Abstract
Aim: Whole body hyperthermia (WBH) has been used clinically as an adjunct to radio- and chemotherapy in patients with various cancers. Recently, it has been reported that an activation of the immune system has recently been reported as a possible contributor to the therapeutic effects of WBH. Conversely, the glycolipid α-galactosylceramide (α-GalCer) is recognized by natural killer (NK) T cells together with the monomorphic MHC-like antigen, CD1d, in mice and humans. This study investigated the antitumor effects of WBH combined with α-GalCer in a mouse subcutaneous tumor model of colon cancer.
Methods: Colon26 cells were inoculated subcutaneously into male BALB/c mice to establish subcutaneous tumor. Colon26-bearing mice were treated with WBH using far infrared rays three times/week. Rectal temperature was maintained for 60 min at 41°C. In some experimental groups, α-GalCer was intraperitoneally injected before WBH. We investigated the therapeutic effects of WBH, α-GalCer and combined therapy.
Results: (1) Compared with controls, WBH alone resulted in significant inhibition of tumor growth. (2) No inhibitory effect on tumor growth was seen with α-GalCer. (3) The combination of WBH and α-GalCer showed significant inhibition of tumor growth and prolongation of survival. (4) Serum IFN-γ increased after 3 h and returned to basal levels by 24 h after α-GalCer administration. (5) CTL activity was enhanced following combination therapy with WBH and α-GalCer.
Conclusion: WBH showed antitumor effects in a mouse subcutaneous tumor model of colon cancer. Addition of α-GalCer increased the efficacy of WBH, probably via enhancement of immune response.
Introduction
Whole body hyperthermia (WBH) has been applied as an adjunctive therapy with various established cancer treatments, such as radio- and chemotherapy, and favorable results have been obtained from several clinical studies Citation[1–4].
Besides the well-known effects of WBH in enhancing the efficacy of radiotherapy and certain anti-cancer agents, stimulation of the immune system might also contribute to therapeutic effects. HT-induced heat shock proteins (HSP) have been found to play important roles in eliciting potent anti-cancer immune responses mediated by T cells, dendritic cells and natural killer (NK) cells Citation[5–8].
Conversely, α-galactosylceramide (α-GalCer), a glycolipid derived from marine sponge, has been found to exhibit potent antitumor activity in a variety of experimental and spontaneous tumor metastasis models Citation[9–12]. The antitumor effects of α-GalCer were first attributed to NK cell activation Citation[13]. Subsequently, α-GalCer was identified as a ligand presented by CD1d to the invariant Vα14 T-cell antigen receptor of mouse NK1.1+αβTCR+ (NKT) cells and the invariant Vα24JαQVβ11TCR of human NKT cells Citation[14], Citation[15]. In particular, upon stimulation, NKT cells have a remarkable capacity to produce immunoregulatory cytokines, including interleukin (IL)-4, IL-10, IL-13 and interferon (IFN)-γ Citation[16], Citation[17], which can powerfully influence the nature of adaptive immune responses. Considering that combination treatment with immunomodulators and HT has achieved significant antitumor activity in clinical and experimental studies Citation[18], Citation[19], we speculated that WBH combined with α-GalCer may produce marked antitumor effects. To investigate this hypothesis, we examined the antitumor effects of WBH combined with α-GalCer in a mouse subcutaneous tumor model of colon cancer.
Materials and methods
Animals
The present study used 8-week-old male BALB/c mice purchased from Shimizu Animal Inc. (Kyoto, Japan). Mice were maintained under our standard laboratory conditions. The Animal Care Committee of Kyoto Prefectural University of Medicine (Kyoto, Japan) approved all the experimental procedures described below.
Cancer cell line
The Colon26 murine colon adenocarcinoma cell line Citation[20] was kindly provided by Dr A. Hagiwara (Department of Digestive Surgery, Kyoto Prefectural University of Medicine), and was maintained in monolayer cultures in RPMI-1640 medium supplemented with 10% fetal bovine serum and L-gulutamine at 37°C in a humidified atmosphere of 5% CO2.
Whole body hyperthermia
We used a WBH device heated by far-infrared rays (Blast, Tokyo, Japan) which was a well established heating system Citation[21]. Mice were anesthetized with the mixture of ketamine (150 mg/kg) and xylazine (7.5 mg/kg) before exposing to WBH for 30 or 60 min. Room temperature of a WBH device and rectal temperatures of mice were continuously monitored by Thermo Recorder TR-71U (T and D, Matsumoto, Japan). We controlled the temperature of far-infrared ray panels at 60°C for the first 10 min. After that, we re-set the panels’ temperature down to 41.5°C for 30 or 60 min, then switched the far-infrared ray panels off. The control group was maintained at a normothermic condition of ambient room temperature (24°C). WBH was performed three times per week for 8–12 mice in WBH-treated group.
