Abstract
Hyperthermic isolated limb perfusion (HILP) and isolated limb infusion (ILI) may play a significant role in the treatment of patients with recurrent or in transit extremity melanoma or sarcoma that is unresectable. These procedures may be indicated when patients are otherwise faced with the possibility of a debilitating amputation. Not entirely benign treatment modalities, HILP and ILI can be associated with regional and systemic toxicities. We conducted a literature search of published studies using HILP and ILI for the treatment of extremity sarcomas and melanomas, and associated toxicities was performed.
The regional toxicities of HILP and ILI are similar. The most common toxicities reported are mild to moderate. However, when severe regional toxicity occurs, albeit infrequently (<5%), fasciotomies or even amputation may be necessary. Some studies have showed a relationship between acute regional toxicities and long term regional morbidity. Systemic toxicity appears to be more frequent when TNF-α is used in combination with other drugs during HILP, however the use of TNF-α in the United States is limited to trials.
Although regional toxicities are similar, systemic toxicity of ILI is minimal compared to HILP. ILI is easier to repeat, technically less complex, and may be more acceptable in infirmed patients. Long term morbidity and outcomes for ILI are still being evaluated. Both of these techniques may be suitable options in patients with unresectable advanced or recurrent, or in transit extremity melanoma or sarcoma.
Introduction
Hyperthermic isolated limb perfusion (HILP) is used for the treatment of in transit metastases from melanoma confined to an extremity and was described by Creech, Krementz, and co-workers in 1958. Its minimally invasive counterpart, isolated limb infusion (ILI), was described by John Thompson from the Sydney Melanoma Group in Australia in the late 1990s. These two techniques have emerged as alternative treatments for patients who present with unresectable (recurrent or in-transit disease) extremity melanoma and soft tissue sarcomas (STS) Citation[1], Citation[2].
HILP is usually performed under hyperthermic conditions, with temperatures ranging from 39° to 42°C Citation[3]. Higher response rates, as well as greater regional toxicity, have been shown to be associated with higher temperatures Citation[4], Citation[5]. During HILP, chemotherapeutic agent leakage into the systemic circulation is monitored by inert compound 99mTc-radiolabeled albumin or erythrocytes injected into the circuit Citation[3], Citation[6].
The regional toxicities seen in HILP range from mild erythema in the perfused leg to soft tissue injury severe enough to require amputation. These toxicities may not be just related to the temperature of the limb/perfusate during the procedure, but also directly related to the type of chemotherapeutic agent used in the perfusate Citation[7–9]. Systemic toxicities seen in HILP are related to the amount of leakage of chemotherapy into the systemic circulation Citation[10]. The leakage rate is usually less than 3–4%, however, with high flow rates leakage can increase leading to gastrointestinal, bone marrow, integument and other constitutional symptoms Citation[10], Citation[11].
The regional side effects after an ILI are similar to those seen after HILP and range from mild to moderate edema to compartment syndrome or, rarely, amputation Citation[12–15]. After an ILI the majority of patients will manifest regional toxicity symptoms in the form of mild to moderate edema to erythema and blistering Citation[12–19]. The rates of compartment syndrome are reported in some series in up to 15% of cases, but most series report compartment syndrome rates less than 4%. Amputations have been reported after an ILI but only associated with repeat infusions and only in one series Citation[13].
Systemic side effects secondary to chemotherapy agent leakage are rarely seen with ILI. These side effects have been shown to be minimal and generally less than those seen with HILP. The most common side effects consist of mild post-operative nausea. Bone marrow, major gastrointestinal and constitutional toxicities are rarely seen in this procedure Citation[12–18]. Systemic heparinization and reversal with protamine after the procedure can lead to potential side effects such as bleeding complications as well as hypotension secondary to protamine usage in both HILP and ILI Citation[20].
Regional vascular complications such as venous thromboembolism (DVT and subsequent pulmonary embolism) as well as arterial thrombosis secondary to vascular cannulation in HILP or catheter placement in ILI are potential complications as well. DVT has been reported between 1.7% to 10% of the cases after HILP Citation[21], Citation[22].
In this manuscript we will review the acute and chronic regional toxicities associated with ILI and HILP as well as the systemic complications that may be seen with each treatment modality.
Regional toxicity of HILP and ILI
Regional toxicity after an HILP or ILI can be broken down into acute (immediately after the procedure to 3 months) and chronic (beyond 3 months). In 1982 Weiberdink and colleagues described a grading system that is commonly used to report acute tissue reactions (regional toxicity) seen after these procedures (). Grade I toxicity is no reaction. Grade II toxicity is described as soft tissue reactions manifesting as slight erythema or edema in the extremity. Considerable erythema or edema with some blistering is seen in grade III toxicity, whereas extensive epidermiolysis or obvious damage to deep tissue occurs in grade IV. Grade IV injury may cause definite functional disturbance with a threat or manifestation of compartment syndrome requiring fasciotomy. Grade V reactions include severe tissue necrosis or vascular catastrophe that may or does result in amputation Citation[19].
Table I. Grading of acute limb toxicity according to Weiberdink et al. Citation[19].
Although the Weiberdink scale is the standard measure of extremity/regional toxicity injury scoring system, a more comprehensive system was proposed by Vrouenraets and colleagues in 1998. This more ‘objective’ regional grading system could include creatine kinase (CK) levels, serial evaluation of intracompartmental pressure, limb circumference measure, and neurological exam Citation[9], Citation[23].
The most common regionally related symptoms are seen within the first 48 and 72–96 hours after HILP and ILI, respectively Citation[9], Citation[10], Citation[12], Citation[13]. These reactions are typically mild to moderate erythema, edema and pain (Weiberdink grades II, and III). Severe blistering after these procedures is not uncommon and can be seen around 7 days post-procedure. Most symptoms are largely resolved by 2–3 weeks post-procedure. However, edema and erythema can persist for months in a mild form. Other acute regional side effects include temporary loss of nails, drying or blistering of skin on the palms and soles, inhibition of hair growth and transient neuralgia Citation[10], Citation[24].
Factors reported to predispose to severe tissue reactions include: pharmacokinetics of the drugs, such as higher doses and peak concentrations in the perfusate, proximal level of isolation, deterioration of venous blood gas values, low flow rates, low tissue oxygen, and tissue temperature greater than 40°C. Temperatures of 42°–43°C produce only slight increase in complete response rates, but are associated with severe extremity damage and may lead to amputations Citation[4], Citation[5], Citation[8], Citation[9]. Other factors related to toxicity are acidic pH, age younger than 60, arteriosclerosis, and female gender (believed to be related to lower muscle-fat ratio in females) Citation[9], Citation[25], Citation[26].
On a molecular level, the glutathione-s-transferase detoxification pathway may play a role in effecting response and regional toxicity after infusion or perfusion with melphalan. In vitro studies have shown that inhibitors of glutathione enhance the cytotoxic effect of melphalan on cells that are resistant to alkylating agents. This increased activity could also potentially translate into an increased incidence of soft tissue toxicity when melphalan is used Citation[27].
Acute regional toxicity and HILP
The HILP technique has been used for almost 50 years. With this experience has come a well documented history of the complications associated with HILP as well as an accepted regional toxicity scoring system. In general, complications associated with HILP are comparable or worse than ILI. The perception that HILP may be worse could be related to the agents used in HILP such as TNF-α and the early use of hyperthermic conditions, which potentiates the local effect of melphalan and other drugs used in the HILP circuits Citation[7], Citation[28].
