and pulse oximetry) where connected to the patient, anesthesia was induced using a central venous catheter, which had been inserted the day before WBH treatment, with 2–2.5 mg/kg propofol 1% (Disoprivan® 1%, B.Braun, Melsungen, Germany) and 0.5 µg/kg/min remifentanil (Ultiva®, GlaxoSmithKline, Munich, Germany). To facilitate endotracheal intubation relaxation was achieved with 0.5 mg/kg rocuronium (Esmeron®, Organon, Oberschleißheim, Germany). Patients received 0.2 mg IV glycopyrronium (Rubinol®, Riemser Arzneimittel, Riems, Germany) to minimize evaporative heat loss and 50–100 mcg/kg odansetron (Zofran®, GlaxoSmithKline, Munich, Germany) to prevent nausea and vomiting after the procedure. Anesthesia was maintained as target controlled infusion (TCI) with remifentanil 0.25–0.4 mcg/kg/min and 6–10 mg/kg/h propofol 1%. Pressure-controlled ventilation maintaining normocapnia (etCO2 35–45 mmHg) and hyperoxia (FiO2 0.5) was provided with positive end-expiratory pressure levels of 8 mbar. In order to maintain a diuresis of ≥ 1 ml/kg body weight/h volume replacement was given by infusion of Ringer's solution (600 ml/h) and in order to mean arterial pressure of least 60 mm Hg hydroxyl ethyl starch 6% 130/0.4 (≤33 ml/kg body weight) (Voluven®, Fresenius Kabi, Bad Homburg, Germany).
Measurement of arterial blood pressure was performed using the pulse contour analysis system (PiCCO, Pulsion Medical Systems, Munich, Germany) in the left or right femoral artery.
Ventilation and oxygenation, acid-base status, electrolytes, concentration of hemoglobin and lactate as well as glucose values were checked regularly with a blood gas analyser (ABL 505, Radiometer, Copenhagen, Denmark). After finishing the WBH treatment, general anesthesia was stopped. Patients were extubated when ventilating adequately and protective laryngeal reflexes had returned. After the treatment patients remained in the ICU for 24 hours.
Whole-body hyperthermia treatment procedure
Whole-body hyperthermia (WBH) is a very demanding treatment applied to the patient. Heating of the patients was performed by a humidified radiant heat device (RHS 7500, Enthermics Medical Systems, Menomonee Falls, USA). Treatment started directly after induction of general anesthesia. A target maximum body-core temperature of 41.8°C was reached after heating for at least two hours and kept for a plateau phase of 60 minutes. All patients received a cytostatic treatment which was administered during the plateau phase of 41.8°C. The following cytostatic agents were used. Patients with colorectal carcinomas were treated with 5-floururacil (425 mg/m2 body surface) in combination with oxaliplatin (100 mg/m2). All other patients received ifosfamid (3.75–5 mg/m2), carboplatin (240 mg/m2) or etoposid (100 mg/m2). Skin temperatures were continuously measured by pre-calibrated skin probes and core temperature was registered using temperature probes in the femoral artery, rectum and urinary bladder.
Measurements
Hemodynamic parameters were measured at four defined time points: M37 (baseline, body temperature 37°C) after induction of general anesthesia, M39 during the warming up phase when temperature has reached 39°C, M41.8 directly after reaching the plateau level of 41.8°C and M38 during the cooling period before extubation at a body temperature of 38°C. Measurements were started after the respective temperature was reached using the femoral artery probe. Heart rate (HR), systolic, mean and diastolic blood pressures, central venous pressure and five-lead electrocardiogram with an analysis of the ST-segments were continuously monitored during the procedure and documented using a clinical documentation system (CliniComp, San Diego, USA).
Transesophageal echocardiography
After induction of anesthesia and endotracheal intubation the transesophageal echocardiography probe (5 Mhz transducer, Sonos 5500, Hewlett Packard, Hamburg, Germany) was inserted into the esophagus and a standardized examination of the heart and the great vessels was performed. The images and Doppler data were recorded on video tape at the defined time points of measurements (M37, M39, M41.8 and M38). At least three consecutive cardiac beats were recorded at end-expiration and the mean of the results was calculated. The recordings were analysed offline by two independent observers blinded to the intraoperative protocol.