Western blotting
Whole cancer cell extracts of subcutaneous tumor were prepared as follows. Tumor specimens were thawed on ice and homogenized at 4°C in a solution of 50 mmol/L Tris-HCL, pH 7.6, 300 mmol/L NaCl, 0.5% Triton X-100, 10 μg/mL aprotinin, 10 μg/mL leuptin, 1 mmol/L phenylmethylsulfonyl fluoride, 1.8 mg/mL iodoacetamide, 50 mmol/L NaF, and 1 mM DTT for extracting total cell protein. Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred on to nitrocellulose (Bio-Rad Laboratories, Hercules, CA). Membranes were probed with specific antibody against Hsp70 (Stressgen, Victoria, Canada). The immune complexes were visualized by Western blotting with a commercial kit (ECL by Amersham, Buckinghamshire, England) according to the manufacturer's recommendations.
Alpha-galactocylceramide
The α-GalCer for this study was kindly provided by Kirin Brewery (Gunma, Japan). An original solution of α-GalCer (220 μg/ml) was dissolved in 0.9% NaCl solution containing 0.5% polysorbate 20 (Nikko Chemical, Tokyo, Japan), then diluted by vehicle (0.5% polysorbate 20 in 0.9% NaCl solution) before use.
Colon26 subcutaneous tumor model
To establish tumors in mice, 1.0 × 106 viable Colon26 cells in 100 μl of saline were injected subcutaneously in the mid-dorsal region. Tumors were allowed to grow for three days without treatment and then WBH with or without α-GalCer treatment was initiated. WBH was performed three times per week after the first treatment. In WBH with α-GalCer treatment, the α-GalCer (100 μg/kg) was administered intraperitoneally immediately before WBH. Control mice were injected with vehicle at the same time points in an identical manner. The major and minor axes of tumors were measured and volumes were calculated using the formula:
Cytotoxicity assay
NK cytolytic activity was measured using the standard 51Cr release assay as previously described Citation[22]. Splenocytes were obtained from tumor-bearing mice 48 h after first treatment, and were used as effector cells. Cells were seeded into microtiter plates at various effector-to-target (E:T) ratios and co-cultured with YAC-1 cells that had been labeled with 370 kBq Na2CrO4 for 60 min at 37°C. After 4 h of incubation at 37°C in 5% CO2/95% humidified air, supernatants were collected and radioactivity was measured on a gamma counter.
For CTL cytotoxicity assay, we obtained splenocytes from mice that had been inoculated with Colon26 tumor on day 14 (after six treatments). Splenocytes were re-stimulated in vitro with irradiated Colon26 (40 Gy) in the presence of 10 U/ml of recombinant human IL-2 for 3 days under standard conditions. Cells were subjected to 51Cr release assay as above using Colon26 as the target. Target cells labeled with Na251CrO4 were cultured with each of the effector cells for 4 h and radioactivity was also measured on a gamma counter.
Spontaneous release was determined by assays of radioactivity released from 51Cr-labeled cells incubated in RPMI without effector cells. For the determination of total 51Cr release, 10% Triton X100 was added to each well. All assays were performed at least in triplicate, and the percentage of specific lysis was calculated using the following formula:
Assay for serum IFN-γ
Mice were bled from the heart. Serum IFN-γ concentrations were measured by enzyme-linked immunosorbent assay (ELISA) using IFN-γ ELISA kits (BioSource International, Amarillo, CA).
Statistical analysis
Results are presented as mean ± standard error of the mean. Data were compared using one-way analysis of variance, and differences were considered significant for values of P < 0.05 according to PLSD or Scheffe's F multiple comparison test. All analyses were performed using Stat View 4.11-J software (Abacus Concepts, Berkeley, CA) on a Macintosh computer.