Skin/soft tissue damage
Skin and soft tissue injury can range from mild to severe regional damage, corresponding to grades I to III on the Weiberdink regional toxicity scale. Weiberdink grades I to III are the most common findings in the perfused extremities postoperatively. Rossi and colleagues evaluated the response of 21 patients with STS who underwent HILP in a phase I/II trial using doxorubicin and TNF-α Citation[29]. Twenty (96%) patients experienced grade III regional toxicity or less Citation[29]. Grünhagen et al. reviewed the use of HILP in 197 patients with STS using TNF-α + melphalan. They reported 76% grade I–II, and 21% grade III regional toxicities seen after HILP Citation[30]. Bonvalot et al. also studied HILP using de-escalating doses of TNF-α with concomitant melphalan in 99 patients with STS. Most regional toxicities were grade II or lower, ranging from 32–48% Citation[31]. In the Noorda study of 49 patients with STS the authors reported grade I-II toxicity in 71% of patients and grade III in 25% Citation[32]. In another study by the same authors, of 130 patients with melanoma treated with melphalan alone versus melphalan plus TNF-α, they reported grade I–II toxicities in 27% and 75% of patients, respectively. Grade III toxicities were similar in both groups Citation[33]. In our small institutional series of 15 patients who underwent HILP for STS with melphalan alone, we encountered mostly grade II and III acute toxicity Citation[34]. Overall, greater than 90% of patients experience Weiberdink regional toxicity scores of III or less.
Pain
In the ACOSOG trial Z0020, 130 patients were treated with HILP using either melphalan alone, or melphalan/TNF-α. The trial was closed early due to a lack of significant benefit seen in the combined group; however, a total of 16 patients reported pain as an adverse event. Pain occurred in 11(17%) of melphalan alone treatments and 5(8%) of the combination arm Citation[35].
Neurovascular injury
The HILP technique involves direct intravascular manipulation of both vein and artery. As a consequence, acute vascular complications are reported in 1%-10% of cases. These injuries/adverse events can range from DVT and possible pulmonary embolus to acute arterial thrombosis necessitating thrombectomy and potential limb loss. These include both arterial and venous thrombosis, hemorrhage and risk of anticoagulation, and complications associated with heparin reversal (use of protamine). Vascular complications are typically associated with higher regional toxicity scores Citation[20], Citation[22], Citation[36], Citation[37].
Arterial and venous injuries
In a study by Eggiman using TNF-alpha, melphalan, and IFN-gamma, one out of 18 patients (5%) required an arterial thrombectomy post-HILP Citation[26]. In one large series, the incidence of thrombosis at the arteriotomy site was 2.5% in 366 patients Citation[38].
Noorda et al. reported their experience in 49 patients who underwent HILP using melphalan + TNF-α. Three (6%) major ILP complications occurred. This included 2 arterial thromboses requiring thrombectomy without amputation or fasciotomy. Chemotherapy and adjuvant radiation may contribute to the incidence of long term arterial stenosis Citation[32].
DVT post-HILP occurs in 1.7%-10% of cases Citation[21], Citation[22]. Recently Hoven-Gondrie et al. reported on long-term locoregional vascular morbidity after HILP and external-beam radiotherapy for STS of the extremity. They reported an incidence of post-operative DVT in 7.7% of 77 patients undergoing HILP Citation[21].
Compartment syndrome
Compartment syndrome has been well documented after HILP for melanoma and STS. The reports in the literature of compartment syndrome necessitating fasciotomy range from 1% to 5% Citation[29–31], Citation[39–42]. One (4%) of 21 patients in the Rossi study underwent fasciotomy for compartment syndrome (Weiberdink grade IV toxicity). This resulted in permanent popliteal nerve palsy for that patient Citation[29]. Grünhagen et al. performed 217 HILP in 197 patients with STS using TNF-α + melphalan. They reported 2% grade IV Weiberdink toxicities Citation[30]. In a study by Eggiman using TNF-alpha, melphalan, and IFN-gamma, one out of 18 patients (5%) ultimately developed compartment syndrome requiring fasciotomy Citation[26]. Noorda et al. reported their experience of 49 patients who underwent HILP using melphalan + TNF-α for STS post XRT. Grade IV toxicity was seen in 2% Citation[30]. In the USA, the ACOSOG randomized multicenter trial Z0020 of 124 patients who underwent HILP with melphalan versus melphalan + TNF-α for locally advanced melanoma reported grade IV toxicity in 1 (2%) and 2 (3%) patients in the melphalan alone and melphalan/TNF- α arms, respectively Citation[35].
Amputation
Treatment related amputation is the most extreme regional complication seen with HILP. Fortunately, this complication is rare. In the USA, the American College of Surgeons Oncology Group (ACOSOG) randomized multicenter trial Z0020 evaluated 124 patients who underwent HILP with melphalan versus melphalan + TNF-α for locally advanced extremity melanoma. This trial showed a higher complication rate in the combined treatment arm without demonstrating enhanced short-term response. Two (3%) toxicity related amputations (grade V) were performed in the combined arm, while none were performed in the melphalan alone arm. These authors also found a greater probability of grade III-V toxicity after HILP with TNF-alpha (36%) than after normothermic (16%) or ‘mild’ hyperthermic (17%) HILP with melphalan alone (p = 0.0038) Citation[35]. In a series published by Vrouenraets and colleagues of 415 patients treated with HILP, two patients (0.5%) underwent treatment related amputation Citation[28]. In our small institutional series of 15 patients who underwent HILP for STS with melphalan alone, one (6%) patient with grade V toxicity, required treatment related amputation of the fifth finger after an upper extremity HILP Citation[34]. In general, most studies in the recent literature report procedural related amputation rates ranging from 0–3.3% Citation[2], Citation[29–31], Citation[39–42].
Reports in the literature documenting acute regional toxicities after HILP or ILI procedures are summarized in .
Table II. Regional toxicity of HILP and ILI.
Long term regional toxicity and HILP
There are few studies reporting long term morbidity after HILP or ILI. In general it is accepted that higher grade regional injury scores result in more tissue damage and increased long-term complications Citation[19], Citation[43]. A report by Vrouenraets of long-term morbidity after HILP in 367 patients showed that 44% had some degree of objective or subjective long-term morbidity. Overall, 55 (15%) patients were left with some degree of limb malfunction. In the majority (46 of 55), this was caused by muscle atrophy or fibrosis and/or neuropathy. In this study, their data suggest that the degree of acute regional tissue injury had a statistically significant effect on the incidence of long-term morbidity (p < 0.001) Citation[43]. In general, long-term effects after HILP are mainly functional and consist of edema, stiffness, functional impairment, and muscle atrophy Citation[32], Citation[44], Citation[45].
Neurovascular
Little is published on long-term vascular complications associated with HILP. In the study by Hoven-Gondrie study researchers showed a time related decrease in ankle-brachial index (ABI) and femoral pulsatility index (PI) in the treated extremity. Both ABI and PI were significantly decreased when compared to the contralateral leg (p = 0.001 and p = 0.011, respectively). Interestingly, there was not a strong concordance in patients with decreased ABI/PI and subjective complaints. In this series partial arterial occlusion was identified in 3% of patients, and total occlusion at the cannulation sight in 6% Citation[21].
Pain
In 1995 Vrouenraets reported on long-term morbidity after HILP. Minimum follow-up in this series was one year. Of 367 patients, 8% reported chronic pain as a result of the procedure Citation[43].