After the appropriate position in the stomach was achieved the left ventricle was represented in the short axis in the midpapillary axis view. By manual marking of the endocardium, the area of the left cardiac ventricle, circumference and volume were measured at end-systole and end-diastole. End-diastole was identified as the peak of the R-wave and end-systole as the minimal left ventricular area. The mean of three consecutive beats was assessed in all cases. End-diastolic and end-systolic areas were measured by planimetry of the left ventricular endocardium using the leading edge method.
Moreover, stroke volume (SV = VTI·AreaLVOT), cardiac output (CO = SV x HR), and systemic vascular resistance (SVR = MAP-CVP x 79.9/CO) were calculated by measuring the Velocity-time integral (VTI) in the left ventricular outflow tract using the pulsed wave Doppler. The area of the LVOT was determined by measuring the diameter of the LVOT and using the formula AreaLVOT = π · (d/2)2. For these measurements the transesophageal probe sector had to be rotated to 120° still in the transgastric position to obtain a long axis view of the left ventricular outflow tract and the aortic valve. Hemodynamic indexes (stroke volume index (SVI), cardiac index (CI)) were determined by relating the values to the body surface area.
Statistical analysis
Statistical analysis was performed using the software SPSS 9.0 (SPSS Inc., Chicago, IL, USA). The data are presented as mean ± SD. A p < 0.05 was considered statistically significant. The results of hemodynamic measurements (HR, blood pressure, CVP, CI, SVR, SVI, FAC, EF) at the three points were compared with the initial value at 37°C using the Wilcoxon signed ranks test.
Results
Whole-body hyperthermia could be performed in all 20 patients (demographic data: age 54 ± 8 years; weight: 80.1 ± 19.6 kg; height: 175 ± 10 cm) without complications. However, one patient had to be excluded from the study because TEE examination could not be performed due to chest and abdominal surgeries prior to therapy.
The results of hemodynamic measurements using TEE after induction of anesthesia at a body temperature of 37°C (M37), at a temperature of 39°C (M39), at the plateau temperature of 41.8°C (M41.8) as well as after cooling down to 38°C shortly before extubation (M38) are summarized in , and and .
Figure 1. Heart rate before, during and after WBH treatment at M37, M39 M 41.8 and M38. Values are mean ± standard deviation. (*p < 0.05 vs. M0; #p < 0.05 vs. M39; + p < 0.05 vs. M41.8).
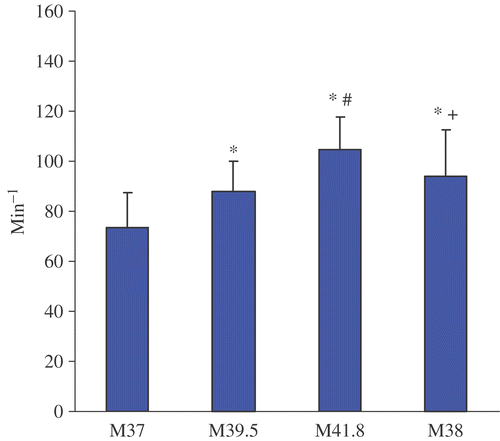
Figure 2. Ejection fraction before, during and after WBH treatment at M37, M39, M41.8 and M38. Values are mean ± standard deviation (*p < 0.05 vs. M0; #p < 0.05 vs. M39; + p < 0.05 vs. M41.8).
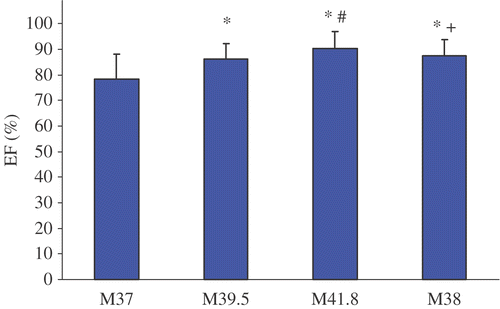
Figure 3. Fractional area change before, during and after WBH treatment at M37, M39, M41.8 and M38. Values are mean ± standard deviation (*p < 0.05 vs. M37; #p < 0.05 vs. M39; + p < 0.05 vs. M41.8).