Results
Rectal temperature of mice during WBH
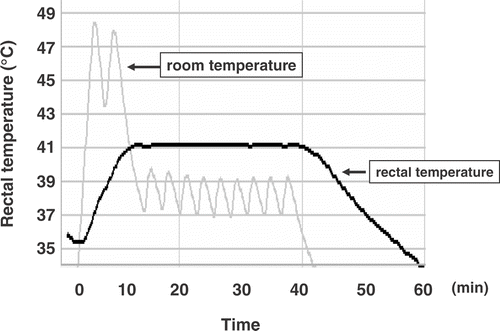
WBH was carried out in a heating box in which far-infrared ray panels were set up on both the floor and ceiling. This heating device was designed to mimic the clinical hyperthermia devic Citation[23], Citation[24]. shows that mouse rectal temperature was increased within 10 min after starting the procedure and maintained at 41°C within the duration of treatment. The rectal temperature of mice that were received WBH combined with alpha-Galcer showed no difference in that of WBH alone treated mice.
Figure 1. WBH was effectively carried out using the far-infrared ray heating device. We controlled the temperature of far-infrared ray panels at 60°C for the first 10 min. After that, we re-set the panels’ temperature down to 41.5°C for 30 min, then switched the far-infrared ray panels off. The rectal temperature of mouse was monitored by Thermo Recorder TR-71U, and was maintained at 41°C for 30 min. Similar results were obtained from three independent experiments.
Accumulation of Hsp70 in Colon26 subcutaneous tumor
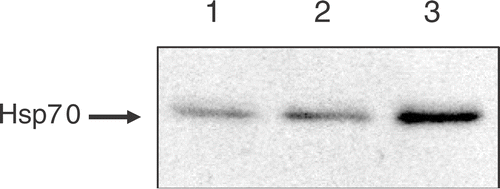
Tumor-bearing mice were treated with WBH for 30 or 60 min. After heat treatment, whole cancer cell extracts of subcutaneous tumor were prepared at 6 h after WBH. In order to investigate accumulation of heat shock protein after WBH, the tumor contents of Hsp70 in the treated mice were assessed by Western blotting. There were substantial increases in the contents of Hsp70 in the tumors of mice who received WBH for 30 min. The higher level of this protein was accumulated in the tumors of mice who received WBH for 60 min (). Similar results were obtained in at least three independent experiments. These results indicated that the heating device could work effectively as a WBH device for mice.
Figure 2. The accumulation of Hsp70 proteins after WBH: tumor-bearing mice were treated with WBH for 30 or 60 min. Whole cancer cell extracts of subcutaneous tumor were prepared at 6 h after WBH. The accumulation of Hsp70 was assessed by Western blotting. Lane 1: untreated control, Lane 2: WBH for 30 min, Lane 3: WBH for 60 min.
Antitumor effect of WBH in the Colon26 subcutaneous tumor model
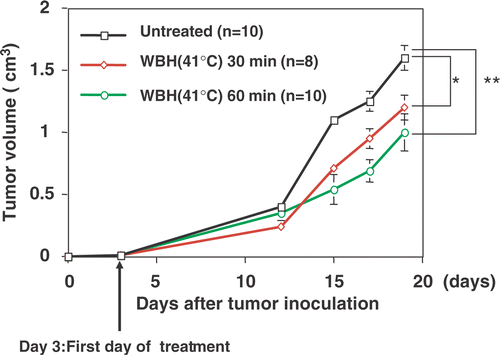
We first investigated whether WBH at a rectal temperature of 41°C resulted in inhibition of tumor growth. The tumor growth curves obtained from mice with no treatment (referred to as untreated control), WBH for 30 min, and WBH for 60 min are shown in . Compared with untreated controls, WBH showed significant inhibition of tumor growth.
Figure 3. Antitumor effect of WBH in the Colon26 subcutaneous tumor model: mice were XYZ injected subcutaneously with Colon26 cells and tumors were allowed to grow for 3 days before initiation of treatment with WBH. Values are the means ± SEM of 8–10 mice. *P < 0.05, **P < 0.01 compared with the untreated group.
Effects of WBH and α-GalCer on subcutaneous tumor growth and survival
We examined the effects of established subcutaneous Colon26 tumor. Tumors rapidly grew in control mice, resulting in the death of 100% of mice by 5 weeks. Compared with untreated controls, WBH alone or combined with α-GalCer treatment showed significant inhibition of tumor growth (p < 0.01 and p < 0.0001, respectively), whereas α-GalCer treatment alone had no such effect. Moreover, significant inhibition of tumor growth was observed in mice treated with WBH combined with α-GalCer compared to WBH alone (p < 0.01; ). shows the Kaplan--Meier survival curve for the mice in the untreated, WBH alone, α-GalCer alone, and WBH combined with α-GalCer groups. As expected from tumor growth results, survival was significantly prolonged in WBH combined with α-GalCer compared to untreated controls (p < 0.001). A tendency toward prolonged survival was also seen in mice treated with WBH alone compared to untreated mice. However, as expected from tumor growth results, no significant difference in survival rate was seen for α-GalCer alone compared to untreated controls.