Decreased range of motion
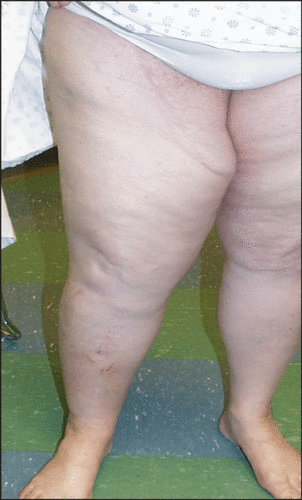
Grünhagen et al. from The Netherlands, reviewed the use of HILP in 197 patients with STS using TNF-α + melphalan. In long-term functional follow-up, 72% of patients had normal function of the involved extremity, 7% were mildly disturbed, and 4% had severely diminished limb function, requiring the use of ambulatory aids Citation[30]. In our small institutional series of 15 patients who underwent HILP for STS with melphalan alone, chronic lymphedema [] and neuropathic pain were persistent at 12% and 6% incidence respectively one year after HILP Citation[34]. These complications directly or indirectly limited the range of motion in those patients.
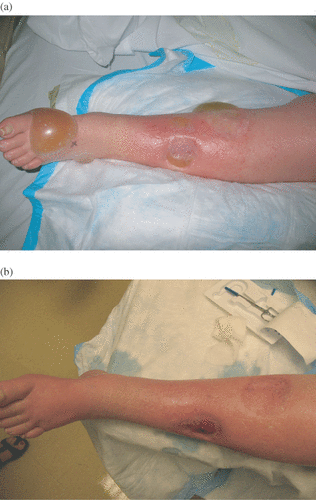
Figure 1. Panel A depicts Weiberdink regional toxicity grade III 6 days after an isolated limb infusion. Notice the erythema, edema and severe blistering of the skin. Panel B shows resolution of these toxicities by the 6 week post-operative visit.
Vrouenraets study of long-term morbidity after HILP in 367 patients showed that 44% had some degree of objective or subjective morbidity. Muscle atrophy and fibrosis occurred in 11% and limb malfunction in 15% of treated patients. Lymphedema, the most commonly reported morbidity, was present in 28% of patients Citation[43]. In a later study by Vrouenraets et al. the authors reported the long term regional morbidity of 65 patients who underwent HILP. The authors found that 24% of patients had atrophy of the muscles in the perfused extremities Citation[46]. Van Geel et al. also reported 20% and 36% chronic lymphedema of the upper extremities and lower extremities, respectively, with severe ankle restriction in 25% of patients with a median follow up of 5 years after HILP Citation[47]. Higher rates of lymphedema have been associated with groin dissection performed at the time of HILP Citation[46].
Altered sensation/neuropathy
Neuropathy has been reported ranging from 1% to 48% of perfused patients Citation[48]. This complication may result from drug choice, compartment syndrome, or excessively tight tourniquet placed at the time of operation. In Rossi's phase I/II trial six patients (29%) had chronic, but reversible neuralgia post-HILP. All six patients were treated with analgesics for 4-8 months with eventual resolution of their symptoms Citation[29]. In the Vrouenraets study of long-term morbidity after HILP in 367 patients the most commonly reported morbidities were neuropathy (4%), and recurrent infections (3%) Citation[43].
Amputation
In a recent study Hoven-Gondrie and colleagues sought to evaluate the long-term locoregional vascular morbidity after HILP and external-beam radiotherapy for STS of the extremity. In this series median follow-up was 88 months. Five of 32 patients underwent leg amputation. Two cases were due to critical leg ischemia 10 years after ILP Citation[21]. In most series late amputation has not been reported very commonly with the HILP technique.
Acute regional toxicity and ILI
The regional toxicity of ILI has been considered and reported to be comparable to HILP. The acute regional side effects seen after ILI are also measured by the Weiberdink regional toxicity scale and range from I to V, with the majority of reports describing acute regional side effects in the grades I to III range Citation[12], Citation[14–17].
Skin/soft tissue damage
As seen with HILP, the majority of the reported regional toxicity effects of immediately after ILI are related to skin and soft tissue damage (Weiberdink grade I–III), with incidences ranging from 38%–100% Citation[14–18]. In a study from the Sydney Melanoma Unit using a drug combination of melphalan and actinomycin D, the authors reported grade II and III toxicity in 41% and 53% of 43 patients respectively Citation[17]. Brady et al. reported their results of a phase II trial of ILI with melphalan and actinomycin D for melanoma and STS. The authors showed that peak morbidity occurred at 2 weeks, most of which was grade II and III toxicities. The majority of these symptoms resolved by 6 weeks Citation[16].
Zager et al. reported similar results after ILI using melphalan and actinomycin D on 64 patients with unresectable recurrent or in transit extremity melanomas. The authors reported the patients’ Weiberdink regional toxicities as grades I–III toxicity in 96% of patients Citation[14]. depicts a Weiberdink grade III reaction at 7 days after ILI (A) and resolution of the symptoms by 3 months post procedure (B).
Vascular injury and ILI
Although not reported in the literature, arterial or venous injury secondary to fluoroscopically placed catheters as well as deep vein thrombosis due to catheter placement or tourniquet placement are two potential complications that can occur during or after an ILI.
Compartment syndrome
Grade IV toxicities requiring fasciotomies has been reported in 1% to 15% of patients treated by ILI. In the study by Zager et al. 4% of patients had grade IV toxicity necessitating fasciotomies, similar to the rate reported by Lindner et al. where 5% of 135 patients had grade IV toxicity Citation[14], Citation[17]. Bonekamp et al. reported the higher fasciotomy rate with 2 (15%) of 13 patients with grade IV toxicity, using fotemustine and dacarbazine as chemotherapeutic agents Citation[18]. In contrast, in the study by Brady et al. where melphalan alone was used as the infusion agent, the patients did not suffer any grade IV or V toxicities Citation[16].
Amputations
Procedurally related amputations after an ILI are uncommon. The only report of ILI related amputations came out of the study by Bonekamp et al. where grade V toxicity (amputation) occurred in 28% (4 out 14 patients), when repeated treatments were performed using fotemustine and dacarbazine Citation[18]. No other procedurally related amputations have been reported using single ILI treatments in the literature.
Long-term regional toxicity and ILI
Since ILI is a relatively new procedure, there are very few reports on long term morbidity form the procedure. It is possible that the long-term morbidities when finally published will mimic those seen with HILP.
The majority of patients in the Brady series experienced grade I/II regional toxicity post-ILI at 3 months follow up. Morbidity greater than grade III was not seen in this cohort. Of note, the highest regional toxicities in the Brady series peaked at 2 weeks and most resolved by 6 weeks after the procedure. These symptoms included Weiberdink grade I to II toxicity, (mild to moderate edema of or erythema) and some minor pain and discomfort with motion. They described few residual side affects after 3 months consisting of mild lymphedema and hyperpigmentation of the affected extremity. The percentages of how many patients experienced long term (>3 months) morbidity were not specified in this study Citation[16].
Repeated treatments
Both HILP and ILI have an inherent re-treatment possibility. This may be considerably more challenging in HILP where the vessels have been previously dissected, isolated, and cannulated. Several studies have evaluated the effect and complication rates of patients undergoing more than one HILP Citation[49–52]. ILI may have a distinct advantage when re-treatment is an option given its less invasive manipulation of the root vessels.