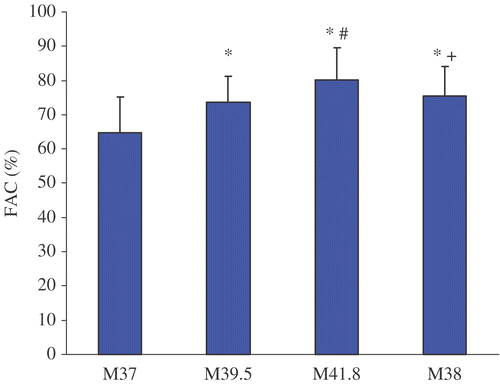
Figure 4. Systemic vascular resistance before, during and after WBH treatment at M37, M39, M41.8 and M38. Values are mean ± standard deviation.(*p < 0.05 vs. M37; #p < 0.05 vs. M39; + p < 0.05 vs. M41.8).
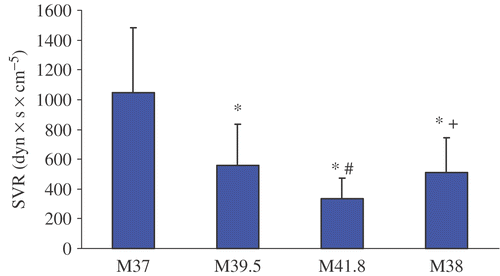
Figure 5. Cardiac index change before, during and after WBH treatment at M37, M39, M41.8 and M38. Values are mean ± standard deviation (*p < 0.05 vs. M37; #p < 0.05 vs. M39; + p < 0.05 vs. M41.8).
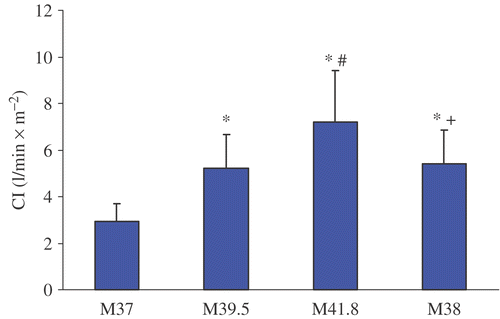
Table I. Hemodynamic changes during whole-body hyperthermia.
Table II. Individual data.
Table III. Individual data.
Before the induction of WBH, heart rate, central venous pressure, cardiac index, stroke volume index, systemic vascular resistance, ejection fraction and fractional area change were within normal limits. In order to patients requirements and to maintain a mean arterial pressure of least 60 mm Hg and a normal right filling pressure (CVD) volume replacement was given by infusion of crystalloid and colloid solutions. During the whole treatment of WBH patients received 5 to 6l of solutions, which guarantee balanced electrolytes and fluid requirement.
Treatment with WBH induced a hyperdynamic cardiovascular reaction with a significant increase of the mean heart rate from 73.6 ± 13.7 min−1 (M37) to 104.6 ± 13.0 min−1 (M41.8) and concomitantly a significant decrease of mean blood pressure from 74.9 ± 15.3 mmHg (M37) to 65.3 ± 11.2 mmHg (M41.8). CI nearly doubled from M37 (3.0 ± 0.8 l/min x m−2) to M41.8 (7.2 ± 2.2 l/min x m−2) and SVI increased significantly from M37 (40.8 ± 5.9 ml x min x m−2) to M41.8 (69.3 ± 17.4 ml x min x m−2). Additionally, significant increases of FAC and EF could be detected. With decreasing temperature CI, SVI, FAC and EF showed a tendency to decrease (M38) in comparison to plateau temperature (41.8°C) but were still elevated compared to pre-treatment values (M37). Only SVR reached pre-treatment values at M38.
During the whole study period neither changes of the ST-segment, any kind of arrhythmia nor wall motion abnormalities were observed. However, 14 patients had dysphagia the following days after WBH.
Discussion
Under therapy of whole-body hyperthermia there is a higher risk of anesthesia for each patient. These risks are not only a result of co-existing heart diseases but also of pharmacological therapy like anesthetic and chemotherapeutic agents Citation[3], Citation[7]. WBH represents the most invasive hyperthermia application, requires a great deal of expenditure and bears the risk of severe toxicity when chemotherapeutic agents are applied Citation[3]. In addition to thermal injuries, cardiovascular stress, rise in cardiac index and decrease of systemic vascular resistance are major problems in WBH Citation[7], Citation[10]. Whole-body hyperthermia is associated with profound cardio-vascular alterations Citation[10], such as tachycardia and a decrease of mean arterial blood pressure Citation[6]. Furthermore, evaluation of hemodynamic changes during WBH treatment using TEE showed characteristic signs of a hyperdynamic circulation. Also, a significant decrease of SVR with increasing body temperature was detected. For compensation of this relative hypovolemia, CI showed an increase of about 140% at M41.8 compared to the pre-treatment level (M 37). Moreover, an increase of cardiac contractility was associated with a significant increase of FAC and EF at a body temperature of 41.8°C. EDA (end-diastolic area) as a surrogate parameter for left ventricular preload Citation[11] increased significantly during WBH treatment. Increase of preload is probably the consequence of intravenous infusion of crystalloids (Ringer's solution 800 ml/h within the first two hours, 600 ml/h within the second two hours of WBH treatment and 200 ml/h for the next 18 hours) and colloid infusion (hydroxyl ethyl starch 6% 130/0.4 500 to 2500 ml during the treatment) during WBH treatment. An explanation for the increase of FAC could be the decrease of SVR Citation[11].