Figure 4. Antitumor effect of WBH combined with α-Galcer in the Colon26 subcutaneous tumor model: mice were injected subcutaneously with Colon26 cells and tumors were allowed to grow for three days before initiation of treatment with WBH and/or α-Galcer. In WBH with α-Galcer treatment, the α-Galcer (100 μg/kg) was administered intraperitoneally immediately before WBH. Values are the means ± SEM of 12 mice. *P < 0.01, **P < 0.0001 compared with untreated group. ***P < 0.01 compared with WBH alone.
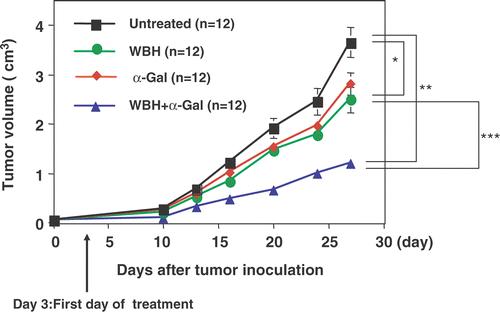
Figure 5. Kaplan--Meier survival curve for the mice in the untreated, WBH alone, α-GalCer alone, and WBH combined with α-GalCer groups. Mice were injected subcutaneously with Colon26 cells and tumors were allowed to grow for three days before initiation of treatment. Each treatment perfomed three times a week for two weeks. WBH combined with α-GalCer group and untreated curves are significantly different (p < 0.001).
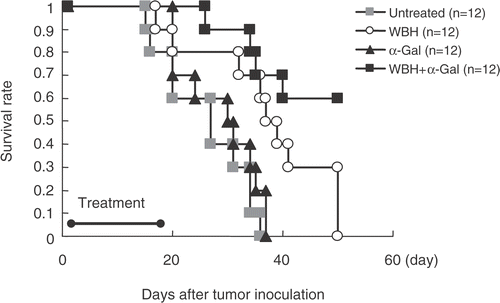
Effects of α-GalCer on serum IFN-γ in BALB/c mice
α-Galcer (100 μg/kg) was administered intraperitoneally to BALB/c mice. Mice were bled from the heart at 0, 3, 6, 12, and 24 h following α-Galcer injection (n = 3). The concentration of serum IFN-γ was measured. Administration of BALB/c mice with α-GalCer resulted in increased serum concentrations of IFN-γ that was apparent at 3 h and maximal at 12 h. Serum IFN-γ returned to baseline levels by 24 h ().
Effect of WBH on α-GalCer-induced serum IFN-γ in BALB/c mice
We investigated whether WBH affected increased serum levels of IFN-γ after administration of α-GalCer. α-GalCer (100 μg/kg) was intraperitoneally injected at 0, 3, 6, 24 h after WBH treatment. At 12 h after α-GalCer administration, mice were bled from the heart to measure serum levels of IFN-γ. Increases of IFN-γ by α-GalCer were unaffected by WBH pretreatment. WBH and α-GalCer thus showed no synergistic effects concerning serum IFN-γ (). On the other hand, WBH alone did not affect the serum level of IFN-γ until 24 h after WBH (data not shown).
Figure 6. Serum IFN-γ after the administration of α-Galcer. The α-Galcer (100 μg/kg) was administered intraperitoneally to mice. Mice were bled from the heart at 0, 3, 6, 12, and 24 h following α-Galcer injection (n = 3). The concentration of serum IFN-γ was measured. Values are the means ± SEM of three mice. *P < 0.01, **P < 0.001 compared with 0 h.
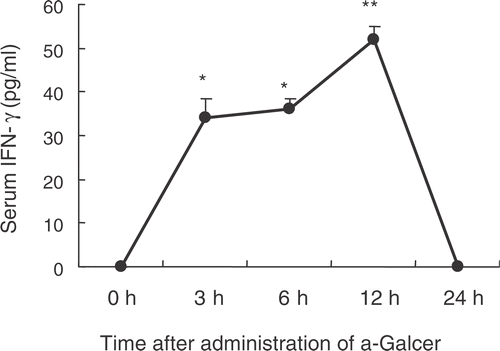
Figure 7. Effects of WBH on α-Galcer-induced increase of serum IFN-γ in BALB/c mice. α-Galcer was injected i.p. 0, 3, 6, 12, 24 h after WBH. The concentration of serum IFN-γ was measured 12 h after α-Galcer administration respectively. Values are the means ± SEM of three mice. #P < 0.01, compared with control.