HILP and repeated treatments
Noorda et al. noted that the acute regional toxicity after repeat HILP using TNF-α and melphalan for recurrent melanoma was not significantly different than first-time HILP in 21 patients. Grade I–II toxicity was seen in 85% of patients undergoing repeat HILP versus 67% (p = NS) in those undergoing a HILP for the first time. Grade III–IV toxicities were not significantly different between initial and repeat perfusions (15 and 33%, respectively). There were no grade V regional toxicities Citation[49]. Grünhagen et al. and Klaase and colleagues also found that regional toxicity was not increased with subsequent HILPs Citation[50], Citation[51]. These results are contradicted by those reported by Kroon et al. Citation[52]. The Kroon study showed an increase in regional toxicity after a second perfusion with a Weiberdink grade III toxicity in 34.8% (15 of 43 patients). In general one or more complications occurred in 28% of patients with a single HILP. The complication rate increased to 51% for patients having two or more HILPS Citation[53]. Furthermore, data published by Klaase and colleagues reported on a more pronounced long-term complication rate after a second ILP Citation[51].
ILI and repeated treatments
ILI allows for a relative short interval between treatments due to the minimal systemic drug leakage therefore systemic side effects as well as the ease of catheter placement and technical aspects of the procedure. Citation[12].
Bonekamp et al. reported on 13 patients who underwent ILI with fotemustine after dacarbazine chemosensitation for recurrent melanoma. These patients had previously failed to respond to an ILI with melphalan and actinomycin D. In this series 4 (28%) patients underwent late amputations. Interestingly, the CPK concentrations remained within normal range in these patients. Local tissue reaction was so minimal that the patients were initially discharged home around day three. However, a second peak of regional toxicity developed in 8 patients 3 weeks after ILI, consisting of skin breakdown, stiffness, and severe pain. Eventually all 8 patients underwent amputation either due to the regional toxicity that was debilitating or development of new tumor nodules throughout the treated limb Citation[18].
Lindner et al. reported that grade III toxicity increased from 48 to 65% for single and repeated ILI treatments with melphalan for melanoma, respectively. Fasciotomies for compartment syndrome (grade IV) were required in 2% of patients on each group in the Lindner study Citation[15].
Systemic toxicity of HILP and ILI
Systemic toxicity is reported based on guidelines of the World Health Organization (WHO) grading system. A five-grade scoring system is used with values ranging from 0 to 4. The systemic disturbances that are frequently measured include hematologic, gastrointestinal, genitourinary, cardiac, pulmonary, neurological, and integumentary symptoms Citation[54]. Common Terminology Criteria for Adverse Events (CTCAE) is a descriptive terminology recommended by the National Institute of Health to report the severity of symptoms related to the use of a procedure. The severity of the adverse event (AE) in an organ or system is graded from 1 (mild) to 5 (death related to AE) Citation[55]. The majority of the authors to date have reported the systemic and regional toxicity using the WHO and Weiberdink grading system respectively, except for the ACOSOG study where the AE terminology was used Citation[35].
HILP systemic toxicity
Systemic toxicity during HILP may occur and is dependent on both flow rate and the degree of drug leakage into the systemic circulation Citation[11], Citation[26]. Optimal isolation of the limb needs to be achieved to help avoid systemic leakage. Continuous monitoring of systemic leakage is mandatory to ensure that the rate of leakage from the limb is less than 5% of the total dosage Citation[8]. The systemic toxicity after an HILP is largely related to the type of chemotherapeutic agent leaked into the systemic circulation. Alternatively, systemic adverse events can be seen after full systemic heparinization prior to the procedure as well as protamine reversal.
ILI systemic toxicity
Systemic toxicity is much less common in ILI. Leakage of drug is minimal during an ILI, however, there are still systemic side effects seen with the procedure Citation[12], Citation[14], Citation[17]. The systemic toxicities seen with ILI can be similar to those described for the specific chemotherapeutic agents, secondary to the administration of heparin and protamine, or from muscle necrosis resulting in systemic enzymatic release in the immediate post-operative period Citation[14].
There are only a few studies reporting details of systemic toxicity and ILI Citation[14–18]. Nausea has been reported in as many as 52% of patients resolving 48h after ILI using melphalan and actinomycin D Citation[14], Citation[16]. In the study by Zager et al. no systemic toxicity beyond mild nausea on post-operative day one was seen Citation[14].
Muscle ischemia from tourniquet isolation under anaerobic conditions may have systemic consequences. In ILI the CPK (creatinine phosphokinase) rises dramatically around post-operative day four secondary to rhabdomyolysis. These values can routinely rise well over 1000 u/L Citation[14], Citation[17],. At CPK levels greater than 1000 u/L patients are at risk for myoglobin-induced renal failure and should be followed very closely with daily blood urea nitrogen and creatinine levels (BUN and Cr). Aggressive hydration with normal saline to obtain a brisk urine output (0.5cc/kg per hour) may reduce the risk of renal failure Citation[12–14].
Zager et al. report routine draws of serum CPK every 12 h until they peak on approximately post-operative day four, and continue to monitor CPK twice daily until CPK levels start to return to baseline. Only then do they stop the intravenous hydration and consider the patients for discharge home Citation[14].
Systemic toxicities attributable to specific chemotherapeutic agents
Hypotension
Hypotension seen during or post-HILP is likely a reflection of chemotherapy leakage during the procedure. In a review of the Cumulative European Multicenter experience by Eggermont et al., 186 sarcoma patients treated in eight cancer centers were treated with at least single HILP using TNF-α and melphalan. The systemic toxicity was considered moderate and easily manageable in general. Cardiovascular grade III–IV toxicity, with a hyperdynamic state and hypotension, occurred in 3% of patients. Symptoms were managed with intravenous hydration Citation[3].
In general the agent most commonly associated with systemic hypotension is TNF-α. Overall, rates of systemic hypotension are reported at ranges of 0–94%. Again, the rates of hypotension have been shown to correlate directly with systemic leakage Citation[11], Citation[26], and Citation[57].
TNF-α
TNF-α targets the tumor microvascularization, resulting in coagulative and hemorrhagic necrosis of tumors Citation[58]. When TNF-α is used, the systemic toxicity rate is increased Citation[9], Citation[26], Citation[59]. Leakage can produce a severe systemic inflammatory response syndrome with shock necessitating vasopressors Citation[28], Citation[57]. Eggiman et al. showed severe hemodynamic effects of high dose TNF-α, interferon-γ, (INF-γ) and melphalan. Seventeen of 18 patients (94%) developed vasomotor shock unresponsive to fluid, needing vasopressors. Transient pulmonary infiltrates were present in 100% of patients, hematologic disturbances in 83%, infection requiring antibiotics in 61%, liver toxicity in 50%, and a decrease in creatinine clearance in 22% of patients. No deaths occurred. They found an early massive leakage rate with peak serum levels of 100 000 pg/ml of TNF-α Citation[26]. Bonvalot's report on systemic toxicity comparing low and high doses of TNF-α for the treatment of STS. They found one of 24 patients in the TNF-α high dose (3mg/4mg) arm of this study required dopamine for sepsis like syndrome, and five other patients developed hypotension that resolved with aggressive intravenous hydration Citation[31].
In the Lans study of HILP in 26 patients with previously irradiated limbs, systemic toxicity was mild to moderate and reported as ‘easily manageable’. Interestingly, in this study there was not a correlation between systemic TNF-α levels (they mentioned >10% systemic leakage in five of these patients) and the severity of the systemic effects. The authors explained these findings by suggesting high drug perfusate flow rates with higher limb venous pressure and insufficient wash out may have favored leakage Citation[60].