During the whole study period no ST-segment changes, any kind of arrhythmia or wall motion abnormalities were observed, which might be explained by a protective potency of general anesthesia in these patients. It has been emphasized by a study, that WBH treatment under general anesthesia induced significantly lower release of adrenaline and noradrenaline than IV sedation protocol in spontaneously breathing patients Citation[1].
The use of cardiotoxic cytostatic agents may cause myocardial ischemia. Especially for 5-flourouracil, which was used to treat patients with colorectal carcinomas, cardiac complications were reported, although most of the respective patients had no history of cardiac disease or any risk factor for cardiac ischemia Citation[12]. Cardiotoxicity of 5-flourouracil therapy has been reported to range from asymptomatic ECG abnormalities to fatal myocardial infarctio Citation[13], Citation[14], with an overall incidence of 1.2 to 18%. In a prospective, multicenter study it has been shown that cardiac events occurred in 29 of 1097 patients receiving 5-flourouracil continuously over 3–5 days with a fatal outcome in three patients. Patients with cardiac disease and advanced tumor stages had a higher risk for cardiac complications Citation[15]. Even for the administration of oxaliplatin hemodynamic complications, e.g. idiosyncratic reactions with hypotension have been described Citation[16]. Coronary artery complications using carboplatin are rare but vasospasms have been described Citation[17].
Although cardiac complications were not observed during this study, there is a potential risk of transient cardiac arrhythmias and electrocardiographic signs of myocardial ischemia as reported in five patients in a prospective study including 45 patients undergoing WBH Citation[1]. All of these episodes occurred during the first WBH treatment at temperatures >41°C. To exclude an underlying coronary artery disease, further diagnostic studying was performed in four patients with negative results (myocardial perfusion imaging under stress conditions). One patient was excluded from a second WBH treatment because of acute heart failure during the treatment-free interval Citation[1]. This underlines the necessity for continuous cardiac monitoring during WBH.
Electrocardiography, pulmonary artery occlusion pressure (PAOP) and TEE are variable methods of detecting intraoperative myocardial ischemia in real time but with a great variation in sensitivity and specificity Citation[18]. Analysis of ST-segment trends offers a simple and non-invasive method of detecting myocardial ischemia and should be used routinely. However, there are baseline abnormalities that make interpretation of the ST-segment difficult or impossible, for example left bundle branch block, which can lead to nonischemic ST-segment changes.
The sensitivity of PAOP changes for detecting myocardial ischemia is poor Citation[18]. Van Daele et al. observed that in consequence of wide variations in PAOP, changes resulted in many false-positive and false-negative observations when a rise in PAOP was used as an indicator for myocardial ischemia. The main reason for low sensitivity might be wide variations of the results despite standardized measurement procedures Citation[19]. Additionally, due to the reported incidence of death attributed to PA catheter ranging from 0.02–1.5% compared to 0.0098% attributed to TEE we avoided the use of PA catheter and monitored WBH treatment usi Citation[8], Citation[20].
But the main advantage of TEE is that new regional wall motion abnormalities associated with myocardial ischemia can be detected before ECG changes are observed. Smith et al. could demonstrate the advantage of two-dimensional transesophageal echocardiography over electrocardiography for intraoperative detection of myocardial ischemia, because acute segmental wall motion abnormalities of the left ventricle are highly sensitive and specific indicators of myocardial ischemi Citation[21], Citation[22].