Enhancement of cytotoxicity, particularly CTL activity by WBH combined with α-GalCer
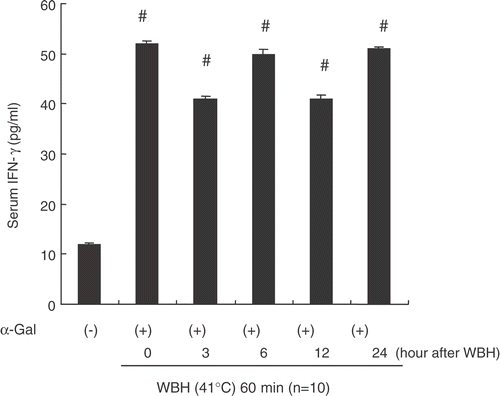
We investigated whether enhancement of NK cell activity and CTL activity contributes to anti-tumor effect of WBH combined with α-GalCer. We analysed the cytolytic activity of NK cells and CTL obtained from the spleen of WBH and/or α-GalCer-treated BALB/c mice. shows NK activity against YAC-1 in various groups. In mice treated with α-GalCer alone, NK activity was significantly increased. In mice treated with WBH combined with α-GalCer, NK activity was also enhanced significantly, although the effect of α-GalCer on NK activity was partially attenuated.
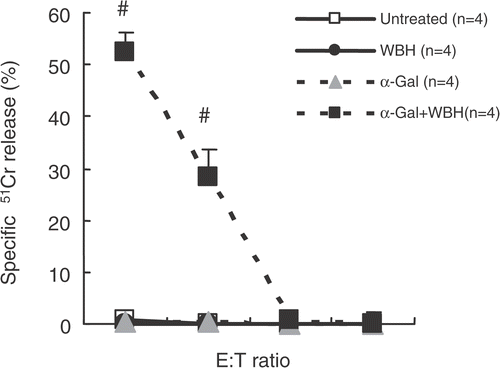
shows CTL activity against Colon26 in various groups. Splenocytes from mice with WBH and α-GalCer only showed significant increase in CTL activity against Colon26.
Figure 8. The cytolytic activity of NK cells obtained from the spleen of WBH and/or α-GalCer-treated BALB/c mice. Spleen cells were collected from mice 48 h after the first each treatment (WBH alone, α-Galcer alone, and WBH combined with α-Galcer). The spleen cells were subjected to the 51Cr release assay using the YAC-1 cells as target. The results are expressed as percent specific lysis at different effector (E)/target (T) cell ratios. Values are the means ± SEM of 4 mice. #P < 0.05, compared with the untreated group. ##P < 0.01, compared with untreated group.
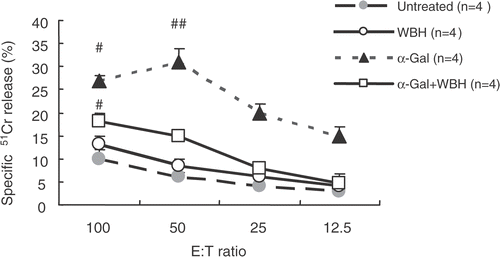
Figure 9. For CTL cytotoxicity assay, we obtained splenocytes from mice that had been inoculated with Colon26 tumor on day 14 (after six treatments). Splenocytes were re-stimulated in vitro with irradiated Colon26 (40 Gy) in the presence of 10 U/ml of recombinant human IL-2 for three days under standard conditions. These splenocytes were evaluated for CTL cytolytic activity assay by using the standard 51Cr release assay. Values are the means ± SEM of four mice. #P < 0.0001, compared with other groups.