Zwaveling reported on 25 patients who received HILP with TNF-α after subcutaneous INFδ. In this study, high plasma TNF-α level directly correlated with a severe inflammatory response/sepsis-like syndrome. Systemic leakage rates of the drug ranged from 0% to 8% (median 2%), depending on flow rate. Respiratory complications occurred in 5 patients (20%), necessitating intubation. Two of these patients remained intubated for >2 weeks, and one patient developed multiorgan system failure. Increased prothrombin time was also correlated with the systemic concentration/leakage of TNF-α Citation[57]. In a study by Sorkin of 15 patients with STS, they evaluated the systemic toxicity of TNF-α using two different flow rates, while monitoring the patients’ hemodynamic and metabolic variables. An important conclusion from this study was that TNF-alpha related systemic toxicity could be reduced by decreasing flow rates (869 ± 122 ml/min versus 286 ± 62 ml/min (p = 0.003)) Citation[11]. Bonvalot's report on systemic toxicity found grade II and III cardiovascular toxicity correlated directly with the TNF-α dose. In their study they compared four groups of patients assigned different dose regimens of TNF (0.5mg, 1 mg, 2 mg and 4 mg) Citation[31]. In the ACOSOG Z0020 trial the authors reported more systemic side effects such as cardiovascular, thrombotic, dermatologic, myelosupression, pulmonary, and musculoskeletal side effects, in patients receiving TNF-α and melphalan. Grade IV CTCAE were identified in 11 (17%) patients in the TNF-alpha-plus-melphalan arm (p < 0.028) Citation[35]. In general, recent reports of TNF-α related hypotension range from rates of 0–7% stating that aggressive hydration is typically all that is required.
Melphalan
Systemic toxicity is rarely reported to be severe with melphalan. Its toxicity has been mostly associated with bone marrow depression, hair loss, maculopapular rashes, pruritus, and gastrointestinal toxicity (stomatitis, diarrhea, nausea and vomiting) Citation[5], Citation[61]. Mortality rates after HILP with melphalan range from 0% to 9%, with deaths occurring mainly in the early experience with HILP, mostly related to systemic toxicity due to significant leakage of the drug. Some of the reported deaths were due to pulmonary embolism, cardiac, renal and bone marrow failure. Generally melphalan is well tolerated Citation[9], Citation[28], Citation[56].
Hoekstra et al. reported that leakage values during HILP of about 15% do not always correlate with systemic toxicity Citation[62]. If systemic leakage exceeds 10–20% the procedure should be stopped and the limb flushed Citation[9], Citation[56]. In the ACOSOG Z0020 trial the authors identified 3 (5%) grade IV CTCAE in the melphalan alone arm Citation[35].
Fontemustine and dacarbazine
In the ILI study by Bonekamp, using fotemustine, systemic drug toxicity was observed in two patients who developed neutropenia and thrombocytopenia 18–20 days after ILI. Both problems were of brief duration and resolved spontaneously. Pulmonary toxicity, which is seen in systemic leakage of fotemustine was not seen in this study, nor were any other significant systemic toxicities Citation[18].
Complications associated with systemic heparinization and reversal with protamine
Both HILP and ILI procedures are performed under full systemic anticoagulation with heparin. Both procedures include isolation of the affected limb from the systemic circulation. This isolation typically occurs after the patient is fully anticoagulated with heparin and activating clotting times are above 300–400 seconds.
Heparin
Bleeding complications may occur in as many as 10–15% of patients when the major arterial system is violated. Patients undergoing HILP undergo systemic heparinization prior to vascular isolation of the affected limb. Although bleeding complications are not a frequent finding in the HILP literature, there is an inherent risk when heparin is used or vessels are cannulated. A meta-analysis performed by Landefeld and Byeth evaluating the risks of systemic heparinization found the mean daily incidences of fatal, major, and major or minor bleeding during heparin therapy were 0.05%, 0.8%, and 2.0%, respectively Citation[63]. Heparin acts as a co-factor for antithrombin, enhancing its activity against several clotting factors Citation[64]. In-house bleeding complications of patients undergoing percutaneous coronary intervention and systemic heparinization may be as high as 6%. Brener et al. combined data from four large percutaneous coronary intervention trials to evaluate the relationship between activated clotting time (ACT) and hemorrhagic complications. In this meta-analysis higher doses of unfractionated heparin (UFH (>5000U, or up to 90 U/Kg)) were independently associated with higher rates of major and minor bleeding at 48 hours. In this series the highest bleeding complication rate (4%) correlated with increased ACT and heparin dose (p = 0.04). Increasing UFH weight-indexed dose was associated with higher bleeding rates (OR 1.04 (1.02–1.07) for each 10U/Kg, P = 0.001) Citation[65]. Heparin-induced thrombocytopenia (HIT) is a clinicopathologic, immune-mediated syndrome of platelet activation that complicates 1% to 3% of adult unfractionated heparin exposures Citation[66], Citation[67]. HIT carries a thrombotic morbidity of 38% to 81% and mortality of approximately 28%. Onset is typically within 5 to 10 days of exposure. Although there are no documented cases of HIT in HILP, there is inherent risk due to the use of heparin in the HILP procedure.
Protamine
Protamine's efficacy is related in part to its charge, but so is its toxicity. This positively charged protein complexes with negatively charged heparin and reverses anticoagulation. In general protamine is known to provoke adverse reactions in approximately 10% of patients Citation[68]. Complications include systemic vasodilation resulting in hypotension and may be associated with the use of protamine in a dose-dependent fashion. The dominant source of information regarding protamine is derived from the cardiac/vascular bypass literature. Rapid administration is associated with a higher complication and side-effect profile Citation[69]. The protamine-heparin interaction has been shown to cause increased pulmonary artery pressure and decrease both systolic and diastolic pressures, cardiac output, systemic vascular resistance, and myocardial oxygen consumption. These multiple cardiovascular effects are compliment and histamine mediated Citation[70]. Risk factors for adverse reactions after protamine include previous cardiopulmonary bypass, insulin treatment, history of fish allergy, and prior vasectomy Citation[71]. None of the studies evaluating HILP report protamine-associated complications. However, it is easy to see that cardiovascular related symptoms associated with protamine might be assumed to correspond to chemotherapy agents used (TNF-α).
Prevention of toxicity
Close monitoring of white blood count, BUN and creatinine as well as CPK values are highly recommended after HILP or ILI. Melphalan, which is the most commonly used agent, has bone marrow suppression side effects. Daily complete blood counts should be drawn to ensure systemic leakage of the melphalan has not led to any bone marrow suppression. These labs should also be redrawn at a 2-week post-op visit to make sure there are no late bone marrow effects. Muscle necrosis can lead to rhabdomyolysis and in turn cause myoglobin-induced renal failure. Therefore, serum CPK values should be measured daily, and if they rise above 1000 u/L, then aggressive measures to prevent myoglobin-induced renal failure should be undertaken. Hydration with normal saline at rates significant enough to produce a urine output of greater than 0.5 cc/kg per hour should be sufficient to prevent myoglobin-induced renal failure. Thompson et al. and Zager et al. suggest that intravenous steroids may help after an ILI to lessen the regional inflammatory response in the muscle from reperfusion injury. Decadron 4 to 6 mg every 6 hours intravenously may lead to less post-operative edema, erythema and extremity pain Citation[12], Citation[14]. The authors usually use steroids after CPK values peak over 1000 u/l or the infused leg develops immediate post-operative grade III regional toxicity. The steroids are tapered as the symptoms abate and the CPK levels come down towards baseline. Careful and constant monitoring of the limb for compartment syndrome should be initiated. Serial neurovascular checks should be undertaken in the affected extremity in order to diagnose and then treat any compartment syndrome at an early time point, prior to permanent neurovascular damage Citation[14].