However, the expertise necessary for interpretation of TEE imaging to detect wall motion abnormalities, the costs of the technology and its predominant use in intubated patients limit the widespread adoption of TEE in noncardiac interventions. However, if a difficult diagnostic dilemma develops during the WBH, insertion of a TEE probe may provide additional information to guide treatment. Besides myocardial ischemia several haemodynamic abnormalities can lead to new segmental wall motion abnormalities, for example acute hypovolemia with reduced cardiac filling pressures or acute changes in afterload Citation[18].
TEE seems to be a more adequate method than ECG or a PA catheter to detect myocardial ischemia leading to a reduced compliance of the left cardiac ventricle and regional wall motions abnormalities Citation[23]. TEE may help to detect ischemia but may continue to exhibit persistent wall motion abnormalities even after ischemia is resolved. The advantage of using TEE is the possibility to achieve a diagnosis rapidly. Moreover, TEE enables the examination of cardiac anatomy, for example aortic and valvular pathology and may reveal new findings with major implications Citation[23]. Other publications could show that results of TEE altered therapeutic management in at least one third of critically ill patients after noncardiac surgery in the ICU population independent of the presence of a pulmonary artery catheter. The authors of this study speculated that patients without supplementary investigation like TEE will be ‘undertreated’ Citation[24].
Intraoperative TEE is thought to be safe and well tolerated in intubated and anesthetized or sedated patients. The complication rate is within a range comparable to the 0.08–0.13% complication rates reported for patients undergoing gastroduodenoscopy Citation[20]. In a prospective study in 7200 adult cardiac surgery patients, TEE safety has been demonstrated. Intraoperative TEE was associated with a morbidity of 0.2% and a mortality rate of 0% Citation[25]. But the gastroesophageal junctions seem to be particularly vulnerable. Several cases of active bleeding from mucosal lesions and tears, especially in patients undergoing chemotherapy, who have a vulnerable mucosa, have been reported. In this study no severe complications like injuries, bleeding or bronchospasm caused by TEE were noted, but 14 patients had dysphagia the following days after WBH. The reason for this minor complication could be the insertion of an endotracheal tube as well as the duration of TEE measurements.
TEE provides valuable information on regional and global ventricular function as well as on its determinants such as preload, afterload and contractility Citation[26]. A weakness of this method is that it is impractical to use TEE before induction of anesthesia and in the postoperative period in patients, who are awake. But both periods are associated with a high risk of ischemia in patients with coronary disease Citation[19].
In conclusion cardiovascular changes during WBH can be monitored with TEE. With respect to cardiac contractility and wall motion abnormalities TEE is an excellent method to monitor the treatment of whole-body hyperthermia especially in patients with cardiac risk.
References
- Hegewisch-Becker S, Gruber Y, Corovic A, Pichlmeier U, Atanackovic D, Nierhaus A, Hossfeld DK. Whole-body hyperthermia (41.8°C) combined with bimonthly oxaliplatin, high-dose leucovorin and 5-fluorouracil 48-hour continuous infusion in pretreated metastatic colorectal cancer: A phase II study. Ann Oncol 2002; 13: 1197–1204
- Bremer K, Meyer A, Lohmann R. Pilot study of whole-body hyperthermia combined with chemotherapy in patients with metastasised pretreated progressive breast, ovarian and colorectal carcinomas. Tumordiagn Ther 2001; 22: 115–120
- Wondergem J, Stephens LC, Strebel FR, Baba H, Ohno S, Siddik ZH, Newman RA, Bull JM. Effect of adriamycin combined with whole body hyperthermia on tumor and normal tissues. Cancer Res 1991; 51: 3559–3567
- Eisler K, Hipp R, Gogler S, Lange J. New clinical aspects of whole body hyperthermia. Adv Exp Med Biol 1990; 267: 393–398