Discussion
This study provided evidence supporting the hypothesis that WBH combined with α-GalCer may produce marked antitumor effects. The present results showing that WBH at a rectal temperature of 41°C induces inhibition of tumor growth for mice colon adenocarcinoma suggest that WBH might have antitumor mechanisms by which WBH shows indirect antitumor effects, rather than direct effects of heat itself on cancer cells. WBH has been demonstrated to enhance antigen presentation and function of dendritic cells Citation[8]. Immunomodulators such as OK-432 demonstrate marked antitumor activities when received after WBH Citation[25–27]. The results of previous studies have shown that α-GalCer enhances NK cell activity more strongly than OK-432 Citation[28]. Given these previous reports, we speculated that combined treatment of WBH and α-GalCer may produce significant antitumor activity against colon cancer. To test this speculation, we examined antitumor activities of combined therapy with WBH and α-GalCer in a Colon26 subcutaneous tumor model. A previous study reported significant antitumor effects of α-GalCer on experimental hepatic metastases in mice, but no antitumor effects on subcutaneous tumor Citation[11]. The present results also showed that α-GalCer alone was ineffective against subcutaneous tumor, and WBH alone showed significant antitumor effect. Surprisingly, the combination of WBH and α-GalCer showed stronger tumor growth inhibition than treatment with WBH or α-GalCer alone. Combination therapy prolonged the survival of mice most potently.
We further investigated the mechanisms by which combined WBH and α-GalCer enhanced the antitumor effects of either therapy alone. α-GalCer-stimulated NKT cells display a remarkable capacity to produce immunoregulatory cytokines, including IFN-γ. We examined the effects of α-GalCer on serum IFN-γ levels in tumor-bearing mice. Serum concentrations of IFN-γ were increased at 3 h and maximal at 12 h, consistent with a previous report Citation[29]. IFN-γ produced by NKT cells is required for the antitumor actions of NK cells. If WBH enhances IFN-γ production induced by α-GalCer from NKT cells, this is a strong candidate for the mechanism by which combined treatment shows stronger tumor growth inhibition than treatments using WBH or α-GalCer alone. To test this speculation, we measured serum IFN-γ levels after α-GalCer treatment with/without WBH in tumor-bearing mice. However, WBH did not enhance production of IFN-γ by α-GalCer. Furthermore, the present examination of NK activity revealed that α-GalCer-induced increases in NK activity were not enhanced by WBH, but attenuated by WBH. That is, α-GalCer and WBH did not show synergistic effects concerning IFN-γ produced by NKT cells and NK activity. NK activity enhanced by α-GalCer was actually slightly decreased by WBH. This phenomenon is supported by another study, which indicated that WBH suppresses the cytolytic activity of NK cells via down-regulation of perforin/granzyme B expression Citation[21]. To clarify the antitumor mechanisms of combination therapy, we measured CTL activity of splenocytes from tumor-bearing mice. Unexpectedly, only splenocytes from tumor-bearing mice treated with both WBH and α-GalCer showed strong CTL activity, with no CTL activity from WBH or α-GalCer treatment alone. IFN-γ is known to play an important role in the induction of CTL Citation[30], Citation[31]. We thus speculate that α-GalCer administration increases serum levels of IFN-γ, inducing the capabilities of CTL activation, and WBH actually induces activation of CTL. WBH and heat-induced heat shock protein (HSP) have been shown to stimulate the immune system by augmenting the activities of dendritic cells and T cells, production of cytokines, and antigen presentation on cancer cells Citation[32–34]. In particular, the important role of tumor-derived Hsp70 peptide complex in the maturation of dendritic cells has been well investigated. Previous studies have demonstrated that tumor-derived HSPs initiate tumor-specific CTL responses Citation[32], Citation[35]. WBH results in de novo synthesis of heat-inducible HSPs. Under this condition, local necrosis occurs in tumor tissue and may result in the release of HSP-tumor antigen peptide complex and uptake by dendritic cells with subsequent processing and presentation of HSP-chaperoned tumor-specific peptides to cytotoxic T cells. Taken together with previous reports, this study suggests that WBH augmented the immune response, particularly CTL activity through the induction of Hsp70 and activation of dendritic cells. However, the present results do not eliminate the involvement of other factors such as regulatory T cells or other cytokines. Further detailed examinations are required to evaluate serum levels of cytokines and immunohistology.
In summary, we demonstrated that WBH combined with α-GalCer was more beneficial against solid tumor than treatment with WBH or α-GalCer alone, with respect to inhibition of tumor growth and prolongation of survival for colon26-bearing mice. We also suggest that combination therapy might induce tumor-specific immunity. Tumor recurrence and distant metastases are frequently seen in patients with advanced cancer. Induction of tumor-specific CTL is thus quite important to prevent tumor recurrence and metastasis. From this perspective, we suggest that combination therapy with WBH and α-GalCer is actually useful for cancer treatment.