Application of a distal tight wrapping of the feet and hands, prior to drug administration, to prevent early pain and edema and late epidermiolysis and desquamation has been described Citation[9]. For repeat HILPs, cannulating the vessels at a more proximal site should be performed if possible to avoid vascular complications such as stenosis of the arteries. Patients with severe atherosclerosis of the extremity vessels are a relative contraindication to HILP and pulseless extremities are a contraindication to both HILP and ILI Citation[9], Citation[14].
Calculation of the drug dose is highly important. Dose reduction in terms of a 10% decrease in obese and/or pre-existing severely edematous extremities can help avoid potential post-op regional complications Citation[16], Citation[72]. Melphalan dosing may and should be corrected for ideal body weight in those patients. Cheng et al. demonstrated in a small series that when the ratio of estimated limb volume (Vesti) to melphalan volume of distribution (Vss) is greater than 4, the incidence of acute regional toxicity is increased. This can potentially be decreased by adjusting the dosing of melphalan to ideal body weight, especially if higher risk patients are treated (i.e. those with pre-existing moderate to severe extremity edema and obese patients) Citation[14], Citation[73].
sHILP or ILI should be stopped if leakage into systemic circulation is >20% Citation[9]. A complete washout is required at the end of the procedure for both HILP and ILI. This eliminates waste products and residual chemotherapeutic agents in the extremity therefore decreasing the risk of systemic and regional toxicity. In HILP the washout typically consists of 2 l isotonic crystalloid solution and 1 l normal saline in an ILI, prior to deflating the tourniquet Citation[14].
Summary
HILP and ILI have been proven to play a significant role in the treatment of patients with unresectable melanoma and sarcomas of the extremities. These procedures are valuable when patients are otherwise faced with the possibility of a debilitating amputation.
The regional toxicities of HILP and ILI are similar. The most common toxicities reported are Weiberdink grade II–III regional toxicities. However, when severe regional toxicities occur, albeit infrequently (<5%), amputation may be necessary. Some studies have shown a correlation with acute toxic regional effects and long term regional morbidity.
Systemic toxicity appears to be more severe when TNF-α is used in combination with other drugs. Use of TNF-α in the USA is limited to IRB approved trials, but is common in European centers. Careful monitoring, appropriate dosing, and maintenance of physiologic parameters are paramount to minimize the systemic side effects. The systemic toxicity is minimal when ILI is used compared to HILP, making ILI more suitable for higher risk patients, those with multiple co-morbidities. ILI also has the advantage over HILP of being easier to perform technically. Therefore, ILI is particularly attractive in those patients where repeat procedures need to be performed after previous HILP or ILI.
References
- Creech O, Krementz ET, Ryan RF, Winblad JN. Chemotherapy of cancer: Regional perfusion utilizing an extracorporeal circuit. Ann Surg 1958; 148: 616–632
- Thompson JF, Waugh RC, Saw RPM. Isolated limb infusion with melphalan for recurrent limb melanoma: A simple alternative to isolated limb perfusion. Ref Cancer Treat 1994; 7: 188–190
- Eggermont AM, van Geel AN, de Wilt JH, ten Hagen TL. The role of isolated limb perfusion for melanoma confined to the extremities. Surg Clin North Am 2003; 83: 371–384
- Di Filippo F, Calabro A, Giannarelli D, Carlini S, Cavaliere F, Moscarelli F, Cavaliere R. Prognostic variables in recurrent limb melanoma treated with hyperthermic antiblastic perfusion. Cancer 1989; 63: 2551–2561
- Kroon BBR, Klaase JM, Ven Geel AN, Eggermont AMM. Application of hyperthermia in regional isolated perfusion for melanoma of the limbs. Reg Cancer Treat 1992; 4: 223–226
- Eggermont AM, Schraffordt Koops H, Klausner JM, Kroon BB, Schlag PM, Liénard D, van Geel AN, Hoekstra HJ, Meller I, Nieweg OE, et al. Isolated limb perfusion with tumor necrosis factor and melphalan for limb salvage in 186 patients with locally advanced soft tissue extremity sarcomas. The cumulative multicenter European experience. Ann Surg 1996; 224: 756–764
- Benckhuijsen C, Kroon BB, van Geel AN, Weiberdink J. Regional perfusion treatment with melphalan for melanoma in a limb: An evaluation of drug kinetics. Eur J Surg Oncol 1988; 14: 157–163
- Hayes AJ, Clark MA, Harries M, Thomas JM. Management of in-transit metastases from cutaneous malignant melanoma. Br J Surg 2004; 91: 673–682
- Vrouenraets BC, Klaase JM, Nieweg OE, Kroon BB. Toxicity and morbidity of isolated limb perfusion. Semin Surg Oncol 1998; 14: 224–231
- Vrouenraets BC, Nieweg OE, Kroon BB. Thirty-five years of isolated limb perfusion for melanoma: Indications and results. Br J Surg 1996; 83: 1319–1328
- Sorkin P, Abu-Abid S, Lev D, Gutman M, Aderka D, Halpern P, Setton A, Kudlik N, Bar-On J, Rudich V. Systemic leakage and side effects of tumor necrosis factor alpha administered via isolated limb perfusion can be manipulated by flow rate adjustment. Arch Surg 1995; 130: 1079–1084
- Thompson JK, Kam PC, Waugh RC, Harman CR. Isolated limb infusion with cytotoxic agents: A simple alternative to isolated limb perfusion. Semin Surg Oncol 1998; 14: 238–247
- Thompson JF, Kam PC. Isolated limb infusion for melanoma: A simple but effective alternative to isolated limb perfusion. J Surg Oncol 2004; 88: 1–3
- Zager JS, Gershenwald JE, Aldrink J, Lin E, Cheng T, Cormier JN, Mansfield PF, Lee JE, Tyler DS, Ross MI. Isolated limb infusion for locally recurrent and in-transit extremity melanoma: A multi institutional experience. Ann Surg Oncol 2006; 13: 75
- Lindner P, Thompson JF, De Wilt JH, Colman M, Kam PC. Double isolated limb infusion with cytotoxic agents for recurrent and metastatic limb melanoma. Eur J Surg Oncol 2004; 30: 433–439
- Brady MS, Brown K, Patel A, Fisher C, Marx W. A phase II trial of isolated limb infusion with melphalan and dactinomycin for regional melanoma and soft tissue sarcoma of the extremity. Ann Surg Oncol 2006; 13: 1123–1129
- Lindner P, Doubrovsky A, Kam P, Thompson J. Prognostic factors after isolated limb infusion with cytotoxic agents for melanoma. Ann Surg Oncol 2002; 9: 127–136