- Heib C, Schmidt HJ, Heib T, Luer B, Stasche N. [Cardiotoxicity of 5-fluorouracil. Clinical relevance of palliative radiochemotherapy of malignant head-neck tumors]. Laryngorhinootologie 2001; 80: 249–252
- Kerner T, Deja M, Ahlers O, Loffel J, Hildebrandt B, Wust P, Gerlach H, Riess H. Whole body hyperthermia: A secure procedure for patients with various malignancies?. Intensive Care Med 1999; 25: 959–965
- Kerner T, Hildebrandt B, Ahlers O, Deja M, Riess H, Draeger J, Wust P, Gerlach H. Anaesthesiological experiences with whole body hyperthermia. Int J Hyperthermia 2003; 19: 1–12
- Practice guidelines for pulmonary artery catheterization. A report by the American Society of Anesthesiologists Task Force on Pulmonary Artery Catheterization. Anesthesiology 1993; 78: 380–394
- Stoddard M, Ammash Nea. Pulsed Doppler transesophageal echocardiographic determination of cardiac output in human beings: Comparison with thermodilution technique. Am Heart J 1993; 126: 956–962
- Wust P, Nadobny J, Szimtenings M, Stetter E, Gellermann J. Implications of clinical RF hyperthermia on protection limits in the RF range. Health Phys 2007; 92: 565–573
- Filipovic M, Skarvan K, Seeberger M. What about the left ventricle? Echocardiograpic evaluation of global left ventricular function in haemodynamic unstable patients. Intensivmed 2005; 42: 413–423
- Robben NC, Pippas AW, Moore JO. The syndrome of 5-fluorouracil cardiotoxicity. An elusive cardiopathy. Cancer 1993; 71: 493–509
- Akhtar SS, Salim KP, Bano ZA. Symptomatic cardiotoxicity with high-dose 5-fluorouracil infusion: A prospective study. Oncology 1993; 50: 441–444
- Becker K, Erckenbrecht JF, Haussinger D, Frieling T. Cardiotoxicity of the antiproliferative compound fluorouracil. Drugs 1999; 57: 475–484
- Sorbette F, Simon I, Bonneterre J, Clavel M, David M, Degardin M, Labat JP, Pinot I, Thyss A, Vignoud J, et al. [Multicenter prospective study of cardiac accidents during treatments with 5-FU]. Therapie 1992; 47: 371–373
- Santini D, Tonini G, Salerno A, Vincenzi B, Patti G, Battistoni F, Dicuonzo G, Labianca R. Idiosyncratic reaction after oxaliplatin infusion. Ann Oncol 2001; 12: 132–133
- Chasen MR, Ebrahim IO. Carboplatin hypersensitivity presenting as coronary vasospasm - A case report. Cancer Chemother Pharmacol 2002; 50: 429–431
- Fleisher LA. Real-time intraoperative monitoring of myocardial ischemia in noncardiac surgery. Anesthesiology 2000; 92: 1183–1188
- van Daele ME, Sutherland GR, Mitchell MM, Fraser AG, Prakash O, Rulf EN, Roelandt JR. Do changes in pulmonary capillary wedge pressure adequately reflect myocardial ischemia during anesthesia? A correlative preoperative hemodynamic, electrocardiographic, and transesophageal echocardiographic study. Circulation 1990; 81: 865–871
- Daniel WG, Erbel R, Kasper W, Visser CA, Engberding R, Sutherland GR, Grube E, Hanrath P, Maisch B, Dennig K, et al. Safety of transesophageal echocardiography. A multicenter survey of 10,419 examinations. Circulation 1991; 83: 817–821
- Smith JS, Cahalan MK, Benefiel DJ, Byrd BF, Lurz FW, Shapiro WA, Roizen MF, Bouchard A, Schiller NB. Intraoperative detection of myocardial ischemia in high-risk patients: Electrocardiography versus two-dimensional transesophageal echocardiography. Circulation 1985; 72: 1015–1021
- Comunale ME, Body SC, Ley C, Koch C, Roach G, Mathew JP, Herskowitz A, Mangano DT. The concordance of intraoperative left ventricular wall-motion abnormalities and electrocardiographic S-T segment changes: Association with outcome after coronary revascularization. Multicenter Study of Perioperative Ischemia (McSPI) Research Group. Anesthesiology 1998; 88: 945–954
- Margreiter J, Hormann C, Mair P. [Possible uses of transesophageal echocardiography in perioperative monitoring]. Anaesthesist 2000; 49: 74–92
- Poelaert JI, Trouerbach J, De M, Everaert J, Colardyn FA. Evaluation of transesophageal echocardiography as a diagnostic and therapeutic aid in a critical care setting. Chest 1995; 107: 774–779
- Kallmeyer IJ, Collard CD, Fox JA, Body SC, Shernan SK. The safety of intraoperative transesophageal echocardiography: A case series of 7200 cardiac surgical patients. Anesth Analg 2001; 92: 1126–1130
- Skarvan K. The role of transesophageal echocardiography for anaesthesists. Curr Opin Anaesthesiol 2000; 13: 667–674