References
- Hildebrandt B, Wust P, Ahlers O, Dieing A, Sreenivasa G, Kerner T, Felix R, Riess H. The cellular and moolecular basis of hyperthermia. Crit Rev Oncol Hematol 2002; 43: 33–56
- Wust P, Hildebrandt B, Sreenivasa G, Rau B, Ge llermann J, Riess H, Felix R, Schlag PM. Hyperthermia in combined treatment of cancer. Lancet Oncol 2002; 3: 487–497
- Yoshikawa T, Oyamada H, Ichikawa H, Naito Y, Ueda S, Tainaka K, Itani K, Seto O, Sugino S, Kondo M. Antitumor effect and indication of chemoembolization using degradable starch microspheres and regional hyperthermia in patients with hepatocellular carcinoma. J Jpn Soc Cancer Ther 1989; 24: 786–792
- Schulze T, Wust P, Gellermann J, Hildebrandt B, Riess H, Felix R, Rau B. Influence of neoadjuvant radiochemotherapy combined with hyperthermia on the quality of life in rectum cancer patients. Int J Hyperthermia 2006; 22: 301–318
- Calderwood SK, Theriault JR, Gong J. How is the immune response affected by hyperthermia and heat shock proteins? Int J Hyperthermia 2005; 21: 713–716
- Manjili MH, Wang XY, Park J, Macdonald IJ, Li Y, Van Schie R, Subjeck JR. Cancer immunotherapy: Stress proteins and hyperthermia. Int J Hyperthermia 2002; 18: 506–520
- Tanaka K, Ito A, Kobayashi T, Kawamura T, Shimada S, Matsumoto K, Saida T, Honda H. Intratumoral injection of immature dendritic cells enhances antitumor effect of hyperthermia using magnetic nanoparticles. Int J Cancer 2005; 116: 624–633
- Baronzio G, Gramaglia A, Fiorentini G. Hyperthermia and immunity. In Vivo 2006; 20: 689–695
- Morita M, Motoki K, Akimoto K, Natori T, Sakai T, Sawa E, Yamaji K, Koezuka Y, Kobayashi E, Fukushima H. Structure-activity relationship of alpha-galactosylceramides against B16-bearing mice. J Med Chem 1995; 38: 2176–2187
- Nakagawa R, Motoki K, Ueno H, Iijima R, Nakamura H, Kobayashi E, Shimosaka A, Koezuka Y. Treatment of hepatic metastasis of the colon26 adenocarcinoma with an alpha-galactosylceramide, KRN7000. Cancer Res 1998; 58: 1202–1207
- Fuji N, Ueda Y, Fujiwara H, Toh T, Yoshimura T, Yamagishi H. Antitumor effect of alpha-galactosylceramide (KRN7000) on spontaneous hepatic metastases requires endogenous interleukin 12 in the liver. Clin Cancer Res 2000; 6: 3380–3387
- Kobayashi E, Motoki K, Uchida T, Fukushima H, Koezuka Y. KRN7000, a novel immunomodulator, and its antitumor activities. Oncol Res 1995; 7: 529–534
- Yamaguchi Y, Motoki K, Ueno H, Maeda K, Kobayashi E, Inoue H, Fukushima H, Koezuka Y. Enhancing effects of (2S,3S,4R)-1-O-(alpha-D-galactopyranosyl)-2-(N-hexacosanoylamino)-1,3,4-octadecanetriol (KRN7000) on antigen-presenting function of antigen-presenting cells and antimetastatic activity of KRN7000-pretreated antigen-presenting cells. Oncol Res 1996; 8: 399–407
- Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Sato H, Kondo E, Harada M, Koseki H, Nakayama T, Tanaka Y, Taniguchi M. Natural killer-like nonspecific tumor cell lysis mediated by specific ligand-activated Valpha14 NKT cells. Proc Natl Acad Sci USA 1998; 95: 5690–5693
- Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, Koseki H, Taniguchi M. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science 1997; 278: 1626–1629
- Godfrey DI, Hammond KJ, Poulton LD, Smyth MJ, Baxter AG. NKT cells: Facts, functions and fallacies. Immunol Today 2000; 21: 573–583
- Smyth MJ, Godfrey DI. NKT cells and tumor immunity a double-edged sword. Nat Immunol 2000; 1: 459–460
- Lejeune FJ, Eggermont AM. Hyperthermic isolated limb perfusion with tumor necrosis factor is a useful therapy for advanced melanoma of the limbs. J Clin Oncol 2006; 24: 4196–4201