- Bonenkamp JJ, Thompson JF, de Wilt JH, Doubrovsky A, de Faria Lima R, Kam PC. Isolated limb infusion with fotemustine after dacarbazine chemosensitisation for inoperable loco-regional melanoma recurrence. Eur J Surg Oncol 2004; 30: 1107–1112
- Weiberdink J, Benckhuysen C, Braat R, Van Slooten EA, Olthuis GA. Dosimetry in isolation perfusion of the limbs by assessment of perfused tissue volume and grading of toxic tissue reactions. Eur J Cancer Clin Oncol 1982; 18: 905–910
- Ovrum E, Lindberg H, Holen EA, Abdelnoor M, Bech J. Systemic and pulmonary circulatory effects of protamine following cardiopulmonary bypass in man. Scand J Thorac Cardiovasc Surg 1991; 25: 19–24
- Hoven-Gondrie ML, Thijssens KM, Van den Dungen JJ, Loonstra J, van Ginkel RJ, Hoekstra HJ. Long-term locoregional vascular morbidity after isolated limb perfusion and external-beam radiotherapy for soft tissue sarcoma of the extremity. Ann Surg Oncol 2007; 14: 2105–2112
- Eroğlu A, Ozcan H, Eryavuz Y, Kocağlu H, Demirci S, Aytac SK. Deep venous thrombosis of the extremity diagnosed by color Doppler ultrasonography after isolated limb perfusion. Tumori 2001; 87: 187–190
- Vrouenraets BC, Kroon BB, Klaase JM. Severe acute regional toxicity after normothermic or ‘mild’ hyperthermic limb perfusion with melphalan for melanoma. Melanoma Res 1995; 5: 425–431
- Guadagni S, Santinami M, Patuzzo R, Pilati PL, Miotto D, Deraco M, Rossi CR, Fiorentini G, Di Filippo F, Valenti M, et al. Hypoxic pelvic and limb perfusion with melphalan and mitomycin C for recurrent limb melanoma: A pilot study. Melanoma Res 2003; 13: 51–58
- Aloia TA, Grubbs E, Onaitis M, Mosca PJ, Cheng TY, Seigler H, Tyler DS. Predictors of outcome after hyperthermic isolated limb perfusion: Role of tumor response. Arch Surg 2005; 140: 1115–1120
- Eggimann P, Chiolero R, Chassot PG, Lienard D, Gerain J, Lejeune F. Systemic and hemodynamic effects of recombinant tumor necrosis factor alpha in isolation perfusion of the limbs. Chest 1995; 107: 1074–1082
- Grubbs EG, Abdel-Wahab O, Cheng TY, Abdel-Wahab Z, Peterson B, Pruitt SK, Colvin OM, Friedman HS, Tyler DS. In-transit melanoma: The role of alkylating-agent resistance in regional therapy. J Am Coll Surg 2004; 199: 419–427
- Vrouenraets BC, Eggermont AM, Hart AA, Klaase JM, van Geel AN, Nieweg OE, Kroon BB. Regional toxicity after isolated limb perfusion with melphalan and tumour necrosis factor- alpha versus toxicity after melphalan alone. Eur J Surg Oncol 2001; 27: 390–395
- Rossi CR, Mocellin S, Pilati P, Foletto M, Campana L, Quintieri L, De Salvo GL, Lise M. Hyperthermic isolated perfusion with low-dose tumor necrosis factor alpha and doxorubicin for the treatment of limb-threatening soft tissue sarcomas. Ann Surg Oncol 2005; 12: 1–8
- Grünhagen DJ, de Wilt JH, Graveland WJ, Verhoef C, van Geel AN, Eggermont AM. Outcome and prognostic factor analysis of 217 consecutive isolated limb perfusions with tumor necrosis factor-alpha and melphalan for limb-threatening soft tissue sarcoma. Cancer 2005; 106: 1776–1784
- Bonvalot S, Laplanche A, Lejeune F, Stoeckle E, Le Pechoux C, Vanel D, Terrier P, Lumbroso J, Ricard M, Antoni G, et al. Limb salvage with isolated perfusion for soft tissue sarcoma: Could less TNF-alpha be better?. Ann Oncol 2005; 16: 1061–1068
- Noorda EM, Vrouenraets BC, Nieweg OE, van Coevorden F, van Slooten GW, Kroon BB. Isolated limb perfusion with tumor necrosis factor-alpha and melphalan for patients with unresectable soft tissue sarcoma of the extremities. Cancer 2003; 98: 1483–1490
- Noorda EB, Vrouenraets BC, Nieweg OE, van Geel B, Eggermont AM, Kroon BB. Isolated limb perfusion for unresectable melanoma of the extremities. Arch Surg 2004; 139: 1237–1242
- Möller MG, Zager JS, Lewis JM, Dessureault D, Kim C, Puleo C, Reintgen D, Letson DG. Single agent experience for hyperthermic isolated limb perfusion of extremity sarcomas. Ann Surg Oncol 2007; 14: 72
- Cornett WR, McCall LM, Petersen RP, Ross MI, Briele HA, Noyes RD, Sussman JJ, Kraybill WG, Kane JM, III, Alexander HR, et al. Randomized multicenter trial of hyperthermic isolated limb perfusion with melphalan alone compared with melphalan plus tumor necrosis factor: American College of Surgeons Oncology Group Trial Z0020. J Clin Oncol 2006; 24: 4196–4201
- Bulman AS, Jamieson CW. Isolated limb perfusion with melphalan in the treatment of malignant melanoma. Br J Surg 1980; 67: 660–662
- Klicks RJ, Vrouenraets BC, Nieweg OE, Kroon BB. Vascular complications of limb perfusion. Eur J Surg Oncol 1998; 24: 288–291
- Klicks RJ. September 11–13, 1995; 46, (Abstract) VII International Congress of Regional Cancer Treatment. Wiesbaden, Germany
- Pennacchioli E, Deraco M, Mariani L, Fiore M, Mussi C, Collini P, Olmi P, Casali PG, Santinami M, Gronchi A. Advanced extremity soft tissue sarcoma: Prognostic effect of isolated limb perfusion in a series of 88 patients treated at a single institution. Ann Surg Oncol 2007; 14: 553–559
- Hayes AJ, Neuhaus SJ, Clark MA, Thomas JM. Isolated limb perfusion with melphalan and tumor necrosis factor alpha for advanced melanoma and soft-tissue sarcoma. Ann Surg Oncol 2007; 14: 230–238
- Grunhagen DJ, de Wilt JH, Graveland WJ, van Geel AN, Eggermont AM. The palliative value of tumor necrosis factor alpha-based isolated limb perfusion in patients with metastatic sarcoma and melanoma. Cancer 2005; 106: 156–162
- Knorr C, Meyer T, Janssen T, Goehl J, Hohenberger W. Hyperthermic isolated limb perfusion (HILP) in malignant melanoma. Experience with 101 patients. Eur J Surg Oncol 2006; 32: 224–227
- Vrouenraets BC, Klaase JM, Kroon BB, van Geel BN, Eggermont AM, Franklin HR. Long-term morbidity after regional isolated perfusion with melphalan for melanoma of the limbs. The influence of acute regional toxic reactions. Arch Surg 1995; 130: 43–47
- Vrouenraets BC, Keus RB, Nieweg OE, Kroon BB. Complications of combined radiotherapy and isolated limb perfusion with tumor necrosis factor alpha +/− interferon gamma and melphalan in patients with irresectable soft tissue tumors. J Surg Oncol 1997; 65: 88–94
- Olieman AF, Schraffordt Koops H, Geertzen JH, Kingma H, Hoekstra HJ, Oldhoff J. Functional morbidity of hyperthermic isolated regional perfusion of the extremities. Ann Surg Oncol 1994; 1: 382–388
- Vrouenraets BC, in't Veld GJ, Nieweg OE, van Slooten GW, van Dongen JA, Kroon BB. Long-term functional morbidity after mild hyperthermic isolated limb perfusion with melphalan. Eur J Surg Oncol 1999; 25: 503–508