- Siddiqui F, Ehrhart EJ, Charles B, Chubb L, Li CY, Zhang X, Larue SM, Avery PR, Dewhirst MW, Ullrich RL. Anti-angiogenic effects of interleukin-12 delivered by a novel hyperthermia induced gene construct. Int J Hyperthermia 2006; 22: 587–606
- Corbett TH, Griswold DP, Jr, Roberts BJ, Peckham JC, Schabel FM, Jr. Tumor induction relationships in development of transplantable cancers of the colon in mice for chemotherapy assay, with a note on carcinogen structure. Cancer Res 1975; 35: 2434–2439
- Koga T, Harada H, Shi TS, Okada S, Suico MA, Shuto T, Kai H. Hyperthermia suppresses the cytotoxicity of NK cells via down regulation of perforin/granzyme B expression. Biochem Biophys Res Commun 2005; 337: 1319–1323
- Gately MK, Glaser M, Dick SJ, Mettetal RW, Jr, Kornblith PL. In vitro studies on the cell-mediated immune response to human brain tumors. I. Requirement for third-party stimulator lymphocytes in the induction of cell-mediated cytotoxic responses to allogeneic cultured gliomas. J Natl Cancer Inst 1982; 69: 1245–1254
- Robins HI, Dennis WH, Neville AJ, Shecterle LM, Martin PA, Grossman J, Davis TE, Neville SR, Gillis WK, Rusy BF. A nontoxic system for 41.8°C whole-bodyhyperthermia: Results of a Phase I study using a radiant heat device. Cancer Res 1985; 45: 3937–3944
- Sawaji Y, Sato T, Takeuchi A, Hirata M, Ito A. Anti-angiogenic action of hyperthermia by suppressing gene expression and production of tumour-derived vascular endothelial growth factor in vivo and in vitro. Br J Cancer 2002; 86: 1597–1603
- Uehara M, Inokuchi T. Hyperthermic photodynamic therapy combined with topical administration of OK-432 in the mouse carcinoma. Oral Oncol 2003; 39: 184–189
- Yamada T, Hayashi Y, Kaneko R, Tohnai I, Ueda M, Ito M. Effect of the combination of a local OK-432 injection and hyperthermia on SCC VII tumors in mice. J Radiat Res 1998; 39: 101–109
- Taradi SK, Taradi M, Urano M. Effect of thermoimmunotherapy with OK-432 on the development of spontaneous lung metastases in mice. Int J Hyperthermia 1992; 8: 221–226
- Kobayashi E, Motoki K, Uchida T, Fukushima H, Koezuka Y. KRN7000, a novel immunomodulator, and its antitumor activities. Oncol Res 1995; 7: 529–534
- Nakagawa R, Nagafune I, Tazunoki Y, Ehara H, Tomura H, Iijima R, Motoki K, Kamishohara M, Seki S. Mechanisms of the antimetastatic effect in the liver and of the hepatocyte injury induced by α-galactcylceramide in mice. J Immunology 2001; 166: 6578–6584
- Lindahl P, Leary P, Gresser I. Enhancement by interferon of the specific cytotoxity of sensitized lymphocytes. Proc Natl Acad Sci USA 1972; 69: 721–725
- Kataoka T, Matsuura N, Oh-hashi F, Suhara Y. Treatment regimen and host T-cell dependent therapeutic effects of interferon in mouse solid tumors. Cancer Res 1985; 45: 3548–3553
- Noessner E, Gastpar R, Milani V, Brandl A, Hutzler PJ, Kuppner MC, Roos M, Kremmer E, Asea A, Calderuood SK, Issels RD. Tumor-derived heat shock protein 70 peptide complexes are cross-presented by human dendritic cells. J Immunology 2002; 169: 5424–5432
- Atanackovic D, Nierhaus A, Neumeier M, Hossfeld DK, Hegewisch-Becker S. 41.8°C whole body hyperthermia as an adjunct to chemotherapy induces prolonged T cell activation in patients with various malignant diseases. Cancer Immunol Immunother 2002; 51: 603–613
- Takahashi T, Mitsuhashi N, Sakurai H, Niibe H. Modifications of tumor-associated antigen expression on human lung cancer cells by hyperthermia and cytokine. Anticancer Res 1995; 15: 2601–2606
- Srivastava P. Interaction of heat shock proteins with peptides and antigen presenting cells: Chaperoning of the innate and adaptive immune responses. Ann Rev Immunol 2002; 20: 395–425