- van Geel AN, van Wijk J, Weiberdink J. Functional morbidity after regional isolated perfusion of the limb for melanoma. Cancer 1989; 63: 1092–1096
- Vrouenraets BC, Eggermont AM, Klaase JM, Van Geel BN, Van Dongen JA, Kroon BB. Long-term neuropathy after regional isolated perfusion with melphalan for melanoma of the limbs. Eur J Surg Oncol 1994; 20: 681–685
- Noorda EM, Vrouenraets BC, Nieweg OE, van Geel AN, Eggermont AM, Kroon BB. Repeat isolated limb perfusion with TNFalpha and melphalan for recurrent limb melanoma after failure of previous perfusion. Eur J Surg Oncol 2006; 32: 318–324
- Grünhagen DJ, van Etten B, Brunstein F, Graveland WJ, van Geel AN, de Wilt JH, Eggermont AM. Efficacy of repeat isolated limb perfusions with tumor necrosis factor alpha and melphalan for multiple in-transit metastases in patients with prior isolated limb perfusion failure. Ann Surg Oncol 2005; 12: 609–615
- Klaase JM, Kroon BB, van Geel AN, Eggermont AM, Franklin HR, van Dongen JA. A retrospective comparative study evaluating the results of a single-perfusion versus double-perfusion schedule with melphalan in patients with recurrent melanoma of the lower limb. Cancer 1993; 71: 2990–2994
- Kroon BB, Klaase JM, van Geel BN, Eggermont AM, Franklin HR, van Dongen JA. Results of a double perfusion schedule with melphalan in patients with melanoma of the lower limb. Eur J Cancer 1993; 29A: 325–328
- Krementz ET, Ryan RF, Carter RD, Sutherland KM, Reed RJ. Hyperthermic regional perfusion of the limbs. Cutaneous melanoma: Clinical management and treatment worldwide, CH Balch, GW Milton. Lippincott, Philadelphia 1985; 171–190
- Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer 1981; 47: 207–214
- National Cancer Institute. Common Terminology Criteria for Adverse Events v3.0 (CTCAE). 2006, Available at: http://ctep.cancer.gov/forms/CTCAEv3.pdf. Accessed 29 October 2007
- Sonneveld EJ, Vrouenraets BC, van Geel Bn, Eggermont Am, Klaase JM, Nieweg OE, van Dogen JA, Kroon BB. Systemic toxicity after isolated limb perfusion with melphalan for melanoma. Eur J Surg Oncol 1996; 22: 521–527
- Zwaveling JH, Maring JK, Clarke FL, van Ginkel RJ, Limburg PC, Hoekstra HJ, Koops HS, Girbes AR. High plasma tumor necrosis factor (TNF)-alpha concentrations and a sepsis-like syndrome in patients undergoing hyperthermic isolated limb perfusion with recombinant TNF-alpha, interferon-gamma, and melphalan. Crit Care Med 1996; 24: 765–770
- Renard N, Liénard D, Lespagnard L, Eggermont A, Heimann R, Lejeune F. Early endothelium activation and polymorphonuclear cell invasion precede specific necrosis of human melanoma and sarcoma treated by intravascular high-dose tumour necrosis factor alpha (rTNF alpha). Int J Cancer 1994; 57: 656–663
- Lewis JM, Sander G, Reintgen D, Letson DG. Hyperthermic limb perfusion: Evolving concepts in the treatment of extremity soft tissue sarcoma. Curr Opin Orthop 2006; 17: 1–5
- Lans TE, Grünhagen DJ, de Wilt JH, van Geel AN, Eggermont AM. Isolated limb perfusions with tumor necrosis factor and melphalan for locally recurrent soft tissue sarcoma in previously irradiated limbs. Ann Surg Oncol 2005; 12: 406–411
- Furner RL, Brown RK. L-phenylalanine mustard (L-PAM): the first 25 years. Cancer Treat Rep 1980; 64: 559–574
- Hoekstra HJ, Naujocks T, Schraffordt Koops H. Continuous leakage monitoring during hyperthermic isolated regional perfusion of the lower limb: Techniques and results. Reg Cancer Treat 1992; 4: 301–304
- Landefeld CS, Byeth RJ. Anticoagulant-related bleeding: Clinical epidemiology, prediction, and prevention. Am J Med 1993; 95: 315–328
- Love DG. Management of hemorrhagic events in patients receiving anticoagulant therapy. J Thromb Thrombolysis 1999; 7: 149–152
- Brener SJ, Moltinero DJ, Lincoff AM, Steinhubl SR, Wolski KE, Topol EJ. Relationship between activated clotting time and ischemic or hemorrhagic complications. Analysis of four recent randomized clinical trials of percutaneous coronary intervention. Circulation 2004; 110: 994–998
- Warkentin TE, Greinacher A. Heparin-induced thrombocytopenia and cardiac surgery. Ann Thorac Surg 2003; 76: 2121–2131
- Warkentin TE, Hematology. Heparin-induced thrombocytopenia: Pathogenesis and management. Br J Haematol 2003; 123: 373–374
- Weiler JM, Gellhaus MA, Carter JG. A prospective study of the risk of an immediate adverse reaction to protamine sulfate during cardiopulmonary bypass surgery. J Allergy Clin Immunol 1990; 85: 713–719
- Wakefield TW, Hantler CB, Wrobleski SK, Crider BA, Stanley JC. Effects of differing rates of protamine reversal of heparin anticoagulation. Surgery 1996; 119: 123–128
- Carr JA, Silverman N. The heparin-protamine interaction. J Cardiovasc Surg (Torino) 1999; 40: 659–666
- Gupta SK, Veith FJ, Ascer E. Anaphylactoid reactions to protamine: An often lethal complication in insulin-dependent patients undergoing vascular surgery. J Vasc Surg 1988; 9: 342–350
- Vrouenraets BC, Hart GA, Eggermont AM, Klaase JM, van Geel BN, Nieweg OE, Kroon BB. Relation between limb toxicity and treatment outcomes after isolated limb perfusion for recurrent melanoma. J Am Coll Surg 1999; 188: 522–530
- Cheng TY, Grubbs E, Abdul-Wahab O, Leu SY, Hung CF, Petros W, Aloia T, Fedrau R, Pruitt SK, Colvin M, et al. Marked variability of melphalan drug levels during regional hyperthermic isolated limb perfusion. Am J Surg 2003; 186: 460–467
- Grünhagen DJ, Brunstein F, Graveland WJ, van Geel AN, de Wilt JHW, Eggermont AM. One hundred consecutive isolated limb perfusions with TNF- and melphalan in melanoma patients with multiple in-transit metastases. Ann Surg 2004; 240: 939–948
- Grünhagen DJ, Brunstein F, Graveland WJ, Van Geel A, De Wilt JW, Eggermont AM. Isolated limb perfusion with tumor necrosis factor and melphalan prevents amputation in patients with multiple sarcomas in arm or leg. Ann Surg Oncol 2005; 12: 1–7
- Grünhagen DJ, de Wilt JH, van Geel AN, Graveland WJ, Verhoef C, Eggermont AM. TNF dose reduction in isolated limb perfusion. Eur J Surg Oncol 2005; 31: 1011–1019
- Rossi CR, Lejeune FJ, Pontes L, Foletto M, De Salvo GL, Pilati PL, Mocellin S, Ribeiro M, Lopes M, Lise M. Phase I–II study on isolation antiblastic fotemustine perfusion after dacarbazine chemosensitisation for advanced melanoma of the extremities. Melanoma Res 2003; 13: 293–297
- Rossi CR, Foletto M, Mocelli S, Pilati P, Lise M. Hyperthermic isolated limb perfusion with low-dose tumor necrosis factor-α and melphalan for bulky in-transit melanoma metastases. Ann Surg Oncol 2004; 11: 173–177